Abstract
Background
There is no data on the prevalence of vitamin D deficiency in children with non-immunoglobulin-E (IgE) mediated gastrointestinal food allergy. The aims of our study were to understand the prevalence of vitamin D insufficiency and deficiency in children with non-IgE mediated gastrointestinal food allergy and identify predisposing factors.
Methods
This was a retrospective study which looked at data from Great Ormond Street Hospital from January 2002 to September 2015. Children 0–18 years old with a confirmed diagnosis of non-IgE mediated gastrointestinal food allergy who had a vitamin D level measured during the course of their disease were included. Low vitamin D levels were defined as <50 nmol/L; insufficient levels were defined as 25–50 nmol/L and deficient levels as <25 nmol/L. Patient characteristics and clinical factors were also recorded.
Results
Ninety-two patients met the study criteria; 49% were female and median age was 10 years 2 months [IQR: 4 years 8 months to 13 years 7 months]. Of the cohort, 26% (24/92) had low vitamin D levels; 16% had insufficient vitamin D levels and 10% had vitamin D deficiency. Gender (p = 0.043) and age (p = 0.035) were significantly associated with low vitamin D levels. Twelve percent of children who were on an amino acid formula (AAF) had low vitamin D compared to 31% of children who were not (p = 0.06). No other clinical factors were found to be significantly associated with low vitamin D levels.
Conclusions
Children with non-IgE mediated gastrointestinal food allergy are at risk of vitamin D insufficiency and deficiency. Further prospective studies need to be performed in all children with non-IgE mediated gastrointestinal food allergies.
Trial registration
The study was registered with the GOSH Research & Development department as a retrospective case note review. The Health Research Authority confirmed that NHS Research and Ethics Committee approval was not required; thus there is no trial registration number.
Similar content being viewed by others
Background
Vitamin D is a fat-soluble vitamin acquired from exposure to sunlight through skin synthesis and diet [1, 2]. The role of vitamin D within the immune system has been well described and its association with food allergy has been of great interest in recent years [1, 3]. Vitamin D is thought to improve antimicrobial defences within the immune system, suppress excessive inflammation and inhibit production of pro-inflammatory cytokines. More specifically, it is thought to help maintain epithelial barrier integrity by regulating tight junction proteins. It has been hypothesised that children with vitamin D deficiency are more likely to have dysbiotic microbial flora, increased risk of gastrointestinal infections and an abnormal intestinal barrier increasing the risk of developing food allergy [4].
Various factors can affect vitamin D levels in children, including dietary intake, ethnicity, sun exposure, being born in the winter and obesity [5]. Dietary intake can lead to low levels through extended breastfeeding without supplementation, late introduction of hen’s egg or a low maternal consumption of oily fish in pregnancy. Varying levels of vitamin D fortification of food and infant supplementation may also impact on vitamin D levels [2, 5].
Immunoglobulin E (IgE)-mediated food allergy tends to have a quick onset presenting with skin, respiratory and gastrointestinal symptoms whereas non-IgE mediated food allergy has a delayed onset of symptoms that are usually skin and gastrointestinal in nature [6]. Previous studies have shown an association between children with vitamin D deficiency and increased risk of IgE-mediated food allergy sensitisation, [4, 7, 8] conversely children with food allergy were at greater risk of vitamin D deficiency [9, 10]. An Australian study established that children with vitamin D insufficiency (<50 nmol/L) were more likely to be sensitised to both egg and peanut, but also at a greater risk of having multiple food allergies [11]. However, the majority of these studies have focused on the role of vitamin D in association with IgE-mediated food allergy with limited studies specifically focusing on the relationship of vitamin D and non-IgE mediated gastrointestinal food allergy. Similar to IgE-mediated food allergy, dietary elimination forms the mainstay of management in non-IgE-mediated food allergy, which can lead to restricted intake [12, 13]. Meyer et al. [13] found that in non-IgE gastrointestinal food allergic children on an elimination diet, there was a high risk of insufficient vitamin D intake especially in children not on a hypoallergenic formula [14]. It may also be that children with non-IgE gastrointestinal food allergy are at risk of developing vitamin D deficiency given the changes that occur to the gut on exposure to food antigens which can impact the role vitamin D metabolites have on the epithelial defences of the gut [4, 15]. In a study by Slack et al. [8], vitamin D levels were found to be low in patients with eosinophilic oesophagitis, especially those that were older and had a higher body mass index.
