Abstract
Background
Heart failure is an ever-growing contributor to morbidity and mortality in the ageing population. Medication adherence rates among the HF population vary widely in the literature, with a reported range of 10–98%. Technologies have been developed to improve adherence to therapies and other clinical outcomes.
Aims
This systematic review aims to investigate the effect of different technologies on medication adherence in patients with heart failure. It also aims to determine their impact on other clinical outcomes and examine the potential of these technologies in clinical practice.
Methods
This systematic review was conducted using the following databases: PubMed Central UK, Embase, MEDLINE, CINAHL Plus, PsycINFO and Cochrane Library until October 2022. Studies were included if they were randomised controlled trials that used technology to improve medication adherence as an outcome in heart failure patients. The Cochrane Collaboration's Risk of Bias tool was used to assess individual studies. This review was registered with PROSPERO (ID: CRD42022371865).
Results
A total of nine studies met the inclusion criteria. Two studies showed statistically significant improvement in medication adherence following their respective interventions. Eight studies had at least one statistically significant result in the other clinical outcomes it measured, including self-care, quality of life and hospitalisations. All studies that evaluated self-care management showed statistically significant improvement. Improvements in other outcomes, such as quality of life and hospitalisations, were inconsistent.
Conclusion
It is observable that there is limited evidence for using technology to improve medication adherence in heart failure patients. Further studies with larger study populations and validated self-reporting methods for medication adherence are required.
Keypoints
-
The evidence in this review shows the potential for technology use in practice to improve health outcomes for patients with heart failure. Still, there is uncertainty in the evidence on its impact on medication adherence.
-
Technologies presented in the studies are generally acceptable for participants, which is informative for future implementations into practice.
-
There was a lack of high-quality studies investigating technology's use on medication adherence in heart failure. More extensive studies with improvements to study design are needed to ultimately conclude the effects on medication adherence.
Similar content being viewed by others
Introduction
Heart failure (HF) is a debilitating chronic condition with high morbidity and mortality affecting over 900,000 people in the UK, with a higher prevalence above age 65 [1,2,3]. Similarly, the population of HF patients per year continues to increase [4]. HF patients are roughly equally split between those with reduced Ejection Fraction (HFrEF) and those with preserved or mildly reduced Ejection Fraction (HFpEF or HFmrEF) [2]. All three types usually require pharmacological therapy to manage symptoms, co-morbidities, and heart function [4]. These include diuretics, Angiotensin-Converting Enzyme (ACE) inhibitors, beta-blockers, and Angiotensin Receptor Blockers (ARBs) [4]. Medication Adherence (MA) is defined as "the extent to which a patient’s action matches the agreed recommendations" [5]. MA rates among the HF population vary widely in the literature, with a reported range of 10–98% [6, 7]. Low adherence rates are associated with worse cardiac event-free survival, all-cause mortality, cardiovascular hospitalisations, and a more significant symptom burden [8, 9]. Studies have shown a greater rate of non-adherence in the elderly population, potentially due to co-morbidities and polypharmacy [10]. On the other hand, high MA is linked to fewer HF symptoms, lower rates of hospitalisation, and fewer deaths [9].
MA is affected by multiple factors, including patient beliefs, socioeconomic influences, and the type of prescribed therapies [11]. Educational interventions to address these issues have been shown to be effective [11]. However, this does not guarantee that medications will be taken, especially if regimens require multiple doses each day or a patient has various medications to take. Interventions to tackle the lack of medication adherence have been the focus of technological advancements, for example, mobile applications, electronic pill boxes, automated telephone calls, and messaging [11]. Previous trials using these technologies have shown significant increases in refilling medications, specifically in patients with cardiovascular diseases (CVD) such as hypercholesterolaemia, hypertension, and coronary heart disease [11].
Systematic reviews have previously evaluated specific technological interventions such as mobile health and application (app) technology; however, they only evaluated CVD as a whole [12]. Even in systematic reviews that studied technology use in HF, MA was not the primary objective [13]. There is also limited evidence assessing MA specifically in HF [13]. Hence, this systematic review aims to assess the impact of technology on MA in HF patients.
Aim
-
1.
To investigate the effect of different technologies on medication adherence in patients with heart failure.
-
2.
To determine the impact of different technologies on other clinical outcomes for heart failure patients and examine the potential of these technologies in clinical practice.
