Abstract
Continuous drug delivery (CDD) is used in moderately advanced and late-stage Parkinson’s disease (PD) to control motor and non-motor fluctuations (‘OFF’ periods). Transdermal rotigotine is indicated for early fluctuations, while subcutaneous apomorphine infusion and levodopa-carbidopa intestinal gel are utilised in advanced PD. All three strategies are considered examples of continuous dopaminergic stimulation achieved through CDD. A central premise of the CDD is to achieve stable control of the parkinsonian motor and non-motor states and avoid emergence of ‘OFF’ periods. However, data suggest that despite their efficacy in reducing the number and duration of ‘OFF’ periods, these strategies still do not prevent ‘OFF’ periods in the middle to late stages of PD, thus contradicting the widely held concepts of continuous drug delivery and continuous dopaminergic stimulation. Why these emergent ‘OFF’ periods still occur is unknown. In this review, we analyse the potential reasons for their persistence. The contribution of drug- and device-related involvement, and the problems related to site-specific drug delivery are analysed. We propose that changes in dopaminergic and non-dopaminergic mechanisms in the basal ganglia might render these persistent ‘OFF’ periods unresponsive to dopaminergic therapy delivered via CDD.
Similar content being viewed by others
Introduction
Response fluctuations (motor and non-motor ‘OFF’ periods) are a common feature of levodopa-treated Parkinson’s disease (PD) and major determinants of quality of life in PD, serving as a main outcome measure in most key clinical trials [1]. They occur in both early and late illness, with reported onset as early as 5–6 months following the initiation of levodopa treatment [2,3,4] and are characterised by ‘OFF’ periods that are clinically heterogeneous. They manifest as ‘wearing OFF’, early morning ‘OFF’, delayed ON, no ON or random/unpredictable ‘ON–OFF’ [5] (Fig. 1). The duration of ‘OFF’ periods, as well as their severity and unpredictability are the features most associated with a more impaired quality of life [6,7,8]. Predictable ‘OFF’ periods can be associated with drug dose and the onset of ‘wearing OFF’ with the end of its effect [9]. Loss of drug efficacy characterising predictable ‘OFF’ periods has been associated with a loss of presynaptic storage of levodopa/dopamine in remaining dopaminergic terminals in the striatum with increased disease severity [5, 10,11,12]. This is, however, unlikely to be the entire explanation, as ‘wearing OFF’ is also reported with dopamine agonists and in animal models of PD without presynaptic involvement [12,13,14,15]. A post-synaptic component affecting basal ganglia output seems likely and this may be associated with the loss of the long-duration response to levodopa, although this has recently been disputed [5, 12, 16,17,18,19]. Unpredictable or random ‘OFF’ periods are a more complex phenomenon and difficult to treat with dopaminergic medications, even when given continuously to achieve continuous drug delivery (CDD) [9]. Factors affecting the peripheral pharmacokinetic profile of levodopa, such as the interference of high-protein meals, gastroparesis, H. Pylori infection or constipation, can contribute to the unpredictability [20, 21]. However, the central mechanisms underlying unpredictable ‘OFF’ periods, such as ‘ON–OFF’, may involve non-dopaminergic pathways, although there has been no preclinical investigation examining whether or why these occur.
Standard therapies for treating ‘OFF’ periods involve alterations of the dose, dose frequency and timing of oral dopaminergic medications, and the use of adjuncts (enzyme inhibitors and agonists) to levodopa to extend its duration of effect [5, 22]. While these reduce ‘OFF’ periods and ‘OFF’ time in the short term, they are not effective in producing a constant restoration of motor or non-motor function. Dosing of levodopa using new slow-release or longer-acting preparations as well as dopamine agonists, has largely failed to provide continuous dopaminergic stimulation (CDS) or to restore ‘ON’ periods without troublesome dyskinesia [23]. Indeed, avoiding the standard oral administration of dopaminergic drugs has been seen as essential in achieving more predictable delivery of dopaminergic medications and more predictable ‘ON’ time. In this respect, the concept of CDS has proven useful [24, 25]. While initially proposed to provide a more physiological dopaminergic response that avoids the onset of motor fluctuations and motor complications, it has morphed in to providing a means of CDD that has proven effective in reducing ‘OFF’ time and lessening the intensity of existing dyskinesia [5], even if it fails to achieve a complete control of motor fluctuations.
