Abstract
Background
Sex hormone-binding globulin (SHBG) levels are low in adult subjects with obesity when compared to normal-weight individuals. Obesity is associated with higher tumor necrosis factor alpha (TNFα) plasma levels and lower adiponectin levels. Moreover, we have recently elucidated the molecular mechanisms by which TNFα and adiponectin regulate hepatic SHBG production.
Aim
The main objective of this study was to assess if the adult associations between TNFα, adiponectin, and SHBG are present in prepubertal children.
Methods
We determined several morphometric and biochemical parameters in normal-weight (n=15) and obese prepubertal (n=51) children, as well as quantified plasma SHBG, TNFα receptor 1 (TNFα-R1), and adiponectin levels.
Results
Our results showed that prepubertal children with obesity had decreased plasma SHBG levels compared to normal-weight controls (67 nmol/L vs 172 nmol/L). Importantly, SHBG plasma levels correlated significantly (P < 0.05) with TNFα (negatively, ßstd= − 0.31) and adiponectin (positively, ßstd= 0.58) suggesting an important role of these two cytokines in determining plasma SHBG levels in prepubertal children.
Conclusions
Our results suggest that plasma adiponectin levels may play a more important role than TNFα in influencing plasma SHBG levels in our prepubertal population with obesity.
Similar content being viewed by others
Background
Childhood obesity prevalence has increased at an alarming rate globally, and the International Association for the Study of Obesity estimates that up to 200 million school-aged children are either overweight or obese [1, 2]. This is important because children and adolescents with obesity are at higher risk of obesity during adulthood [3, 4], which is often linked to serious conditions including cardiovascular disease, type 2 diabetes, metabolic syndrome, and several types of cancer [3,4,5,6,7,8].
It is well-known that adult individuals with obesity show lower plasma sex hormone-binding globulin (SHBG) levels than normal-weight subjects [9, 10]. SHBG is a protein produced and secreted into the circulation by the liver that binds androgens and estrogens with high affinity. In blood, SHBG acts as a carrier of these sex steroids and regulates their bioavailability and access to target tissues and cells [11]. Interestingly, we have previously described that low-grade inflammation occurring in obesity is an important regulator of plasma SHBG levels [12]. Obesity is associated with higher tumor necrosis factor alpha (TNFα) plasma levels and lower adiponectin levels in adults [13, 14]. Moreover, we have recently elucidated the molecular mechanisms by which TNFα and adiponectin regulate hepatic SHBG production [15,16,17]. Low plasma SHBG levels have been also found in children with obesity; however, the reasons for such reduction need to be yet determined.
In the present work, we aimed to study whether these regulatory mechanisms were already present in prepubertal children. For this purpose, we determined several morphometric and biochemical parameters in normal-weight and prepubertal children with obesity, as well as quantified plasma SHBG, TNFα receptor 1 (TNFα-R1), and adiponectin levels.
Methods
Study participants
The study was approved by the Hospital’s Ethic Committee (PIC-21-12). Subsets of data from this cohort have been reported elsewhere [18, 19]. Children with obesity attending the Hospital Sant Joan de Déu, Barcelona (Spain) were recruited between the months of January 2013 and December 2014. Inclusion criteria were children (a) age from 7 to 10 years old; (b) prepubertal, defined as Tanner stage I breast development for girls and testicular volume less than 4 ml in boys; (c) with BMI-SDS for a given age and sex using the World Health Organization (WHO) standards greater than 2 standard deviations for the obesity group, and between −1 and 1 standard deviations for the normal-weight group. Exclusion criteria included any form of endogen obesity, major congenital or chronic disease, drug-induced obesity, use of drugs for weight loss, and involvement in another weight-loss program. All parents signed the informed consent document. Blood samples were obtained from the hospital’s biobank and were available from 15 normal-weight controls and from 51 of the 53 subjects with obesity initially included in the study.