Methods
This systematic review was conducted according to PRISMA guidelines and was registered with PROSPERO (registration ID: CRD42022371865).
Search strategy
An electronic search of the following databases was conducted for research articles: PubMed Central UK, Embase (Ovid), MEDLINE (Ovid), CINAHL Plus (EBSCO), PsycINFO (Ovid) and Cochrane Library, from 2000 until October 2022. The keywords' heart failure', 'medication adherence', and 'technology' were used in this review. Filters for Randomised Controlled Trials (RCTs) and the English language were applied. The MeSH term's function was utilised to generate other keywords for each database (see example search strategy in Additional file 1: Table S1. Reference lists of included studies were reviewed to identify additional relevant RCTs.
Study inclusion criteria
Studies that were RCTs and included technology as an intervention to improve adherence to HF medication were included. Studies were required to have a usual care control group. Studies with patients aged 18 or over were included, and participants could be of any class of the New York Heart Association (NYHA) classification of HF. Studies needed to include patients taking at least one medication related to HF and have a precise MA outcome measure. Studies involving patients under the age of 18 and non-RCTs: observational studies, systematic reviews, meta-analyses, grey literature, abstract available only, unpublished studies, and quasi-randomised trials, were excluded, along with those that were not in the English language.
Study selection and data extraction
All the identified studies were imported and intensively analysed to remove duplicate records manually. The first author (CC) screened all titles and abstracts using the inclusion criteria. This was checked by another author (ZJ). All researchers screened the full texts of the remaining articles independently to identify eligible studies (CC, GD, and ZJ). Any studies that displayed uncertainty were discussed among all the authors to reach a consensus. All relevant studies underwent data extraction for country of origin, number of study arms, intervention description, length of intervention, outcome measures, sample size, and results for comparison.
Outcomes assessed
The primary outcome assessed was MA in HF. Secondary outcomes were quality of life, self-care/self-management behaviours, HF knowledge, number of hospital admissions, mortality, health status, self-efficacy, depression, clinical follow-up attendance, patient satisfaction, physiological measures (e.g., NT-proBNP and HbA1C), and physical activity.
Quality assessment
Quality assessment was conducted using the Cochrane Collaboration's Risk of Bias Tool to assess each study's risk of bias according to the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting bias, and other bias [14]. Each study's domains were allocated to low-risk, unclear-risk, or high-risk of bias [14]. The risk of bias summary graphs were generated using RevMan software version 5.4.
Results
Search results
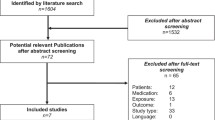
The initial search of databases identified 1157 records. No additional studies were identified from the reference lists of included studies. Once duplicates were removed, and RCT filters were applied, 166 studies were screened using titles and abstracts. After exclusion, 19 studies were screened using full-text, and ten studies were excluded due to reasons listed in the PRISMA diagram in Fig. 1. Subsequently, nine studies were included [15,16,17,18,19,20,21,22,23]. Six of the nine studies were RCTs [18,19,20,21,22,23], two studies were pilot RCTs [15, 16], and one study was a randomised controlled feasibility trial [17]. A meta-analysis was considered; however, because of the heterogeneity of the outcome measure tools (specifically the self-reporting tools used to measure medication adherence), and variations in other factors such as sample size and intervention length, this made it difficult to bring together an appropriate statistical analysis.
Study characteristics and design
Included studies were published between 2004 and 2022. Seven studies were conducted in the United States [15,16,17, 19, 20, 22, 23]. One study was conducted in the Netherlands and another in Argentina [18, 21]. All studies involved one or more intervention arms against a control arm [15,16,17,18,19,20,21,22,23]. All study controls were usual care, defined as participants undergoing follow-up without the intervention [15,16,17,18,19,20,21,22,23]. However, three studies gave their usual care participants the intervention technology dice to either measure medication adherence as a study outcome or programmed it to be silent [16, 17, 19] All nine studies used a similar study design [15,16,17,18,19,20,21,22,23]. The sample size varied between 29 and 382 participants, with 1032 participants and intervention duration ranging from 28 days to 12 months [15,16,17,18,19,20,21,22,23]. Table 1 provides a summary of study characteristics.