Three current distinct approaches to non-oral CDD have been introduced: the transdermal delivery of rotigotine, predominantly used in early PD, the continuous subcutaneous infusion of apomorphine (CSAI), and the intraduodenal delivery of levodopa-carbidopa intestinal gel (LCIG), recently available in a new formulation with entacapone (levodopa–entacapone–carbidopa intestinal gel) [26], indicated for the treatment of advanced PD [25, 27]. Additionally, novel formulations of subcutaneously-delivered levodopa are currently under investigation [28]. The use of alternative routes to the oral one, is likely to be the key factor for improved outcomes, as they avoid the gastrointestinal route and thus overcome drug delivery bottlenecks [29].
In early PD, rotigotine transdermal patches are employed as a mono- or adjunctive therapy to improve motor function as well as motor and non-motor fluctuations, in particular ‘wearing OFF’ [30,31,32]. This strategy reduces but does not abolish ‘OFF’ periods [33]. In later PD, CSAI and LCIG are used, and controlled data from pivotal studies suggest a reduction in ‘OFF’ time and an increase in ‘ON’ time without troublesome dyskinesia in the short term [34,35,36,37]. However, after a variable “honeymoon” period, most patients still experience ‘OFF’ episodes despite receiving CDD [38]. Although the technologies involved, the device-related issues or the complications at the site of infusion may explain the emergent and “refractory” ‘OFF’ periods, they are unlikely the sole reason. The possibility of involvement of non-dopaminergic pathways needs to be explored.
The problem of drug-resistant motor fluctuations
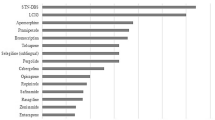
There remains a fundamental issue as to why drug-resistant fluctuations and ‘OFF’ periods occur despite CDD. As an example, multiple randomised, double-blind, placebo-controlled trials have reported improved motor and non-motor symptoms with transdermal rotigotine as an early monotherapy [30, 32, 39, 40] and as an adjunctive therapy in advanced PD [33, 41,42,43,44,45]. This strategy is based on preclinical studies where sustained delivery of rotigotine provided stable extracellular drug levels in the striatum, resulting in continuous stimulation of dopamine receptors [46]. When translated into PD patients, continuous transdermal drug delivery of rotigotine results in stable plasma levels over a 24-h period [47], but whether this translates into CDS in the basal ganglia remains unclear, even though preclinical data underpin this concept. While there is a sustained improvement in ‘OFF’ time and reduction in absolute ‘OFF’ time with rotigotine, motor fluctuations are not abolished and more than 50% of motor ‘OFF’ time remains across the patient population studied (Table 1). The effects of transdermal rotigotine on non-motor fluctuations, with the exception of pain [48], are unknown and need to be evaluated [49, 50], although it is presumed that the non-motor fluctuations are likely also to be prevalent during these emergent “refractory” ‘OFF’ periods, as non-motor fluctuations are often associated with motor fluctuations, particularly with the ‘OFF’ state [1, 51]. Specific reasons for a failure to eliminate ‘OFF’ periods could be application site reactions, which also represent the major reason for discontinuation [52], or reduced patch adhesion [53]. Additionally, there could potentially be differences in the extent of rotigotine delivery through the skin and to the systemic circulation and then to the brain between individual PD patients, but these have not been defined. It could be argued that the rotigotine dose employed in the patches is inadequate to provide plasma/brain levels of the drug to abolish ‘OFF’ periods, but these also persist in patient populations receiving concomitant oral dopaminergic therapy. Finally, it is reasonable to assume that the “refractory” ‘OFF’ periods during rotigotine treatment are qualitatively more severe than those present with apomorphine or levodopa. In fact, despite the lack of specific studies comparing ‘OFF’ quality between the different regimes, it is well known that levodopa treatment is associated with better motor function and quality of life when compared to dopamine agonists [54, 55], with the exception of apomorphine which has been confirmed to be as effective as levodopa [56, 57].