The main aim for this study was to understand the prevalence of vitamin D insufficiency and deficiency in children with non-IgE mediated gastrointestinal food allergy. We also wanted to identify predisposing factors for vitamin D deficiency in these children.
Methods
A retrospective study was performed at Great Ormond Street Hospital (GOSH), United Kingdom, from January 2002 to September 2015. The study was registered and approved by GOSH Research and Development department. All children aged 0–18 years old with a diagnosis of non-IgE mediated gastrointestinal food allergy established through an elimination diet [16] of at least one of the common allergic foods (i.e. cow’s milk, soya, egg, wheat, gluten, nuts, fish) were identified from the gastroenterology department’s clinical database of patients. Of this group, those who had a vitamin D level performed at any point during the course of their disease were included. Patients with concomitant non-atopic co-morbidities that could influence vitamin D intake or mechanism (chronic kidney or liver disease, history of prematurity, comorbidities affecting food intake (i.e cerebral palsy, history of bowel surgery) were excluded. Included patients had their demographics, clinical details and blood results entered into an anonymized, secure electronic database. This included data on gastrointestinal symptoms (diarrhoea, rectal bleeding, reflux/vomiting, constipation, abdominal pain, flatus, bloating/distension, back arching, food aversion), food elimination diets and/or hypoallergenic formulas and any evidence of histology results from endoscopy. Blood tests recorded included vitamin D levels, Parathyroid Hormone, Alkaline phosphatase, Ferritin, Haemoglobin, vitamins A, E and B12, Folate, Zinc, Selenium and Copper. If multiple micronutrient blood results were available, the test closest to the date the vitamin D sample was taken was recorded. Weight and height/length measurements were recorded at the time their vitamin D level was captured. Z-scores were calculated using the World Health Organisation (WHO) Anthro (Version 3.2.2) or AnthroPlus (Version 1.0.4) software according to age. We used the WHO guidelines to define undernutrition at ≤ -2- z-score and overnutrition ≥ 2 z-score [17]. Low vitamin D was defined as <50 nmol/L, more specifically vitamin D insufficiency was defined as 25–50 nmol/L and vitamin D deficiency as <25 nmol/L [11].
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22 (Armonk, NY). Continuous variables are presented as medians with interquartile ranges (IQR) or means where appropriate and categorical variables are presented as percentages. Mann Whitney U-tests were used to compare age and total number of dietary foods eliminated between the two groups. Pearson’s chi square test or Fisher’s Exact test were used where appropriate to compare gastrointestinal symptom presentation, atopic comorbidities, the type of dietary foods eliminated, the type of hypoallergenic formulas used, ethnicity and other vitamin and mineral bloods between children with normal vitamin D levels and those with insufficient levels. All tests were two-sided and significance level was set to 0.05.
Results
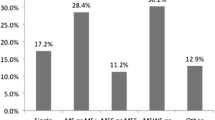
The medical records of 711 children that were within the inclusion criteria were reviewed; 114 children were excluded due to concomitant non-atopic co-morbidities as described in the methodology. Of the 505 children remaining, 92 (18%) had a vitamin D level measured. Of this group 49% (45/92) were female and the median age was 10 years 2 months [IQR: 4 years 8 months to 13 years 7 months] at the time their vitamin D level was measured. Within the group, 71% of the children were Caucasian, 13% Asian, 4% Black Afro-Caribbean and 12% did not specify their ethnicity. Information regarding foods eliminated, formulas the children were on, gastrointestinal symptoms experienced and atopic co-morbidities is shown in Table 1. The number of foods eliminated for the cohort is shown in Fig. 1. The median weight-for-age z-score for the children who had anthropometric data available at the time their vitamin D level was measured (n = 51/92, 55%) was: -0.82 [IQR: -1.64 to 0.79] and median height-for-age z-score was -1.1 [IQR: -1.93 to 0.26] (n = 60/92, 65%). Ten children (20%) had weight-for-age z-scores lower than -2 and 14 children (23%) had height-for-age z-scores lower than -2. There were no significant differences in z-scores between children with normal or low vitamin D levels, p = 0.905 and p = 0.422 respectively.