Technology characteristics
A range of technologies was used in the included studies: Remote medication monitoring systems, telemonitoring devices, electronic pill boxes, apps/text-messaging, electronic pill organiser reminders, and web-based technologies [15,16,17,18,19,20,21,22,23]. Six studies used their technology interventions as a form of patient education to increase MA [16, 18,19,20,21, 23]. The other three studies used medication reminders via the technologies to improve MA [15, 17, 22].
Three studies used single interventions (their respective technology devices). Still, participants were contacted by telephone if they were identified as non-adherent to their medication [15, 16] or classed as high-risk based on their adherence data or answers about symptoms, HF knowledge, and medication-taking behaviours [18]. One study used medication event monitoring system (MEMS) feedback in addition to a counselling intervention known as the 'Theory of Planned Behaviour (TPB)' [19]. Another study used a home telemonitoring system entailing an app with multiple functionalities such as physiological measure monitoring and education [21]. One study used various interventions such as face-to-face self-management sessions, telephone reinforcement sessions, and a self-management toolkit (with devices such as a weight scale and an electronic pill organiser reminder) [22]. Web-based technology in one study created a Web-interface for participants with three components: patient's medical record, education guide, and messaging system with practice nurses [23]. Goldstein et al. used a 2 × 2 study design, where a telehealth intervention versus a control group took place alongside a mobile health intervention against a control group [17]. The mobile health intervention in Felker et al. involved two aspects: personalised text messages to summarise physical activity performance and goal setting and an electronic tool to provide educational materials on medications [20].
Assessment of medication adherence
MA was measured in various ways across the studies. Six studies solely used self-reporting tools or questionnaires [15, 18, 20,21,22,23]. Two studies used the modified Morisky scale or questions derived from it [21, 23]. Other self-reporting measures included an adherence questionnaire from Voils et al. [20], the 'Heart Failure Compliance Scale' [18], a self-reporting question from the Medical Outcomes Study (MOS) [15] and a self-reporting question of the day's medication was missed in the last seven days [22]. Two studies exclusively used a dose count from the technological intervention device as their measure outcome [16, 19]. One study used two outcome measures depending on the intervention- the telehealth groups used a dose count percentage, whilst the mobile health groups used a self-reported measure [17]. The study that used a question from MOS also used a dose count from the intervention device, but only for the intervention group [15]. The outcome measures for each study are shown in Table 2.
Effect of medication adherence
Eight of the nine studies showed improvement in MA following the intervention [15,16,17,18,19, 21,22,23] (Table 2). The remaining study is unclear as they did not report the direction of their scoring system for medication adherence [20]. However, only two studies showed a statistically significant improvement in MA. The study by Wu et al. used MEMS feedback as an educational tool for the patient. It showed 74% of the intervention group adherent to medication versus 36% in the control group [19]. The study by Young et al. used an electronic pill organiser reminder as a medication reminder intervention and found improvement with a marginal mean of 0.8 days if any medication was missed in the last seven days for the control group versus 0.3 days in the intervention group [22].
Effect on clinical and non-clinical outcomes
A table of other clinical outcomes examined in included studies can be found in Additional file 1: Table S2. These included: quality of life, self-care/self-management behaviours, HF knowledge, number of hospital admissions, mortality, health status, self-efficacy, depression, clinical follow-up attendance, patient satisfaction, physiological measures, and physical activity.
Quality of life
Table 3 shows only one of the three studies that assessed the quality of life showed significant improvement in quality of life [20]. Another study showed significance, but the quality of life was worse in the intervention group than in control [15]. A-value was not available in the previous research [19].
Hospitalisation
Hospitalisations showed variations in the results, with two of five studies showing a significant difference in the number of hospital admissions [15, 19] (Table 4).
Health-status
One study evaluated health status and did not show significance [15].
Self-care management
Four studies showed significant improvement in self-care/self-management or general adherence to self-care [18, 21,22,23] (Table 5).
Patient satisfaction with the intervention
Two studies evaluated device rating/patient satisfaction as an outcome towards the intervention- one study observed that patients statistically significantly preferred m-health over telehealth [17]. The other resulted in 88% of participants rating the device as somewhat or very easy to use [16]. Ross et al. measured patient satisfaction with doctor patient-communication, with improvements seen in some domains, but which were not significant [23].
Other outcomes
One study assessed depression and did not show improvement [15]. Follow-up attendance did not improve in another study [16]. Boyne et al. showed statistically significant improvement in disease-specific knowledge at the end of follow-up [18]. Three studies assessed self-efficacy, two of which did not improve [18, 23], while one did [22]. Two trials measured physical activity- one showed significant improvement in mean daily step count [20], whilst the other did not in activity minutes, calories burnt, or daily activity counts [22].