CSAI is indicated for patients with unpredictable or prolonged ‘OFF’ time, motor fluctuations and dyskinesia [58]. The efficacy of CSAI, in monotherapy or as add-on to levodopa, in reducing the ‘OFF’ time has been demonstrated in several uncontrolled open-label series (Table 2). The average ‘OFF’ time reduction was 60%, while the average reduction in dyskinesia severity was 30% [59]. This was confirmed in the TOLEDO study, the first-ever randomised, parallel-group, double-blind, placebo-controlled, multi-centre trial examining CSAI over 12 weeks in advanced PD [36]. The study showed that apomorphine significantly reduced ‘OFF’ time compared to placebo (− 2.47 vs − 0.58 h/day), without increasing troublesome dyskinesias, and allowed a dose reduction of concomitant oral antiparkinsonian medications. These effects were maintained in the open-label extension of the TOLEDO study over 52 weeks [60]. The effects of CSAI were comparable to LCIG in terms of efficacy in treating motor fluctuations in advanced PD [61], but once again without the abolition of ‘OFF’ time, despite optimisation of both apomoprphine delivery and concommitant oral or transdermal therapy. Although apomorphine is also beneficial in treating some non-motor symptoms of PD, such as mood or cognition [62, 63], there is no evidence on the treatment of non-motor fluctuations occurring during the ‘OFF’ state. The persistence of ‘OFF’ periods despite the clear efficacy of CSAI in advanced PD, can be due, as with rotigotine, to the under-dosing, although ‘OFF’ periods are emergent even with high-dose infusion of apomorphine, either in monotherapy or as an add-on to oral levodopa, as in most of the studies which have evaluated apomorphine efficacy [64]. It is feasible that occasional technical issues related to the device, such as pump failure, line blockage or inaccurate needle insertion, can cause a reduction of CSAI efficacy [61, 65]. The extent of apomorphine delivery from the subcutaneous site to the systemic circulation may be determined by the site of injection (the abdomen seems to be the best site), the state of the skin (warm skin increases the absorption compared to cold skin), and volume and depth of the injection (a greater volume is associated with a better absorption) [61, 66]. But these are unlikely to explain the persistence of ‘OFF’ periods across the patient populations studied. Finally, the occurrence of subcutaneous nodules due to an inflammatory reaction [61, 67] can mechanically affect local absorption directly or by altering the blood flow [61]. But again, this seems unlikely a cause of the persistence of significant ‘OFF’ time.
In patients with advanced PD and severe motor fluctuations, continuous delivery of LCIG directly to its site of absorption in the duodenum avoids the typical fluctuations in plasma profile associated with conventional oral levodopa formulations, which lead to a non-physiological pulsatile stimulation of striatal dopamine receptors and are associated with levodopa-induced motor fluctuations and complications [24]. Pharmacokinetic studies have shown a significantly reduced intra-subject variability in plasma levels over the period of infusion compared to that during oral treatment [95, 96]. The continuous delivery of levodopa is expected to markedly reduce ‘OFF’ periods in advanced PD compared to oral therapy, and this has been confirmed in a systematic review and meta-analysis [97], and in an exhaustive qualitative analysis of 27 studies with mixed designs in which ‘OFF’ time outcome measures were reported ≥ 12 months after the initiation of LCIG [98] (Table 3). The ‘OFF’ time showed a mean relative reduction of 47%–82% at 3–6 months of follow-up and up to 83% at 3–5 years of follow-up [98]. However, as with other continuous delivery systems, ‘OFF’ time was not abolished and similarly, the effects on non-motor fluctuations have not been studied, despite the proven efficacy on specific non-motor symptoms such as sleep, fatigue, and gastrointestinal symptoms [62, 63]. Despite the reductions of ‘OFF’ time, the persistence of ‘OFF’ periods during LCIG therapy can be due to several factors, mostly overlapping with those described for CSAI. The LCIG device-related issues such as tube dislocation and pump malfunctioning, or peristomal complications such as the presence of granulation tissue, skin problems or local infection, might further contribute to ‘OFF’ persistence [99, 100]. In addition, comorbid H. Pylori infection as well as alterations of gut microbiota, including small intestinal bacteria overgrowth (SIBO) and high prevalence of tyrosine decarboxylase-producing bacteria, can interfere with LCIG absorption, ultimately leading to motor complications and persistence of ‘OFF’ periods [29, 101,102,103]. Constipation might contribute to LCIG erratic gut absorption [104], and protein-rich meals (containing large neutral amino acid) can worsen the parkinsonian symptoms, because of levodopa fluctuations in the brain [103, 105]. On the other side, fasting can also contribute to the worsening of motor fluctuations. A recent study has demonstrated that the maintenance dose of LCIG is strongly correlated with the mean plasma concentration of levodopa in the absence or presence of lunch, and comparison of the pharmacokinetic parameters showed that the coefficient of variation is significantly greater in fasting patients than in those that did eat [106]. Finally, it has been demonstrated that LCIG treatment is associated with high plasma levels of 3-O-methyldopa (3-OMD), a metabolite of levodopa (converted by catechol-O-methyltransferase [COMT]) which competes with levodopa itself for brain penetration, a phenomenon that can be counteracted by the concomitant administration of a COMT inhibitor [107, 108]. However, even when all these factors are taken into consideration for optimization of LCIG administration, the persistence of ‘OFF’ periods is still commonly observed in everyday clinical practice. It should be noted that subcutaneous levodopa infusion, which is currently under investigation, might be a valuable alternative option to LCIG, as it does not require intrajejunal tube insertion nor is influenced by some of the aforementioned issues, such as H. Pylori infection or SIBO. Preliminary data have indeed shown its efficacy in reducing daily OFF time by at least 2 h [28, 109, 110].