Of the cohort, 26% (24/92) had low vitamin D levels as per our criteria (<50 nmol/L) but more specifically, 16% (15/92) had insufficient vitamin D levels (25–50 nmol/L) and 10% (9/92) were vitamin D deficient (<25 nmol/L).
Analysis of the data indicated that age was significantly associated with low vitamin D levels (p = 0.035). More specifically, we found that children with low vitamin D were older (median age 12 years 5 months [IQR: 9 years 2 months to 14 years 4 months) than children with normal vitamin D levels (median age: 8 years 5 months [IQR: 4 years 4 months to 12 years 9 months]. Being female was also associated with low vitamin D (p = 0.043). However, we did not find any statistical significance between low vitamin D levels and ethnicity or the type and number of foods eliminated. Children on an amino acid formula (AAF) were less likely to have a low vitamin D level compared to children not on an AAF, 31% versus 12% respectively (p = 0.06). The median age of the children on an AAF was 8.1 years compared to 10.7 years for those not on an AAF. The majority of the type of gastrointestinal symptoms at presentation, atopic co-morbidities and abnormal histology from biopsy results were also found not to be associated with low vitamin D. However, significantly more children who did not have vomiting as a presenting gastrointestinal symptom (42%) had a low vitamin D level compared to those who had vomiting symptoms (16%) (p = 0.006).
We also collected data on other nutritional bloods from these patients. Of those who had nutritional bloods available, 71% had high folate levels, but up to 19% had vitamin A, selenium, copper and zinc deficiencies (Table 2). There was no significant association found between low vitamin D and having any abnormal nutritional bloods (p > 0.232).
Discussion
There is evidence suggesting that food allergic children are at risk of developing vitamin D deficiency but there is paucity of data in children with non-IgE mediated gastrointestinal food allergies [9, 10]. To our knowledge this is the first study that has specifically looked at vitamin D levels in a cohort of non-IgE mediated gastrointestinal food allergic children. In this retrospective study, we found that over 26% of our cohort had low vitamin D levels and 10% of the 26% were vitamin D deficient.
Two factors were significantly associated with vitamin D insufficiency: age and gender. It is already known that females, particularly teenagers and young women [2, 18] are at a higher risk of developing this deficiency and our data suggest that young girls with food allergy are also at a higher risk, although more boys have food allergy (64%) [19]. Our data indicates that girls are at greater risk of low vitamin D in gastrointestinal food allergy.
Although children less than 5 years old in the general population are at higher risk for vitamin D deficiency [2, 20], we found that in our allergic cohort older children were at higher risk. Older children who have non-IgE mediated gastrointestinal food allergy tend to be the children with more persistent and complex allergic disease. They often have more investigations performed and may be on elimination diets for longer. Some of the common foods eliminated (i.e. milk, egg) are high in vitamin D and if not adequately substituted can increase the risk of low vitamin D levels. Slack et al. [8] investigated vitamin D levels in adult and paediatric patients with eosinophilic oesophagitis and found that in their cohort, patients with insufficient vitamin D levels were older compared to those with sufficient levels, median age 25.5 and 16.2 years respectively. The UK recommends multivitamins with vitamin D for children below the age of 5 years, providing these for free for those with insufficient financial means [21]; however, for older children there is no such recommendation and no free vitamin supplementation scheme which may also explain the greater risk of deficiency. In a US-based study performed on food allergic children, 25% of children consumed less than 67% of the Recommended Dietary Allowance of vitamin D and calcium and they were also found to be shorter [22]. Although not statistically significant, 23% of our entire cohort had z-scores lower than -2. Studies have shown that food allergic children have a greater risk of impaired growth [12, 23, 24], but the fact that this may also be associated with poor growth in the non-IgE mediated cohort requires further attention.
We also found that a lower percentage of the children on an AAF had vitamin D deficiency compared to those children who were on alternative feeds (12% versus 31%). The median age of the children in the whole cohort on AAF was 8.1 years which is relatively high to still be on an AAF, likely indicating the severity and persistence of their disease. In addition vitamin D levels in hypoallergenic formulas and over the counter milks vary greatly, which may explain our findings, preferring an AAF for vitamin D. The bioavailability and source of vitamin D may also play a role. Although the UK does not routinely fortify foods with vitamin D, it could be that the fortification of hypoallergenic foods suitable for older children and routine vitamin D supplementation for children with non-IgE mediated food allergy could be beneficial.
There were no other nutritional blood tests that were significantly associated with low vitamin D levels. However, up to 19% had micronutrient deficiencies and many of these children were on multiple food elimination diets. Previous studies looking at food allergy have shown these children to be at risk of nutritional deficiencies, with low levels of vitamin A, selenium and zinc [13, 25], which have also been seen in children with other atopic conditions such as eczema [26]. These micronutrients have important immunomodulatory effects in the body and should be monitored in children with gastrointestinal food allergy [13, 25].
Limitations
One of the key limitations in this study was that it was retrospective and our data relied on the quality of the clinical notes available. The timing at which the vitamin D level was measured varied from patient to patient and was clinician dependant, which could have biased the results. There are also other variables that could have affected vitamin D levels such as sun exposure, seasonal timing of when the test was performed, other dietary factors which would be useful to consider in future studies in this population of children. Our cohort was also relatively small with a total of 92 patients included, which may have been why we were unable to identify any factors that had a significant impact on vitamin D levels. However, the fact that 10% of the children were vitamin D deficient is a novel finding and needs addressing in the future. There is also no current policy for vitamin D testing; in our study, the decision to measure vitamin D was based on suspicion of nutritional deficiency by the clinician reviewing the child. There is the possibility that children who are deficient are being missed further emphasising the importance of more research into this cohort of patients.
Conclusion
Children with non-IgE mediated gastrointestinal food allergy seem to be at risk of low vitamin D levels especially older, female patients. It is worth checking the levels in these children with persistent allergy, irrespective of the number of foods eliminated, but especially if they are not on an an AAF. Further prospective research needs to be performed in all children with non-IgE mediated gastrointestinal food allergies, not only children deemed at risk by clinical judgement.
Abbreviations
- AAF:
-
Amino acid formula
- GOSH:
-
Great Ormond Street Hospital
- IgE:
-
Immunoglobulin-E
- WHO:
-
World Health Organisation
References
Suaini NH, Zhang Y, Vuillermin PJ, Allen KJ, Harrison LC. Immune Modulation by Vitamin D and Its Relevance to Food Allergy. Nutrients. 2015;7(8):6088–108.
(SACN) SACoN. Draft Vitamin D and Health Report. 2015.
Della Giustina A, Landi M, Bellini F, Bosoni M, Ferrante G, Onorari M, et al. Vitamin D, allergies and asthma: focus on pediatric patients. World Allergy Organ J. 2014;7(1):27.
Vassallo MF, Camargo Jr CA. Potential mechanisms for the hypothesized link between sunshine, vitamin D, and food allergy in children. J Allergy Clin Immunol. 2010;126(2):217–22.
Vuillermin PJ, Ponsonby AL, Kemp AS, Allen KJ. Potential links between the emerging risk factors for food allergy and vitamin D status. Clin Exp Allergy. 2013;43(6):599–607.
Centre for Clinical Practice at N. National Institute for Health and Clinical Excellence: Guidance. Food Allergy in Children and Young People: Diagnosis and Assessment of Food Allergy in Children and Young People in Primary Care and Community Settings. London: National Institute for Health and Clinical Excellence (UK), National Institute for Health and Clinical Excellence; 2011.
Baek JH, Shin YH, Chung IH, Kim HJ, Yoo EG, Yoon JW, et al. The link between serum vitamin D level, sensitization to food allergens, and the severity of atopic dermatitis in infancy. J Pediatr. 2014;165(4):849–54.e1.
Slack MA, Ogbogu PU, Phillips G, Platts-Mills TA, Erwin EA. Serum vitamin D levels in a cohort of adult and pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2015;115(1):45–50.
Yu JW, Pekeles G, Legault L, McCusker CT. Milk allergy and vitamin D deficiency rickets: a common disorder associated with an uncommon disease. Ann Allergy Asthma Immunol. 2006;96(4):615–9.