Lastly, two studies evaluated physiological parameters such as HbA1c and NT-proBNP with no significant differences shown [20, 22]. One of those studies also evaluated metabolic profiling with significant changes observed for the intervention in 13 metabolites [20].
Risk of bias of included trials
The risk of bias summary graphs were generated using RevMan 5.4 software (Figs. 2, 3). Six studies reported appropriate random sequence generation [16,17,18, 21,22,23], with the other three showing unclear risk [15, 19, 20]. In contrast, six studies showed unclear allocation concealment [15, 18,19,20,21, 23], two with low risk [16, 22], and one reporting a high risk of bias [17]. Blinding of participants and personnel showed a high risk of bias for all studies as it wouldn't have been possible to blind participants, due to the type of intervention involved. However, blinding personnel could have been possible, with only one of the studies mentioning that the study team members were blinded to group assignments [16]. Six studies reported an unclear risk of bias for blinding of outcome assessment [15, 17, 18, 20, 21, 23], with the other three showing low risk [16, 19, 22]. Assessment of incomplete outcome data showed six studies with a low risk [15,16,17,18, 21, 22], two with unclear risk [19, 20], and one study being a high risk for attrition bias [23]. Selective reporting assessment displayed seven studies with a low risk of bias [15, 16, 18, 20,21,22,23] and two studies for unclear risk [17, 19]. Lastly, seven studies exhibited a low risk of other bias [15,16,17, 19,20,21, 23], specifically on whether the tools they used as outcome measurements for MA were reported as being validated, known to be validated, or showed a quantitative measure of adherence. The other two studies did not show this, therefore, were classed as high risk for bias [18, 22].
Discussion
This systematic review aimed to determine technology's impact on MA in HF patients. This review involved nine RCTs with a total of 1032 participants and used some form of technology to support MA and evaluate this as an outcome [15,16,17,18,19,20,21,22,23]. Two RCTs showed statistically significant improvement in MA, both of which used mixed interventions [19, 22]. Six RCTs showed improvement, but results were not statistically significant [15,16,17,18, 21, 23], and one RCT showed unclear improvement [20]. Depending on the study, the technologies provided different purposes- patient education or medication reminders (with no known comparisons on which is more effective in MA). To our knowledge, no other reviews assess technology for MA, specifically in HF.
It is worth noting that the two RCTs that showed significant improvement did not use technology in isolation [19, 22]. They were used in conjunction with non-technology components, for example, synchronous communication with healthcare professionals (HCPs), remote logging of symptoms, and behavioural change therapy (TPB) [19, 22]. Multi-disciplinary involvement was common in most studies [16, 18, 19, 21,22,23]. Previous evidence shows that combined approaches are more likely to improve MA in HF patients, specifically in older adults [24]. This supports the notion that technology if used in practice, should be part of a multi-disciplinary approach to the care of an HF patient.
Where similar improvements have been seen in past reviews for specific technologies in CVD, most have attributed a lack of significant improvement to small sample sizes [12, 25]. Our findings also showed two pilot studies [15, 16], one feasibility study [17], and four studies that had a relatively small sample size [19, 21,22,23]. These studies noted they were not sufficiently powered to test the intervention's actual effect. In addition, limited research assesses MA technology use in HF. This was observed in mobile health-specific reviews by Coorey et al. and Al-Arkee et al., both only included one RCT relating specifically to HF and commented on small sample sizes as a limitation [12, 25].
MA was assessed mainly using self-reporting tools or questionnaires in seven studies [15, 17, 18, 20,21,22,23]. Some tools, however, were not validated or widely known [18, 20, 22]. Whilst self-reporting methods provide a cost-effective approach to predict clinical outcomes, they have the potential to produce the 'Hawthorne effect' whereby certain behaviours evaluated in studies differ between participants due to their awareness of being assessed [26, 27]. This leads to overestimations of proper adherence [26, 27]. One study in our review exemplified this, which attributed overestimations to 'socially acceptable answers' [18], and two others note self-reporting use in various outcomes as a limitation [15, 17]. MEMS/digital monitoring- used in three studies [16, 17, 19], is considered a more accurate way of measuring MA [28]. However, it does not guarantee the correct dose is taken. Hence, results should be interpreted with caution.