Determinants of persistent ‘OFF’ periods in CDD therapies
In this review, we have summarised the evidence for the effectiveness of the three most commonly used therapies employed to provide CDD in treating ‘OFF’ periods—transdermal delivery of rotigotine, CSAI and LCIG. The conclusion reached is that even with CDD, significant amounts of ‘OFF’ time remain, appearing unresponsive to dopaminergic therapy. We have examined in detail the potential reasons why each CDD-based non-oral therapy might fail to abolish ‘OFF’ periods, in relation to the technologies underlying drug delivery. In individual patients, these are perfectly viable reasons for the persistence of motor fluctuations and should be addressed to optimise therapy.
However, a number of caveats need to set out before discussing the findings in greater detail:
-
(1)
For each therapy, the major clinical parameter used to assess efficacy has relied on ‘OFF’ time, but the definition of ‘OFF’ can be vague and inconsistent and surprisingly, remains poorly defined. The ‘OFF’ time is often based on patients’ Hauser diaries, which might lack accuracy, as for other ‘ON/OFF’ diaries [143]. An alternative option for objective measurement of ‘OFF’ periods is the use of home-monitoring devices, such as Parkinson's KinetiGraph™ (PKG) or KinesiaU (Great Lakes Neurotechnology), whose full potential in the clinical practice is still under investigation [144,145,146]. Additionally, the quality and the severity of the ‘OFF’ periods, together with the involvement of motor and non-motor components, need more clarity.
-
(2)
It is unclear as to whether these therapies improve both predictable and unpredictable ‘OFF’ periods, or whether it is predictable ‘OFF’ periods that respond and unpredictable that persist.
-
(3)
The response is highly variable among individual patients and a complete abolition of ‘OFF’ periods can be achieved in some, while others still exhibit marked ‘OFF’ time.
-
(4)
It seems unclear whether additional standard oral dopaminergic therapy or a further increase in the dose for CDD, further reduces ‘OFF’ periods, as this may be masked by the severity of dyskinesia.
-
(5)
The changes in the temporal pattern of ‘OFF’ periods over the day and over time after introduction of CDD remain to be fully mapped.
Having defined the caveats that complicate the data presented for each of the continuous therapies, there are potentially important conclusions that can be reached by a comparison of their individual profiles. The fact that the continuous therapies involve different drugs with differing modes of action and different routes of administration using different technologies allows for exclusion of the drug delivery parameters set out in Table 4 to be responsible for the persistence of ‘OFF’ time.
The major arguments are set out below:
-
(1)
Despite the use of differing technologies to provide CDD, the overall finding of persistent ‘OFF’ time is common to all. This suggests that a technology-based explanation is not viable, with the exception of dose/time. A dose-based reason for remaining ‘OFF’ time is feasible, but persistent ‘OFF’ time is seen with differing periods of daily drug delivery for levodopa and apomorphine, and rotigotine is delivered 24 h per day but still fails to remove ‘OFF’ periods.
-
(2)
Site-specific reasons for persistent ‘OFF’ periods also appear unlikely based on the differing modes of drug delivery. Only levodopa is administered through the gastro-intestinal tract, and none of the multiple potential reasons underlying remaining ‘OFF’ periods would apply to rotigotine or apomorphine delivery.
-
(3)
In contrast to few studies on oral levodopa [147,148,149], there is no evidence that plasma drug levels change during the day to coincide with remaining ‘OFF’ periods or for other changes in the peripheral pharmacokinetics of levodopa, apomorphine or rotigotine. This leads to the conclusion that the remaining ‘OFF’ periods are uncontrolled because of events occurring within the brain.
-
(4)
There is no evidence for altered penetration of drugs into the brain that would coincide with ‘OFF’ periods. Only levodopa shows potential change of active uptake linked to competition from dietary large neutral amino acids [103, 105]. Rotigotine and apomorphine penetrate by simple diffusion [150, 151].