Physiologic and biochemical measurements
Blood samples were obtained in the morning after an overnight fast (8–10 h of fasting). We measured weight (kg) and height (m) with light clothing in a calibrated scale and rigid stadiometer. Body mass index (BMI) was calculated, and BMI-SDS was obtained by using the “Anthro Plus” software (WHO). Blood pressure was measured in the right arm using an automated system with the appropriate sleeve size for the arm diameter. Waist circumference was determined as middle point between the last rib and iliac crest. Blood samples were taken in tubes containing EDTA, and plasma was immediately separated, aliquoted, and stored at −80 °C until further use. Glucose, insulin, glycated hemoglobin (HbA1c), and the lipid profile (total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides) were measured using standard protocols at the Hospital’s clinical laboratory. An oral glucose tolerance test (1.75 g glucose/kg with a maximum of 75 g per subject) was performed in a subset of children with obesity (n=47); glucose and insulin levels were measured at 0, 30, 60, 90, and 120 min post-glucose load. The insulinogenic index (IGI) and the whole-body insulin sensitivity index (WBISI) were calculated from the tolerance test data as previously described [20]. Plasma levels for SHBG, adiponectin, and TNFα-R1 were measured by ELISA (R&D Systems, Madrid, Spain).
Statistical analysis
Data are shown as mean (SD) for normally distributed data, and median [IQR] for non-normally distributed data; groups were compared using two-paired t-test or Mann-Whitney U test, respectively. Normality was tested with the Shapiro-Wilk W test. SHBG levels were log-transformed to achieve normality before testing correlations. Associations between variables were determined by standard least-square regression, with sex, age, and BMI-SDS as covariates where indicated. Data analysis and calculations were performed in JMP v14.3 (SAS Institute Inc., Cary, NC). A p value below 0.05 was considered significant.
Results
Circulating SHBG levels are decreased in prepubertal children with obesity
The main clinical and biochemical features of normal-weight (n=15) and children with obesity (n=51) are displayed in Table 1. All morphometric parameters were significantly elevated in subjects with obesity compared with lean subjects, including weight, BMI-SDS, and waist circumference (Table 1). Regarding the glycemic parameters, no differences were found between lean and obese subjects in glucose and HbA1c levels; however, insulin concentration and HOMA-IR values were significantly increased in children with obesity (Table 1). Regarding the lipid profile, while total cholesterol was not altered, we observed increased LDL-cholesterol and decreased HDL-cholesterol, as well as higher triacylglyceride (TAG) levels in the group with obesity (Table 1). Moreover, subjects with obesity had significantly higher C-reactive protein (CRP) and TNFα-R1 and reduced SHBG and adiponectin plasma levels compared to the normal-weight controls (Table 1).
SHBG correlations with anthropometric and biochemical parameters in prepubertal children
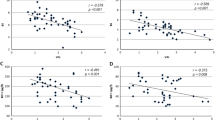
After analyzing all the measured parameters in our study population, our results showed that SHBG levels were negatively correlated to BMI-SDS, waist circumference, insulin, HOMA-IR, TAG, TNFα-R1, and CRP plasma levels adjusting for sex and age. In addition, plasma SHBG levels correlated significantly and positively with adiponectin and HDL plasma levels (Table 2). No correlation was found between SHBG and glucose, HbA1c, total cholesterol, or LDL levels (Table 2). Interestingly, while further adjustment for BMI-SDS did not substantially modify SHBG correlations with insulin, HOMA-IR, and adiponectin, its association with inflammation markers TNFα-R1 and CRP plasma levels was completely lost (Table 2).
SHBG plasma level correlations in prepubertal children with obesity
Given that obesity is intimately associated with SHBG levels, insulin resistance, and inflammation markers, we analyzed SHBG correlations with all measured parameters only in prepubertal children with obesity to avoid this confounding factor. Our results showed that SHBG plasma levels correlated significantly and negatively with waist circumference, insulin, and TAG concentration, while they correlated positively with adiponectin (Table 3). No correlation was found between SHBG and BMI-SDS, % fat mass, TNFα-R1, CRP glucose, total cholesterol, LDL, or HDL plasma levels (Table 3). Further adjustment by BMI-SDS did not substantially modify these correlations (Table 3).