The study population of some studies may have contributed to potential ceiling effects observed in this review. For instance, one study reported a baseline adherence of 98% [18], leaving little room for the intervention to produce a significant effect. Five of the studies reported using the majority of already adherent/motivated patients [16,17,18,19, 22], where it was unclear if this was intentional (except Goldstein et al. which attributed this to their recruitment methods). Therefore, this largely excluded the non-adherent population- the ideal targets of these interventions. Participant recruitment methods including advertisements and mailing, may have automatically pooled these patient cohorts [17]. Previous literature suggests that a willingness to participate in trials through such methods voluntarily can be indicative of the motivation and education the patient already has [29]. Therefore, the selection of these patients is more likely to result in ceiling effects. The opposite has been suggested for non-adherent patients, where significant differences are more likely to be seen [30].
Despite all trials assessing a range of other clinical outcomes, there was much variation in the results, especially for the quality of life and hospital admissions. These findings are consistent with a review from Allida et al., which evaluated the use of mobile health in HF patients- limited evidence was found for the effectiveness of technology interventions on quality of life and hospital admission [13]. Variations for these two outcomes (shown in Tables 3 and 4) could be explained by the heterogeneity of the measurement tools used [13]. Our self-care/management and general adherence results differed from Allida et al., where all trials that assessed it showed statistical significance, mainly using validated or well-known tools [18, 21,22,23]. This demonstrates these technologies' potential to impact remote non-pharmacological care in HF positively. Another review on the use of smartphones in healthcare applications raised the importance of technology in providing education, self-management, and remote monitoring [31]. This links to the multi-intervention aspects of most studies in our review that may have contributed to improvements in self-care/management and general adherence. Three out of four trials involving self-care used educational-based technologies [18, 21, 23], and two of those studies monitored patient symptoms via the technologies [21, 22]. Therefore, these technologies must have multiple functions to improve MA and non-pharmacological behaviours.
Only four RCTs assessed patient satisfaction with the intervention technology [15,16,17, 21]; however, all of them showed most participants finding their technologies easy to use or helpful for their treatment plans. These results are useful for informing technology utilisation in a clinical setting, specifically in a disease population with an older age demographic (all studies showed participants over the age of 50), and where previous research has suggested links between HF patients and susceptibility to cognitive impairment [32]. It is worth noting all studies did not explicitly state the degree of cognitive impairment in their patient characteristics but stated the need for their technologies to be appropriate for them.
Our findings accord with a cardiac tele-rehabilitation review which showed the technologies' high usability, utility, and acceptability, especially in the COVID-19 climate [33]. The review also noted potential preferences for types of technologies [33], but they still appeared usable and satisfactory to patients. A study in our review observed the preference for mobile health over telehealth due to the easy integration of the mobile app into their daily routines [17]. This indicates that patients may prefer technologies that are easy to use and are already familiar. A previous review on mobile health notes that portability and multi-feature access (such as viewing patient records and monitoring) is helpful for patients and their healthcare providers [34]. Additionally, tailored approaches based on the individual's needs and behaviours should be considered [35], particularly motivation- a known contributor to adherence [36], which was not addressed in most studies.
Strengths and limitations
This systematic review was conducted in accordance with PRISMA guidelines to reduce researcher bias. However, it was often difficult to ascertain if any improvements in MA and other outcomes were solely due to the technology or the other non-technology intervention components. Other studies that may have been relevant were excluded, such as grey literature, non-RCTs, and studies not in English. However, we used RCTs, which are well-known to place at the highest level of the hierarchy of evidence [37]. Some studies tested the intervention on a certain number of medications; for e.g., Wu et al. provided MEMs feedback for only one of the medications [19]. HF patients take multiple medications; therefore, total adherence may have differed.
Recommendations for policy and practice
Technology can potentially improve MA in HF patients; however, our review has shown insufficient evidence for this. The RCTs included in this review showed uncertain and inconsistent results for the quality of studies and effectiveness of the technologies. On the other hand, these technologies were generally easy to use or helpful for patients. Therefore, recommendations for clinical practice cannot be made without solid evidence from good-quality studies.
Future research
Our findings from this review indicate the need for further research. Future RCTs should be sufficiently powered, primarily targeting non-adherent patients, and use blinded outcome assessors. Using validated self-reporting tools, in addition to electronic monitoring, should be used together to increase the accuracy of results. Future studies should continue to assess usability and patient satisfaction with technologies and explore the most effective mechanisms for supporting MA, such as whether providing education or medication reminders are more effective. Using similar validated self-report tools will allow future systematic reviews to include meta-analyses to generate more robust conclusions on intervention effectiveness.
Conclusion
Evidence for the effectiveness of technology in medication adherence is currently weak. Whilst it has indicated positive improvements for some outcomes, particularly self-care, further evidence is needed for its impact on MA. More powered trials that include larger sample sizes and mostly non-adherent cohorts are required to build on existing studies and inform the future incorporation of technology into routine clinical practice.
Availability of data and materials
Further supplementary material is available on request.
Abbreviations
- HF:
-
Heart failure
- HFrEF:
-
Heart failure with reduced ejection fraction
- HFpEF or HFmrEF:
-
Heart failure with preserved or mildly reduced Ejection Fraction
- ARBs:
-
Angiotensin Receptor Blockers
- MA:
-
Medication adherence
- app:
-
Mobile health and application
- NYHA:
-
New York Heart Association
- RCTs:
-
Randomised Control Trials
- MEMS:
-
Medication event monitoring system
- HCPs:
-
Healthcare professionals
References
British heart foundation. Fact and figures, information for journalist-BHF. 2022. https://www.bhf.org.uk/what-we-do/news-from-the-bhf/contact-the-press-office/facts-and-figures. Accessed 18 Dec 2022.
National institute for health and care excellence. Prevalence, background information, Heart failure–chronic, CKS, NICE. 2022. https://cks.nice.org.uk/topics/heart-failure-chronic/background-information/prevalence/. Accessed 18 Dec 2022.
National institute for health and care excellence. Prognosis, background information, heart failure–chronic, CKS, NICE. 2022. https://cks.nice.org.uk/topics/heart-failure-chronic/background-information/prognosis/. Accessed 18 Dec 2022.
National institute for health and care excellence. Management, Heart failure–chronic, CKS, NICE. 2022. https://cks.nice.org.uk/topics/heart-failure-chronic/management/. Accessed 18 Dec 2022.
National Institute for Health and Care Excellence. Introduction, medicines adherence: medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. 2009. https://www.nice.org.uk/guidance/cg76/chapter/introduction. Accessed 18 Dec 2022.
Shah D, Simms K, Barksdale D, Wu J-R. Improving medication adherence of patients with chronic heart failure: challenges and solutions. Res Rep Clin Cardiol. 2015;6:87.
Rasmussen AA, Wiggers H, Jensen M, Berg SK, Rasmussen TB, Borregaard B, et al. Patient-reported outcomes and medication adherence in patients with heart failure. Eur Heart J Cardiovasc Pharmacother. 2021;7:287–95.
Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc. 2016;5: e002606.
Wu J-R, Moser DK. Medication adherence mediates the relationship between heart failure symptoms and cardiac event-free survival in patients with heart failure. J Cardiovasc Nurs. 2018;33:40–6.
Smaje A, Weston-Clark M, Raj R, Orlu M, Davis D, Rawle M. Factors associated with medication adherence in older patients: a systematic review. Aging Medicine. 2018;1:254–66.
Simon ST, Kini V, Levy AE, Ho PM. Medication adherence in cardiovascular medicine. BMJ. 2021;374:n1493.
Al-Arkee S, Mason J, Lane DA, Fabritz L, Chua W, Haque MS, et al. Mobile apps to improve medication adherence in cardiovascular disease: systematic review and meta-analysis. J Med Internet Res. 2021;23: e24190.
Allida S, Du H, Xu X, Prichard R, Chang S, Hickman LD, et al. mHealth education interventions in heart failure. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD011845.pub2.
Cochrane. 8.5 The Cochrane Collaboration’s tool for assessing risk of bias. 2022. https://handbook-5-1.cochrane.org/chapter_8/8_5_the_cochrane_collaborations_tool_for_assessing_risk_of_bias.htm. Accessed 21 Dec 2022.
Hale TM, Jethwani K, Kandola MS, Saldana F, Kvedar JC. A remote medication monitoring system for chronic heart failure patients to reduce readmissions: a two-arm randomized pilot study. J Med Internet Res. 2016;18: e91.
Gallagher BD, Moise N, Haerizadeh M, Ye S, Medina V, Kronish IM. Telemonitoring adherence to medications in heart failure patients (TEAM-HF): a pilot randomized clinical trial. J Card Fail. 2017;23:345–9.
Goldstein CM, Gathright EC, Dolansky MA, Gunstad J, Sterns A, Redle JD, et al. Randomized controlled feasibility trial of two telemedicine medication reminder systems for older adults with heart failure. J Telemed Telecare. 2014;20:293–9.
Boyne JJ, Vrijhoef HJ, Spreeuwenberg M, De Weerd G, Kragten J, Gorgels AP. Effects of tailored telemonitoring on heart failure patients’ knowledge, self-care, self-efficacy and adherence: a randomized controlled trial. Eur J Cardiovasc Nurs. 2014;13:243–52.
Wu J-R, Corley DJ, Lennie TA, Moser DK. Effect of a medication-taking behavior feedback theory-based intervention on outcomes in patients with heart failure. J Card Fail. 2012;18:1–9.
Felker GM, Sharma A, Mentz RJ, She L, Green CL, Granger BB, et al. A randomized controlled trial of mobile health intervention in patients with heart failure and diabetes. J Card Fail. 2022;28:1575–83.
Yanicelli LM, Goy CB, del González VC, Palacios GN, Martínez EC, Herrera MC. Non-invasive home telemonitoring system for heart failure patients: a randomized clinical trial. J Telemed Telecare. 2021;27:553–61.
Young L, Hertzog M, Barnason S. Effects of a home-based activation intervention on self-management adherence and readmission in rural heart failure patients: the PATCH randomized controlled trial. BMC Cardiovasc Disord. 2016;16:176.
Ross SE, Moore LA, Earnest MA, Wittevrongel L, Lin C-T. Providing a web-based online medical record with electronic communication capabilities to patients with congestive heart failure: randomized trial. J Med Internet Res. 2004;6: e12.
Andrews AM, Russell CL, Cheng A-L. Medication adherence interventions for older adults with heart failure: a systematic review. J Gerontol Nurs. 2017;43:37–45.
Coorey GM, Neubeck L, Mulley J, Redfern J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol. 2018;25:505–21.
Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5:470–82.
McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–77.
Wu J-R, Moser DK, Chung ML, Lennie TA. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. J Card Fail. 2008;14:203–10.
van Onzenoort HAW, Menger FE, Neef C, Verberk WJ, Kroon AA, de Leeuw PW, et al. Participation in a clinical trial enhances adherence and persistence to treatment. Hypertension. 2011;58:573–8.
Allemann SS, Nieuwlaat R, Navarro T, Haynes B, Hersberger KE, Arnet I. Congruence between patient characteristics and interventions may partly explain medication adherence intervention effectiveness: an analysis of 190 randomized controlled trials from a Cochrane systematic review. J Clin Epidemiol. 2017;91:70–9.
Mosa ASM, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJV, et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017;23:464–75.
Ramachandran HJ, Jiang Y, Teo JYC, Yeo TJ, Wang W. Technology acceptance of home-based cardiac telerehabilitation programs in patients with coronary heart disease: systematic scoping review. J Med Internet Res. 2022;24: e34657.
Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17: e52.
George J, Elliott RA, Stewart DC. A systematic review of interventions to improve medication taking in elderly patients prescribed multiple medications. Drugs Aging. 2008;25:307–24.
Aubeeluck E, Al-Arkee S, Finlay K, Jalal Z. The impact of pharmacy care and motivational interviewing on improving medication adherence in patients with cardiovascular diseases: a systematic review of randomised controlled trials. Int J Clin Pract. 2021;75: e14457.
Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–10.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Study conception and design CC, GD, ZJ, NA; database searches CC, GD, ZJ; study selection and data extraction CC, GD, ZJ.; analysis, CC, GD, ZJ.; manuscript drafting CC, GD, ZJ, NA; manuscript revision and editing CC, GD, ZJ,NA. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Example search strategy- EMBASE. Table S2. Other clinical outcomes and their effects.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cheng, C., Donovan, G., Al-Jawad, N. et al. The use of technology to improve medication adherence in heart failure patients: a systematic review of randomised controlled trials. J of Pharm Policy and Pract 16, 81 (2023). https://doi.org/10.1186/s40545-023-00582-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40545-023-00582-9