Fox AT, Du Toit G, Lang A, Lack G. Food allergy as a risk factor for nutritional rickets. Pediatr Allergy Immunol. 2004;15(6):566–9.
Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol. 2013;131(4):1109-16, 16.e1-6.
Sova C, Feuling MB, Baumler M, Gleason L, Tam JS, Zafra H, et al. Systematic review of nutrient intake and growth in children with multiple IgE-mediated food allergies. Nutr Clin Pract. 2013;28(6):669–75.
Meyer R, De Koker C, Dziubak R, Skrapac AK, Godwin H, Reeve K, et al. A practical approach to vitamin and mineral supplementation in food allergic children. Clin Transl Allergy. 2015;5:11.
Meyer R, De Koker C, Dziubak R, Godwin H, Dominguez-Ortega G, Shah N. Dietary elimination of children with food protein induced gastrointestinal allergy - micronutrient adequacy with and without a hypoallergenic formula? Clin Transl Allergy. 2014;4(1):31.
Leung J, Beukema KR, Shen AH. Allergic mechanisms of Eosinophilic oesophagitis. Best Pract Res Clin Gastroenterol. 2015;29(5):709–20.
Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res (New York, NY). 2011;31(1):61–75.
de Onis MB, Monika. WHO Global Database on Child Growth. Geneva: World Health Organization; 1997.
Wood CL, Cheetham TD. Vitamin D: increasing supplement use among at-risk groups (NICE guideline PH56), Archives of disease in childhood Education and practice edition. 2015.
Kelly C, Gangur V. Sex Disparity in Food Allergy: Evidence from the PubMed Database. J Allergy. 2009;2009:159845.
Organization WH. Vitamin D Supplementation in infants 2015 [updated 22/07/2015. Available from: http://www.who.int/elena/titles/vitamind_infants/en/. Accessed 8 Feb 2016.
Health Do. Healthy Start 2014 [updated March 2014]. Available from: https://www.healthystart.nhs.uk/privacy-policy/. Accessed 8 Feb 2016.
Christie L, Hine RJ, Parker JG, Burks W. Food allergies in children affect nutrient intake and growth. J Am Diet Assoc. 2002;102(11):1648–51.
Berry MJ, Adams J, Voutilainen H, Feustel PJ, Celestin J, Jarvinen KM. Impact of elimination diets on growth and nutritional status in children with multiple food allergies. Pediatr Allergy Immunol. 2015;26(2):133–8.
Beck C, Koplin J, Dharmage S, Wake M, Gurrin L, McWilliam V, et al. Persistent Food Allergy and Food Allergy Coexistent with Eczema Is Associated with Reduced Growth in the First 4 Years of Life. J Allergy Clin Immunol Pract. 2015;4(2):248-56.e3.
Kamer B, Wasowicz W, Pyziak K, Kamer-Bartosinska A, Gromadzinska J, Pasowska R. Role of selenium and zinc in the pathogenesis of food allergy in infants and young children. Arch Med Sci. 2012;8(6):1083–8.
Toyran M, Kaymak M, Vezir E, Harmanci K, Kaya A, Ginis T, et al. Trace element levels in children with atopic dermatitis. J Investig Allergol Clin Immunol. 2012;22(5):341–4.
Acknowledgements
Not applicable.
Funding
There was no funding required for this study.
Availability of data and materials
All data in relation to this work is stored on a secure electronic database at Great Ormond Street Hospital. If further information is required, please contact the corresponding authors for more information.
Authors’ contributions
RXF, RM, RD and NS were involved in the study design, application for ethics approval, data collection, data analysis and drafting of the manuscript. STH and RN were involved in data collection. ACL, HG and KR were involved in reviewing multiple drafts of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was registered with the Great Ormond Street Hospital Research and Development department of which no further ethics approval was required. All data used was obtained retrospectively from previous records and anonymised for the purpose of this study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Foong, RX., Meyer, R., Dziubak, R. et al. Establishing the prevalence of low vitamin D in non-immunoglobulin-E mediated gastrointestinal food allergic children in a tertiary centre. World Allergy Organ J 10, 4 (2017). https://doi.org/10.1186/s40413-016-0135-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40413-016-0135-y