Discussion
Childhood obesity has become a major health concern in the last decades and has increased its prevalence worldwide, in both developed and developing countries [21,22,23]. It has been already described that childhood and adolescent obesity is associated with an increase in BMI and excess of adiposity [5, 24] which was also evident in our study since prepubertal children with obesity showed higher BMI-SDS and waist circumference when compared with lean children. This adiposity increase occurring in obesity is also associated with insulin resistance, hypertension, and dyslipidemia, which in turn increase risk of developing atherosclerotic vascular disease and type 2 diabetes [5, 24]. Our results corroborated the latter since obese prepubertal children had higher insulin plasma levels together with an increase in HOMA-IR and higher LDL and TAG plasma levels when compared with lean prepubertal children.
An excess of adiposity may also influence various aspects of pubertal development, such as timing of pubertal initiation and hormonal parameters during puberty. How obesity may perturb several hormonal aspects of puberty is currently under debate; however, it may involve sex hormone-binding globulin (SHBG), a liver-produced glycoprotein that binds sex steroids with high affinity in blood regulating their bioavailability [11]. Interestingly, SHBG plasma levels change through human life-span, remaining high during infancy and decreasing when puberty approaches [25]. It is important to mention that low plasma SHBG levels are found in children with obesity; this is in agreement with our results showing that prepubertal children with obesity had half the plasma SHBG levels present in normal-weight prepubertal children.
Low plasma SHBG levels are also present in adult subjects with obesity [9, 10]. The reason why this may occur has been investigated during the last three decades; initially, it was suggested that hyperinsulinemia was the main factor reducing plasma SHBG levels [26, 27]. In fact, a negative correlation between plasma SHBG and insulin levels has been previously described [28, 29], which we also found in our study. However, insulin as a major factor downregulating SHBG production does not come along with the fact that obese subjects are characterized by insulin resistance [30, 31], and, to the best of our knowledge, the molecular mechanism by which insulin reduces SHBG production has not yet been described. In this regard, we have demonstrated that insulin does not regulate hepatic SHBG production [15, 32]. Nevertheless, we found strong correlations between SHBG and insulin plasma levels when analyzing our study population or children with obesity alone. Furthermore, SHBG was strongly associated with both insulin sensitivity (HOMA-IR and WBISI) and insulin secretory (IGI) indexes in children with obesity.
Accumulated evidence over the last decade points to inflammation as one of the critical processes associated with the development of obesity, insulin resistance, and type 2 diabetes [33, 34]. In fact, obesity is considered a state of chronic low-grade inflammation [35, 36]. This is of importance since we have reported that alterations in proinflammatory and anti-inflammatory cytokines occurring in obesity [37, 38] play an important role in regulating hepatic SHBG production [12, 15,16,17, 39]. Specifically, using in vitro and in vivo models as well as human samples, we have shown that hepatic SHBG production was reduced by TNFα and increased by adiponectin [15,16,17]. Mechanistically, we have determined that TNFα decreases SHBG production by reducing the protein levels of hepatocyte nuclear receptor 4 alpha (HNF-4α), the main activator of SHBG gene expression [15, 16]. On the other hand, adiponectin increases SHBG production via HNF-4α levels by inhibiting hepatic lipogenesis and stimulating hepatic β-oxidation [17]. Interestingly, we showed that plasma TNFα-R1 levels correlated inversely with plasma SHBG levels in adult obese subjects [15]. Remarkably, prepubertal children with obesity from our study had higher TNFα-R1 plasma levels than lean children, as described previously [40]. Importantly, SHBG plasma levels correlated significantly and negatively with TNFα-R1 plasma levels. However, this correlation was lost after adjusting for BMI-SDS. This is of importance since TNFα levels could be an important factor downregulating SHBG production during early stages of obesity development, but once children reach a certain point of inflammation, TNFα plasma levels may not influence SHBG production.
Plasma adiponectin levels are lower in subjects with obesity, both adult [41] and children, compared to normal-weight controls [42]. These findings were corroborated in our study, since prepubertal children with obesity had lower levels of adiponectin when compared with lean subjects. In addition, it has been previously reported that adiponectin levels were inversely associated with SHBG [43, 44], and this correlation was also found in our prepubertal population. Moreover, this correlation between SHBG and adiponectin was maintained after adjusting for BMI-SDS. More importantly, this SHBG correlation with adiponectin was also found when analyzing only subjects with obesity.
In conclusion, the results obtained in this study show that prepubertal children with obesity had decreased plasma SHBG levels compared to normal-weight controls. Importantly, SHBG plasma levels correlated significantly with TNFα (negatively) and adiponectin (positively), suggesting that adiponectin and TNFα could play a role in determining plasma SHBG levels in prepubertal children. In addition, our results suggest that plasma adiponectin levels may play a more important role than TNFα in influencing plasma SHBG levels in our prepubertal population with obesity.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI-SDS:
-
Body mass index standard deviation score
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- HDL:
-
High-density lipoprotein
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- LDL:
-
Low-density lipoprotein
References
Wang Y, Lim H (2012) The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psychiatry 24(3):176–188
Fruhstorfer BH, Mousoulis C, Uthman OA et al (2016) Socio-economic status and overweight or obesity among school-age children in sub-Saharan Africa - a systematic review. Clin Obes 6(1):19–32
The NS, Suchindran C, North KE et al (2010) Association of adolescent obesity with risk of severe obesity in adulthood. JAMA 304(18):2042–2047
Pi-Sunyer X (2009) The medical risks of obesity. Postgrad Med 121(6):21–33
Biro FM, Wien M (2010) Childhood obesity and adult morbidities. Am J Clin Nutr 91(5):1499S–1505S
Harrell JS, Jessup A, Greene N (2006) Changing our future: obesity and the metabolic syndrome in children and adolescents. J Cardiovasc Nurs 21(4):322–330
Bjerregaard LG, Jensen BW, Ängquist L et al (2018) Change in overweight from childhood to early adulthood and risk of type 2 diabetes. New England Journal of Medicine 378:1302–1312
Juonala M, Magnussen CG, Berenson GS et al (2011) Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 365:1876–1885
De Moor P, Joossens JV (1970) An inverse relation between body weight and the activity of the steroid binding-globulin in human plasma. Steroidologia 1(3):129–136
Kopelman PG, Pilkington TR, White N et al (1980) Abnormal sex steroid secretion and binding in massively obese women. Clin Endocrinol (Oxf) 12(4):363–369
Siiteri PK, Murai JT, Hammond GL et al (1982) The serum transport of steroid hormones. Recent Prog Horm Res 38:457–510
Simó R, Sáez-López C, Barbosa-Desongles A et al (2015) Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab 26(7):376–383
Katsuki A, Sumida Y, Murashima S et al (1998) Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 83(3):859–862
Weyer C, Funahashi T, Tanaka S et al (2001) Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86(5):1930–1935
Simo R, Barbosa-Desongles A, Lecube A et al (2012) Potential role of tumor necrosis factor-alpha in downregulating sex hormone-binding globulin. Diabetes. 61:372–382
Simo R, Barbosa-Desongles A, Sáez-Lopez C et al (2012) Molecular mechanism of TNFalpha-induced down-regulation of SHBG expression. Mol Endocrinol 26:438–446
Simó R, Saez-Lopez C, Lecube A et al (2014) Adiponectin upregulates SHBG production: molecular mechanisms and potential implications. Endocrinology 155(8):2820–2830
Leal-Witt MJ, Ramon-Krauel M, Samino S et al (2018) Untargeted metabolomics identifies a plasma sphingolipid-related signature associated with lifestyle intervention in prepubertal children with obesity. Int J Obes 42:72–78
Leal-Witt MJ, Llobet M, Samino S et al (2018) Lifestyle intervention decreases urine trimethylamine N-oxide levels in prepubertal children with obesity. Obesity (Silver Spring) 26:1603–1610
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Institute of Medicine (2012) Accelerating progress in obesity prevention: solving the weight of the nation. National Academies Press, Washington, DC
US Department of Health and Human Services (2010) The surgeon general’s vision for a healthy and fit nation. US Dept., Health and Human Services, Rockville, MD
Must A, Hollander SA, Economos CD (2006) Childhood obesity: a growing public health concern. Expert Rev Endocrinol Metab 1(2):233–254
Adair LS (2008) Child and adolescent obesity: epidemiology and developmental perspectives. Physiol Behav. 22;94(1):8-16.
Hammond GL (2011) Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod 85:431–441
Plymate SR, Matej LA, Jones RE et al (1988) Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J. Clin. Endocrinol. Metab 67:460–464
Loukovaara M, Carson M, Adlercreutz H (1995) Regulation of production and secretion of sex hormone binding globulin in HepG2 cell cultures by hormones and growth factors. J. Clin. Endocrinol. Metab 80:160–164
Haffner SM, Katz MS, Stern MP et al (1988) The relationship of sex hormones to hyperinsulinemia and hyperglycemia. Metabolism 37:683–688
Pugeat M, Cousin P, Baret C et al (2000) Sex hormone-binding globulin during puberty in normal and hyperandrogenic girls. J. Pediatr. Endocrinol. Metab 13:1277–1279
Bonadonna RC, Groop L, Kraemer N et al (1990) Obesity and insulin resistance in humans: a dose-response study. Metabolism 39(5):452–459
Dunaif A, Segal KR, Futterweit W et al (1989) Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38(9):1165–1174
Selva DM, Hogeveen KN, Innis SM et al (2007) Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest 117:3979–3987
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115(5):1111–1119
Olefsky JM, Glass CK (2010) Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72:219–246
Mathieu P, Lemieux I, Després JP (2010) Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 87(4):407–416
Hotamisligil GS (2003) Inflammatory pathways and insulin action. Int J Obes 27:S53–S55
Yudkin JS (2003) Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes 27(3):S25–S28
Pirola L, Ferraz JC (2017) Role of pro- and anti-inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World J Biol Chem 26;8(2):120-128.
Simó R, Barbosa-Desongles A, Hernandez C et al (2012) IL1β down-regulation of sex hormone-binding globulin production by decreasing HNF-4α via MEK-1/2 and JNK MAPK pathways. Mol Endocrinol 26(11):1917–1927
Dzienis-Straczkowska S, Straczkowski M, Szelachowska M et al (2003) Soluble tumor necrosis factor-alpha receptors in young obese subjects with normal and impaired glucose tolerance. Diabetes Care 26(3):875–880
Oh KW, Lee WY, Rhee EJ et al (2005) The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol (Oxf) 63:131–138
Weiss R, Dufour S, Groszmann A et al (2003) Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. J Clin Endocrinol Metab 88(5):2014–2018
Vanbillemont G, Lapauw B, De Naeyer H et al (2012) Sex hormone-binding globulin at the crossroad of body composition, somatotropic axis and insulin/glucose homeostasis in young healthy men. Clin Endocrinol (Oxf) 76:111–118
Yasui T, Tomita J, Miyatani Y, et al (2007) Associations of adiponectin with sex hormone-binding globulin levels in aging male and female populations. Clin Chim Acta 386:69–75.
Acknowledgements
We are indebted to the families and children who participated in the study. We thank the “Biobanc de l’Hospital Infantil Sant Joan de Déu per a la Investigació” integrated in the Spanish Biobank Network of Instituto de Salud Carlos III for sample procurement.
Funding
This work was supported by grants from the Instituto de Salud Carlos III (DMS) PI12/01357, CIBERDEM (CIBER de Diabetes y Enfermedades Metabólicas Asociadas) an initiative of Instituto de Salud Carlos III (DMS), European Regional Development Funds (ERDF), and Marie Curie FP7-PEOPLE-2011-CIG (CL).
Author information
Authors and Affiliations
Contributions
MRK: researched data, contributed to discussion, reviewed/edited manuscript. MJLW: contributed to discussion, reviewed/edited manuscript. OOC: contributed to discussion, reviewed/edited manuscript. MAB: contributed to discussion, reviewed/edited manuscript. CL: researched data, contributed to discussion, wrote manuscript, reviewed/edited manuscript. DMS: researched data, contributed to discussion, wrote manuscript, reviewed/edited manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All parents signed the informed consent document. The study was approved by the Hospital’s Ethic Committee (PIC-21-12).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramon-Krauel, M., Leal-Witt, M.J., Osorio-Conles, Ó. et al. Relationship between adiponectin, TNFα, and SHBG in prepubertal children with obesity. Mol Cell Pediatr 8, 3 (2021). https://doi.org/10.1186/s40348-021-00113-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40348-021-00113-z