-
(5)
Involvement of conversion and storage of dopamine is only relevant to levodopa [152, 153] and does not apply to the dopamine agonists. Similarly, presynaptic storage and the involvement of a long duration response only relate to levodopa [11] and would not explain ‘OFF’ periods resistant to apomorphine or rotigotine.
-
(6)
Alterations in dopamine receptor stimulation would provide a common link for the drugs administered by continuous delivery. All are ‘broad-spectrum’ dopamine agonists or levodopa-based and have effects on the major post-synaptic dopamine receptor subtypes—at least in vitro [154]. Changes in dopamine receptor density may occur in PD although the direction of change is disputed. CDD may ‘desensitise’ dopamine receptors, leading to reduced efficacy. Indeed, Quinn and colleagues reported that 24-h intravenous infusion of levodopa leads to a ‘tolerance’ after some days [155]. This has to be balanced against a lack of evidence for development of a similar tolerance after continuous 24-h delivery of rotigotine. However, alterations in receptor sensitivity occur relatively slowly rather than being transient and rapidly reversible, and as such would not explain the resistant short-duration ‘OFF’ periods. An exception to this could be the more rapid changes that are thought to occur in D-1 dopamine receptors linked to alterations in receptor trafficking [156].
-
(7)
In LCIG-treated patients, worsening of parkinsonian symptoms and ‘OFF’ periods occur mainly in the afternoon/evening despite no change in levodopa plasma level [107]. A similar change in the duration of response also occurs in response to apomorphine bolus injections [157], although it is unknown whether this is also seen with subcutaneous infusions of apomorphine. A role for physiological diurnal patterns might explain this phenomenon since motor function in patients with PD fluctuates throughout the day, often being worse in the afternoon [158,159,160,161] even in de novo PD [162], suggesting that this motor fluctuation is independent of dopaminergic medication. But this does not reflect the clinical reality of continuing unpredictable ‘OFF’ periods seen when using CDD.
Possible explanations behind the persistence of ‘OFF’ periods during CDD therapies
All of the above increasingly seems to lead to the conclusion that the causes of persistent ‘OFF’ periods are not the drugs or the delivery technology, but rather the disease itself. It appears that despite the best efforts being made to deliver drugs in compliance with the concepts of CDD and CDS [163], dopaminergic therapy is not able to control the persistent ‘OFF’ periods that remain. An obvious explanation for this, is that the persistent ‘OFF’ periods are non-dopaminergic in origin. At first glance, this would seem to challenge the accepted view of ‘wearing OFF’ as a common manifestation of motor fluctuations being due to the loss of presynaptic dopaminergic terminal storage, related post-synaptic dopamine receptor changes and responsiveness to improved dopaminergic drug delivery. However, another view would be that there are predictable ‘OFF’ periods, such as ‘wearing OFF’ which are dopaminergic in nature and respond to continuous delivery therapies, but that in addition, there are unpredictable ‘OFF’ periods that are non-dopaminergic in origin and do not respond to CDD. The non-dopaminergic basis for some ‘OFF’ periods would not be out of line with similar views on the underlying cause of motor complications in PD, notably dyskinesia being due to changes in basal ganglia circuitry beyond the dopaminergic inputs [164, 165].
If persistent ‘OFF’ periods are non-dopaminergic in origin, then a non-dopaminergic approach to treating them should be explored, in the same way that non-dopaminergic treatments have been evaluated for dyskinesia, with a role for glutamatergic, noradrenergic, and serotonergic pathways among others [165, 166]. For example, amantadine, zonisamide, istradefylline and safinamide, which have a mixture of pharmacological actions that are non-dopaminergic in nature, all reduce ‘OFF’ time when used as an adjunct to dopaminergic therapies [68,69,70, 167,168,169,170,171,172,173,174,175]. This illustrates that the occurrence of motor fluctuations and ‘OFF’ periods is largely beyond than can be explained simply by inadequate stimulation of dopaminergic function.
The conclusion of the evaluation of the use of CDD in early and late PD in relation to ‘OFF’ periods that appear resistant to further alterations in dopaminergic therapy, is that to explain their presence we need to look beyond dopamine and dopaminergic therapies. As a final caveat, it should be noted that the proposed explanation for persistent ‘OFF’ periods has at least one substantial flaw, that is related to the fact that they can be explained with a single mechanism across everyone affected. An alternative view might be that they have multiple causes, differentially applicable across the biological universe of PD. Future studies should also investigate whether introducing CDD at an early point in PD—before motor fluctuations and motor complications occur—would prevent their development and as a result, the drug-resistant ‘OFF’ periods would not become the problem that they currently pose.
A speculative view of future investigation of ