Abstract
Background
Patients with sepsis-associated encephalopathy (SAE) have higher mortality rates and longer ICU stays. Predictors of SAE are yet to be identified. We aimed to establish an effective and simple-to-use nomogram for the individual prediction of SAE in patients with sepsis admitted to pediatric intensive care unit (PICU) in order to prevent early onset of SAE.
Methods
In this retrospective multicenter study, we screened 790 patients with sepsis admitted to the PICU of three hospitals in Shandong, China. Least absolute shrinkage and selection operator regression was used for variable selection and regularization in the training cohort. The selected variables were used to construct a nomogram to predict the risk of SAE in patients with sepsis in the PICU. The nomogram performance was assessed using discrimination and calibration.
Results
From January 2017 to May 2022, 613 patients with sepsis from three centers were eligible for inclusion in the final study. The training cohort consisted of 251 patients, and the two independent validation cohorts consisted of 193 and 169 patients. Overall, 237 (38.7%) patients developed SAE. The morbidity of SAE in patients with sepsis is associated with the respiratory rate, blood urea nitrogen, activated partial thromboplastin time, arterial partial pressure of carbon dioxide, and pediatric critical illness score. We generated a nomogram for the early identification of SAE in the training cohort (area under curve [AUC] 0.82, 95% confidence interval [CI] 0.76–0.88, sensitivity 65.6%, specificity 88.8%) and validation cohort (validation cohort 1: AUC 0.80, 95% CI 0.74–0.86, sensitivity 75.0%, specificity 74.3%; validation cohort 2: AUC 0.81, 95% CI 0.73–0.88, sensitivity 69.1%, specificity 83.3%). Calibration plots for the nomogram showed excellent agreement between SAE probabilities of the observed and predicted values. Decision curve analysis indicated that the nomogram conferred a high net clinical benefit.
Conclusions
The novel nomogram and online calculator showed performance in predicting the morbidity of SAE in patients with sepsis admitted to the PICU, thereby potentially assisting clinicians in the early detection and intervention of SAE.
Similar content being viewed by others
Introduction
Sepsis is a major cause of morbidity and mortality in children worldwide [1]. Sepsis-associated encephalopathy (SAE) is a diffuse brain dysfunction that excludes direct central nervous system infections, structural abnormalities, and metabolic encephalopathy [2,3,4]. Patients with SAE are characterized by a series of neurological disturbances, ranging from drowsiness and mild delusions to coma, and relatively rarely by convulsions, tremors, or myoclonus. Owing to the differences in the study populations, different studies have reported its incidence to be from 8% to more than 70% [5]. Patients with SAE tend to have a higher 28-day mortality, spend more time on a ventilator, and have a longer intensive care unit (ICU) stay [6]. Moreover, long-term cognitive impairment and functional disability can persist in many survivors of severe sepsis [7]. SAE can be an early feature of infection and appear before other systemic manifestations of sepsis [4]. As SAE is not a direct infection of central nervous system, the treatment focus remains on the proper management of sepsis [4]. Therefore, early identification of SAE, even before sepsis is diagnosed, is critical for timely investigation for and intervention of infection, thus reducing the associated morbidity and mortality.
Patients with SAE often have significantly higher heart rate, blood lactate, and serum sodium levels, as well as lower platelet count, serum albumin, and serum pH than patients without SAE [6, 8]. Some evidence suggests that electroencephalography (EEG) and magnetic resonance imaging (MRI) are useful for detecting the occurrence of SAE and assessing brain injury, but further clinical investigations are needed to confirm this conclusion [9, 10]. To date, most of the aforementioned research on SAE prediction has been performed in adults, whereas studies on pediatric patients are relatively lacking. Developing a prediction model that is widely used in critically ill children admitted to the pediatric intensive care unit (PICU) can contribute to a decline in the morbidity and mortality of pediatric patients with SAE.
The nomogram model, as a visualization tool, has been widely used in clinical prognosis research among critically ill and cancer patients [11]. In the present study, we collected multiple data points from patients with sepsis admitted to the PICU in three medical institutions to screen the risk factors of SAE and developed a novel prediction nomogram for the morbidity of SAE.
Methods
Study design and participants
We conducted a multicenter retrospective observational study involving the patients with sepsis admitted to the PICU of three hospitals in China from January 2017 to May 2022. Patient inclusion criteria were as follows: (1) age > 28 days and ≤ 18 years; (2) diagnosis of sepsis according to the sepsis 3.0 definition evaluated by pediatric sequential organ failure assessment scores (pSOFA) [12], which is the life-threatening organ dysfunction caused by a dysregulated host response to infection [13]. Patients with the following underlying conditions that may affect brain function and symptomatic diagnosis were excluded: (1) primary central nervous system disease, including traumatic brain injury, cerebral vascular disease, intracranial infection, autoimmune encephalitis, and epilepsy; and (2) metabolic encephalopathy caused by diabetic ketoacidosis, hypoglycemia, hepatic encephalopathy, and inherited metabolic diseases. Patients treated with mechanical ventilation during the first 24 h after PICU admission and patients with missing laboratory data were also excluded. This study was approved by the Institutional Ethics Review Board of Qilu Hospital of Shandong University (KYLL-202202-027-1). The requirement for individual patient consent was waived due to the retrospective design of the study, and all patient information was handled anonymously. The Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statement was used as reporting guideline (Additional file 1: Table S1) [14].
Data collection
All data were reviewed and extracted from the electronic medical record systems of the three hospitals by a single data collector who was blinded to the study design. For each patient included in the study, the following variables were collected: (1) demographic data including age and gender; (2) the first data of vital signs during the first 2 h after PICU admission; (3) suspected infection site and micro-organism; (4) the use of vasopressors (adrenaline, norepinephrine, dopamine, dobutamine, and other vasopressors) during PICU stay time; (5) advanced life support during PICU stay time, including mechanical ventilation and renal replacement therapy; (6) outcomes, including the occurrence of multi-organ dysfunction and septic shock, hospital stay time, PICU stay time, and in-hospital mortality; (7) severity scores on PICU admission, namely the pediatric critical illness score (PCIS) [15]; (8) the first laboratory data after PICU admission. All patients underwent routine laboratory tests upon admission to PICU. For patients treated with renal replacement therapy during the first 24 h after PICU admission, we used the laboratory data before the treatment.
Sepsis-associated encephalopathy
SAE is defined as the combination of sepsis with an altered mental status with cognitive or behavioral abnormalities accompanied by Glasgow Coma Scale (GCS) ≤ 14 during PICU stay [2, 4]. Cognitive and neuropsychiatric disorders of SAE include inattention, disorientation, agitation, irritability, decreased psychomotor activity, somnolence, stupor, and coma, which were clearly documented in medical record by doctors and nurses. Patients with changes in consciousness caused by primary central nervous system disease, metabolic encephalopathy, and toxicosis were excluded. For patients treated with medications that may cause a decrease in level of consciousness (e.g., sedative or analgesic drugs), clinical examination as well as neuroimaging, neurophysiological testing and serum biomarkers such as neuron-specific enolase (NSE) and S100 calcium-binding protein B (S100B) were used together to evaluate the diagnosis of SAE. Brain computer tomography (CT) and MRI findings vary from normal in mild cases of SAE to non-specific structural changes, such as vasogenic edema, leukoencephalopathy, ischemic lesions, and changes in subcortical and cerebellar regions in serious cases [16, 17]. EEG alterations in SAE patients include slow waves, triphasic waves, focal seizures, generalized suppression, and burst suppression [17]. Elevated serum levels of NSE and S100B are proven to be associated with brain injury in patients with sepsis [18], and the thresholds are 16.3 ng/mL for NSE and 0.105 µg/L for S100B in our centers. We checked the daily data in electronic records and once the diagnostic criteria were met, SAE could be diagnosed.
Statistical analysis
Shapiro–Wilk tests were used to test the normal distributions of the variables. Continuous variables with normal distribution were tested by unpaired Student’s t test and presented as the mean ± standard deviation (SD), whereas non-normal distributed continuous variables were tested by Mann–Whitney U-test and presented as the median (interquartile range, IQR). Categorical variables were tested using Chi-square analysis or Fisher’s exact test and are described as numbers (percentages). Univariate and multivariate regression analyses were used to identify independent risk factors associated with SAE in the training cohort. Variables were omitted when more than 30% of the values were missing. Multicollinearity between continuous variables was detected by the variance inflation factor (VIF), and an arithmetic square root of VIF ≤ 2 was considered as non-collinearity.
For the model-building process, least absolute shrinkage and selection operator (LASSO) regression was used for variable selection and regularization in the training cohort. The selected variables were used to build a nomogram to predict the risk of SAE in patients with sepsis admitted to the PICU. The nomogram performance was assessed using discrimination and calibration. We used the receiver operating characteristic curve to assess the discriminative performance of the nomogram and then assessed the area under the curve (AUC). Calibration was performed using the bootstrap method with 1000 re-samplings to analyze the association between the observed incidence and predicted probability. We evaluated the clinical usefulness and net benefit of the new predictive model by decision curve analysis (DCA) in the training set and the validation sets. All statistical analyses were performed using R version 3.6.3 (https://www.r-project.org; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-sided P-value < 0.05.
Results
Baseline and clinical characteristics of the cohorts
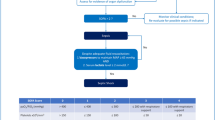
The recruitment of the study population is presented in Fig. 1. Among a total of 7673 PICU patients, 790 patients met the diagnosis criteria of sepsis 3.0. After excluding 177 patients, 613 patients with sepsis from three centers in Jinan and Jining were included in the final study. Of these, 251 patients from Qilu Hospital of Shandong University were included as the training cohort, while 193 patients from Jinan Children’s Hospital of Shandong University and 169 patients from Jining First People's Hospital were included as independent external validation cohorts. The patient characteristics of the training and validation cohorts are shown in Additional file 2: Table S2. There were no significant differences in age or sex between the training and validation cohorts (P > 0.05). The data on when SAE was diagnosed are shown in Additional file 3: Table S3. Most of the SAE patients were diagnosed within 72 h after PICU admission, while others were diagnosed longer after PICU admission. The earliest time for SAE diagnosis was 8 h, and the average time were 41.0 (interquartile range [IQR] 26.0–61.5) hours, 58.0 (IQR 31.5–91.0) hours, and 48.0 (IQR 25.0–105.0) hours for the training and validation cohorts, respectively. Of the 251 patients with sepsis in the training cohort, 90 (35.9%) were diagnosed with SAE. The baseline and outcome characteristics of the training cohort are presented in Table 1. No significant differences in age or sex were identified between the SAE and non-SAE groups (P = 0.773 and P = 0.372, respectively). The average age of the patients with SAE was 46.5 (IQR 8.2–99) months, and 53 patients (58.9%) were males. Heart rate (HRs) and respiratory rates (RRs) on PICU admission were significantly higher in the SAE group than in the non-SAE group (HR: 126.5, IQR 109.2–149 vs. 115, IQR 102–131, P = 0.004; RR: 30, IQR 25–40 vs. 25, IQR 22–30, P < 0.001). However, there were no significant differences between the two groups in other vital signs including systolic pressure, diastolic pressure, and temperature > 38 ℃. No significant differences were observed in suspected infection focus and blood culture positivity between the groups (P > 0.05), and both patients with and without SAE displayed a high incidence of respiratory infection (51.1% and 46%, respectively). The main pathogens detected in this population were Staphylococcus, Enterococcus, Streptococcus, Escherichia coli, Acinetobacter, Pseudomonas, Klebsiella, and fungi. Patients with SAE seemed to have a higher rate of Gram-negative infections than those without (16.7% vs. 8.7%); however, the difference was not statistically significant (P = 0.091). Patients with SAE were more likely to use vasopressors during the PICU stay (36.7% vs. 1.9%, P < 0.001). Moreover, patients in the SAE group had a higher incidence of mechanical ventilation during the PICU stay than those in the non-SAE group (33.3% vs. 3.1%). As shown in Table 1, patients with SAE were more likely to suffer from multi-organ dysfunction (87.8% vs. 29.8%, P < 0.001), and thus had a higher in-hospital mortality rate (35.6% vs. 0, P < 0.001). There were no significant differences in the lengths of hospital and PICU stay (P = 0.717 and P = 0.892, respectively).
Laboratory information of patients on PICU admission in training cohort
As shown in Table 2, alanine transaminase (ALT), total bilirubin (TBIL), creatine kinase-MB (CK-MB), serum creatinine (Scr), blood urea nitrogen (BUN), lactic dehydrogenase (LDH), prothrombin time (PT), activated partial thromboplastin time (APTT), lactate, arterial partial pressure of carbon dioxide (PaCO2), and blood glucose levels were significantly higher in the SAE group than in the non-SAE group (P < 0.05). Moreover, lower platelet (PLT) count, albumin, fibrinogen level, and arterial oxygen partial pressure (PaO2) were observed in the SAE group (P < 0.05). The PCIS was assessed upon PICU admission to evaluate the severity of illness in patients with sepsis. Patients in the SAE group had lower PCIS scores than those in the non-SAE group (90, IQR 80.5–96 vs. 94, IQR 90–96, P < 0.001).
Independent risk factors associated with the occurrence of SAE
Univariate and multivariate logistic regression showed that independent risk factors for SAE were RR (OR 1.048, 95% CI 1.010–1.090, P = 0.015), BUN (OR 1.192, 95% CI 1.079–1.330, P = 0.001), APTT (OR 1.108, 95% CI 1.052–1.176, P < 0.001), PaCO2 (OR 1.056, 95% CI 1.024–1.092, P = 0.001), and PCIS (OR 0.954, 95% CI 0.912–0.996, P = 0.033) (Table 3).
Development of a prediction nomogram
Based on the training cohort, a LASSO regression model was established to screen variables for the SAE nomogram (Fig. 2). RR, BUN, APTT, PaCO2, and PCIS were identified as predictors in the nomogram. These factors can be used to predict the probability of developing SAE in patients with sepsis, and were presented as a visualization nomogram (Fig. 3). The model established a scoring criteria and assigned a score to each level of prognostication. By summing the scores from each variable, projecting the total scores onto the scale, and drawing a straight line to the bottom of the scale, the nomogram allows users to easily estimate the probability of SAE according to the individual patient characteristics.
Construction of SAE prediction nomogram in the training cohort. The points of all features were added to obtain the total points, and a vertical line was drawn on the total point to obtain the corresponding ‘predicted value’, which indicates the risk of SAE. PaCO2: arterial partial pressure of carbon dioxide; PCIS: pediatric critical illness score; RR: respiratory rate; BUN: blood urea nitrogen; APTT: activated partial thromboplastin time.
Validation of the nomogram
Our model yielded an AUC value of 0.82 (95% CI 0.76–0.88) in the training cohort, with a sensitivity and specificity of 65.6% and 88.8%, respectively (Fig. 4A). Moreover, the calibration plot for the nomogram showed excellent agreement in the SAE probabilities between the observed and predicted values, as both the logistic and nonparametric calibration curves deviated slightly from the ideal line (Fig. 5A). Consistent with the training cohort, in validation cohort 1, the AUC was 0.80 (95% CI 0.74–0.86) for patients, with a sensitivity of 75.0% and a specificity of 74.3% (Fig. 4B). In validation cohort 2, the AUC was 0.81 (95% CI 0.73–0.88) for patients, with a sensitivity of 69.1% and specificity of 83.3% (Fig. 4C). The calibration curves also showed good agreement between the prediction and observation of the risk of SAE in the two external validation cohorts (Fig. 5B, C).
Clinical use of the nomogram
The DCA curves for the prediction nomogram in the training and validation cohorts are shown in Fig. 6. According to the DCA, the SAE model of the net benefit had a wide range of threshold probabilities in the training and validation cohorts. To facilitate clinical use, an Excel-based tool or web calculator (https://johny2020.shinyapps.io/Dyn_SAE/) was also developed for the convenience of clinicians (Fig. 7).
Decision curve analysis (DCA) of the nomogram of SAE. DCA compares the net benefits of three scenarios in predicting the risk of SAE: A perfect prediction model (grey line), screen none (horizontal solid black line), and screen based on the nomogram (ride line). The DCA curves were depicted in the training cohort (A), validation cohort 1 (B), and validation cohort 2 (C)
An Internet browser-based online calculator for the SAE prediction nomogram (website: https://johny2020.shinyapps.io/Dyn_SAE/)
Discussion
Our study evaluated several variables in 613 patients with sepsis in the PICU from multiple centers. RR, BUN, APTT, PaCO2, and PCIS were included as predictors of SAE in the nomogram established using a LASSO regression model. The nomogram shows early predictive performance compared with the actual diagnostic time for SAE. A previous study among adult patients with sepsis found that acute renal failure, hypoglycemia, hypercapnia, hypernatremia, and S. aureus infection were independent predictors of SAE [19], and the different results from our study demonstrated a difference between adult and pediatric patients. Another retrospective single-center study of 210 pediatric patients with sepsis showed that procalcitonin, Ca2+, septic shock, pediatric logistic organ dysfunction score 2 (PELOD-2), and midazolam were independent risk factors for SAE; however, no comprehensive predictive model has been developed [20]. Moreover, our study showed that SAE was associated with higher in-hospital mortality and higher use of ICU resources, confirming the early predictive value of SAE at PICU admission.
PaCO2, a vital predictive variable of SAE, was incorporated into the prediction model in our study. Similar results were also reported in another retrospective multicenter analysis, which showed that hypercapnia > 45 mmHg was an independent risk factor for SAE in patients with sepsis upon ICU admission [19]. Carbon dioxide is known to affect cerebral autoregulation in patients with sepsis or septic shock, which may explain why higher PaCO2 levels induce SAE [21, 22]. In fact, impaired autoregulation induces increased blood flow in the brain by reducing capillary resistance in the early stage of sepsis, and in the later stages, cerebral hypoperfusion predominates and appears to be a cause of SAE [17]. However, Thees et al. [23] found that carbon dioxide reactivity was not impaired in patients with sepsis syndrome. Therefore, further clinical studies and animal experiments are required to identify the mechanism of carbon dioxide in the occurrence and development of SAE.
As one of the most commonly used indicators of the endogenous coagulation system in clinical practice, APTT was previously shown to be significantly increased in children with SAE compared to those without [20]. Similarly, in the present study, APTT was significantly associated with the occurrence of SAE (P < 0.001) and was included in the prediction nomogram because of its strong influence. Several studies have found that increased APTT is a risk factor for death in critically ill children and adults with sepsis [24, 25]. Currently, coagulation disorders are widely considered important causes of microcirculation disturbances, organ dysfunction, and multiple organ failure in sepsis. Overall, APTT was identified as a predictive factor for SAE in our study, and normal coagulation function may be critical in improving the outcomes of patients with SAE.
BUN is a representative indicator of renal function, reflecting the degree of kidney injury and accumulation of metabolic waste, including neurotoxic substances such as antibiotics and hypnotics [19]. Several studies have shown that the accumulation of neurotoxic substances caused by renal insufficiency is one of the risk factors for SAE [7, 19]. Acute renal failure is associated with specific biological alterations, such as severe acidosis and uremia, which may influence brain function [19]. In the present study, we found that BUN had a high predictive value for SAE and was thus used in the prediction model. Another study which included 9126 patients with sepsis reported that BUN was an independent and easily available predictor of 28-day mortality in critically ill patients with sepsis admitted to the ICU [26]. Furthermore, BUN was found to be correlated with the severity and mortality of SAE [20, 27, 28] and proven to be a risk factor related to the in-hospital mortality of patients with sepsis in other models [25, 29]. Accordingly, it is advisable that normal renal function is maintained to reduce the occurrence of SAE and mortality in patients with SAE.
The respiratory rate was also closely associated with SAE in our study. Tachypnea is a compensatory mechanism for tissue hypoxia and metabolic acidosis and reflects the severity of metabolic disorders and tissue hypoxia to a certain extent. As is well known, metabolic disorders, hypoxia and inflammatory cytokine storm are involved in the process of cell damage and permeability increase of endothelial cells, which may be responsible for the occurrence of SAE [17, 30]. Respiratory rate ≥ 22 per minute in sepsis patients can be rapidly identified as an indicator for poor outcome, which was a main part of quick sequential organ failure assessment score (qSOFA) [31]. Respiratory rate has also been included in a model predicting 28-day mortality in patients with severe sepsis and septic shock in the emergency department [32]. Recently, some studies have found that the respiratory rate also shows good predictive value for mortality in patients with SAE [8, 28]. The conclusions of the previous studies further support our results. Therefore, more attention should be paid to patients with sepsis with tachypnea, and measures should be promptly taken to redress their hypoxia and metabolic acidosis, thus reducing the incidence and mortality of SAE.
The PCIS is a quick scoring tool with simple calculations and indices that is widely used for critically ill children in China [33]. Previous studies have shown that PCIS is a valuable predictor of early diagnosis and outcomes in pediatric patients with sepsis [34]. A study of 193 hospitalized children with severe sepsis found that the PCIS and pSOFA had similar predictive values in evaluating patient prognosis [35]. Consistent with previous studies, the present study found that PCIS was a vital risk factor for SAE development in children with sepsis. Moreover, the PCIS showed good accuracy and discriminatory ability in the prognostic prediction of many other critical diseases, such as pneumonia-related bacteremia, hand-foot-and-mouth disease, acute paraquat poisoning, and acute leukemia [36,37,38,39]. However, data on the correlation between PCIS and SAE are limited, and future high-quality studies are needed for further exploration.
Other variables were significantly different between the SAE and non-SAE groups, including lactate levels. Elevated serum lactate levels indicate an oxygen supply/demand mismatch and microcirculatory impairment, inducing tissue ischemia and hypoxia in sepsis [40]. Previous studies have suggested that lactate is an important indicator for predicting the prognosis of patients with sepsis and is often used to evaluate disease severity and guide treatment [41]. In patients with septic shock, monitoring serum lactate levels can guide fluid resuscitation and reduce mortality [42]. Many studies have also reported that high lactate levels are significantly associated with mortality in patients with SAE [6, 43]. Moreover, cerebrospinal fluid (CSF) lactate levels in patients with SAE were also increased (2.8–7.9 mmol/L) [17]. In general, the serum lactate level is an important indicator for evaluating the morbidity and prognosis of sepsis and SAE. For patients with lactate acidosis, physicians should provide timely rehydration and other effective treatments to improve tissue perfusion and hypoxia and reduce the morbidity and mortality of SAE.
In the present study, patients with SAE were more likely to use vasopressors during their PICU stay. Vasopressor use indicates the critical state of patients with circulatory dysfunction and hypotension. However, the use of vasopressors, especially for more than 6 h aiming to a relatively higher blood pressure, has been proven to be closely associated with poor prognosis in patients with sepsis [44]. A study related to transcranial Doppler in healthy volunteers showed that norepinephrine, despite increasing arterial pressure, did not increase cerebral perfusion pressure [45]. Another study in healthy subjects suggested that a high dose of norepinephrine may have a negative effect on cerebral oxygenation [46]. Therefore, individualized vasopressor therapy with an appropriate dosage should be applied to patients with SAE.
In our study, respiratory and blood infections were common in children with SAE. However, the primary sites of infection in adult patients with SAE were the respiratory and gastrointestinal tracts [8, 47]. Moreover, Gram-positive bacteria accounted for the largest proportion of adult patients with SAE [8], while Acinetobacter baumannii and fungi were more likely found in elderly patients with SAE [48]. However, we found that the most common organisms in children with SAE were Gram-negative bacteria, which was different from that in adult patients. Therefore, clinicians should consider these differences among different age groups when administering antibiotics to patients with SAEs.
This study has some limitations. First, although this was a multicenter study, the vast majority of patients were from Shandong Province, and the sample size was not particularly large. Further studies involving different populations and larger cohorts are required to validate our findings. Second, the retrospective nature of this observational study suggests that unidentified confounding factors may have influenced the results. For example, the vital signs and laboratory data were greatly affected by age, which was not included in the nomogram model. However, we did not build the specific prediction model for each age group because of the small sample size. More patients will be included in our study in order to establish more accurate models for different age groups in the future. Third, a cytokine storm in the central nervous system with an impaired BBB is considered an important pathophysiological mechanism in the development of SAE [17]. Unfortunately, data regarding CSF cytokine were available only in a few of the studied patients due to the fact that this test was not routinely conducted in many hospitals. We built the nomogram model using predictive factors that are easier to obtain.
Conclusion
In summary, a prediction nomogram for SAE based on PaCO2, APTT, BUN, RR, and PCIS was developed and validated externally. The nomogram and the corresponding online calculator can be conveniently used to accurately predict the morbidity of SAE. This may be beneficial for the early recognition and management of SAE, and ultimately improve the prognosis of patients with SAE.
Availability of data and materials
The datasets used or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–30.
Mazeraud A, Righy C, Bouchereau E, Benghanem S, Bozza FA, Sharshar T. Septic-associated encephalopathy: a comprehensive review. Neurotherapeutics. 2020;17(2):392–403.
Chung HY, Wickel J, Brunkhorst FM, Geis C. Sepsis-associated encephalopathy: from delirium to dementia? J Clin Med. 2020;9(3):703.
Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–66.
Menon K, Schlapbach LJ, Akech S, Argent A, Biban P, Carrol ED, Chiotos K, Jobayer Chisti M, Evans IVR, Inwald DP, Ishimine P, Kissoon N, Lodha R, Nadel S, Oliveira CF, Peters M, Sadeghirad B, Scott HF, de Souza DC, Tissieres P, Watson RS, Wiens MO, Wynn JL, Zimmerman JJ, Sorce LR, Pediatric Sepsis Definition Taskforce of the Society of Critical Care Medicine. Criteria for pediatric sepsis—a systematic review and meta-analysis by the pediatric sepsis definition taskforce*. Crit Care Med. 2022;50(1):21–36.
Zhang L-N, Wang X-T, Ai Y-H, Guo Q-L, Huang L, Liu Z-Y, Bo Y. Epidemiological features and risk factors of sepsis-associated encephalopathy in intensive care unit patients: 2008–2011. Chin Med J. 2012;125(5):828–31.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94.
Yang Y, Liang S, Geng J, Wang Q, Wang P, Cao Y, Li R, Gao G, Li L. Development of a nomogram to predict 30-day mortality of patients with sepsis-associated encephalopathy: a retrospective cohort study. J Intensive Care. 2020;8(1):45.
Stubbs DJ, Yamamoto AK, Menon DK. Imaging in sepsis-associated encephalopathy–insights and opportunities. Nat Rev Neurol. 2013;9(10):551–61.
Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, de la Grandmaison GL, Aboab J, Gray F, Menon D, Annane D. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33(5):798–806.
Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155(4):1793.
Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10): e172352.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 2015;13:1.
Song G, Ren X. Pediatric risk of mortality III score and pediatric Critical illness score. Appl Clin Pediatr. 2006;21:382.
Molnár L, Fülesdi B, Németh N, Molnár C. Sepsis-associated encephalopathy: a review of literature. Neurol India. 2018;66(2):352–61.
Tauber SC, Djukic M, Gossner J, Eiffert H, Brück W, Nau R. Sepsis-associated encephalopathy and septic encephalitis: an update. Expert Rev Anti Infect Ther. 2021;19(2):215–31.
Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, Huyghens L. Elevated serum levels of S-100β protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock*. Crit Care Med. 2006;34(7):1967–74.
Sonneville R, de Montmollin E, Poujade J, Garrouste-Orgeas M, Souweine B, Darmon M, Mariotte E, Argaud L, Barbier F, Goldgran-Toledano D, Marcotte G, Dumenil AS, Jamali S, Lacave G, Ruckly S, Mourvillier B, Timsit JF. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 2017;43(8):1075–84.
Chen Y, Hu Y, Li X, Chen P, Wang C, Wang J, Wu J, Sun Y, Zheng G, Lu Y, Guo Y. Clinical features and factors associated with sepsis-associated encephalopathy in children: retrospective single-center clinical study. Front Neurol. 2022;13: 838746.
Berg RM, Plovsing RR. Effects of short-term mechanical hyperventilation on cerebral blood flow and dynamic cerebral autoregulation in critically ill patients with sepsis. Scand J Clin Lab Invest. 2016;76:1502–7686.
Taccone FS, Castanares-Zapatero D, Peres-Bota D, Vincent JL, Berre J, Melot C. Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care. 2010;12:1556–961.
Thees C, Kaiser M, Scholz M, Semmler A, Heneka MT, Baumgarten G, Hoeft A, Putensen C. Cerebral haemodynamics and carbon dioxide reactivity during sepsis syndrome. Crit Care. 2007;11:1466–609.
Niederwanger C, Bachler M, Hell T, Linhart C, Entenmann A, Balog A, Auer K, Innerhofer P. Inflammatory and coagulatory parameters linked to survival in critically ill children with sepsis. Ann Intensive Care. 2018;8:2110–5820.
Jiang Z, Bo L, Xu Z, Song Y, Wang J, Wen P, Wan X, Yang T, Deng X, Bian J. An explainable machine learning algorithm for risk factor analysis of in-hospital mortality in sepsis survivors with ICU readmission. Comput Methods Programs Biomed. 2021;204:1872–7565.
M. Harazim, K. Tan, M. Nalos, and M. Matejovic, “Blood urea nitrogen - independent marker of mortality in sepsis,” Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, no. 1804–7521 (Electronic), 2023.
Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA. 1996;275:0098–7484.
Peng L, Peng C, Yang F, Wang J, Zuo W, Cheng C, Mao Z, Jin Z, Li W. Machine learning approach for the prediction of 30-day mortality in patients with sepsis-associated encephalopathy. BMC Med Res Methodol. 2022;22:1471–2288.
Ren Y, Zhang L, Xu F, Han D, Zheng S, Zhang F, Li L, Wang Z, Lyu J, Yin H. Risk factor analysis and nomogram for predicting in-hospital mortality in ICU patients with sepsis and lung infection. BMC Pulm Med. 2022;22:1471–2466.
Gao Q, Hernandes M. Sepsis-associated encephalopathy and blood-brain barrier dysfunction. Inflammation. 2021;44:1573–2576.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:1538–3598.
Seo MH, Choa M, You JS, Lee HS, Hong JH, Park YS, Chung SP, Park I. Hypoalbuminemia, low base excess values, and tachypnea predict 28-day mortality in severe sepsis and septic shock patients in the emergency Department. Yonsei Med J. 2016;57:1976–2437.
Zhang L, Huang H, Cheng Y, Xu L, Huang X, Pei Y, Tang W, Qin Z. Predictive value of four pediatric scores of critical illness and mortality on evaluating mortality risk in pediatric critical patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:2095–4352.
Teng QL, Ju M, Liu ZY, He XC. Establishment of a nomogram model for the early diagnosis of childhood sepsis. Zhongguo Dang Dai Er Ke Za Zhi. 2022;24:1008–8830.
Zhou LB, Chen J, Du X-C, Wu S-Y, Bai Z-J, Lyu HT. Value of three scoring systems in evaluating the prognosis of children with severe sepsis. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21:1008–8830.
Lin J, Zhang Y, Song A, Yang N, Ying L, Dai J. Comparison of a new predictive model with other critical scores for predicting in-hospital mortality among children with pneumonia-related bacteremia. J Investig Med. 2021;69:1708–8267.
Dang HX, Liu CJ, Li J, Chen SJ, Xu F. Clinical significance and prognostic effect of serum 25-hydroxyvitamin D concentrations in critical and severe hand, foot and mouth disease. Nutrients. 2017;9:2072–6643. https://doi.org/10.3390/nu9050478.
Song Y, Wang H, Tao YH. Risk factors and optimal predictive scoring system of mortality for children with acute paraquat poisoning. World J Clin Cases. 2022;10:2307–8960.
Wu LA-O, Jin M, Wang R, Yang L, Lai X, Yu L, Lin D, Huang L, Zhang Y, Zhang J, Liao X, Zi J, Yuan Y, Zeng Y, Cheng M, Tao S. Prognostic factors of sepsis in children with acute leukemia admitted to the pediatric intensive care unit. Pediatr Blood Cancer. 2023. https://doi.org/10.1002/pbc.30382.
Yajnik V, Maarouf R. Sepsis and the microcirculation: the impact on outcomes. Curr Opin Anaesthesiol. 2022;35:1473–6500.
Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149:1931–3543.
Hernández G, Ospina-Tascón GA, Damiani LP. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: The ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;327:1538–3598.
Zhao L, Li Y, Wang Y, Gao Q, Ge Z, Sun X, Li Y. Development and validation of a nomogram for the prediction of hospital mortality of patients with encephalopathy caused by microbial infection: a retrospective cohort study. Front Microbiol. 2021;12:1664–2302.
Lamontagne F, Day AG, Meade MO, Cook DJ, Guyatt GH, Hylands M, Radermacher P, Chrétien JM, Beaudoin N, Hébert P, D’Aragon F, Meziani F, Asfar P. Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med. 2018;44:1432–1238.
Moppett IK, Sherman RW, Wild MJ, Latter JA, Mahajan RP. Effects of norepinephrine and glyceryl trinitrate on cerebral haemodynamics: transcranial Doppler study in healthy volunteers. Br J Anaesth. 2008;100:1471–6771.
Brassard P, Seifert T, Secher NH. Is cerebral oxygenation negatively affected by infusion of norepinephrine in healthy subjects? Br J Anaesth. 2009;102:1471–6771.
Chen J, Shi X, Diao M, Jin G, Zhu Y, Hu W, Xi S. A retrospective study of sepsis-associated encephalopathy: epidemiology, clinical features and adverse outcomes. BMC Emerg Med. 2020;20:1471–2227.
Jin G, Wang S, Chen J, Hu W, Zhu Y, Xi S. Identification of sepsis-associated encephalopathy risk factors in elderly patients: a retrospective observational cohort study. Turk J Med Sci. 2022;52:1303–6165.
Funding
This work was supported by the ECCM Program of the Clinical Research Center of Shandong University (No. 2021SDUCRCB013) and the National Natural Science Foundation of China (No. 82171352).
Author information
Authors and Affiliations
Contributions
GW and XJ contributed to the study conception and design, wrote the first draft and revised the manuscript. YF performed the data analysis. YG contributed to the data collection. QJ, EG, and HH revised the manuscript. XL contributed to the conception and design of the study, and revised the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Review Board of Qilu Hospital of Shandong University (KYLL-202202-027-1). The requirement for individual patient consent was waived due to the retrospective design of the study, and all patient information was handled anonymously.
Competing interests
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
TRIPOD Checklist: prediction model development.
Additional file 2: Table S2.
Baseline and clinical characteristics of the study cohorts.
Additional file 3: Table S3.
Grouping of the patients with SAE by the timing of SAE diagnosis after PICU admission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, G., Jiang, X., Fu, Y. et al. Development and validation of a nomogram to predict the risk of sepsis-associated encephalopathy for septic patients in PICU: a multicenter retrospective cohort study. j intensive care 12, 8 (2024). https://doi.org/10.1186/s40560-024-00721-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-024-00721-7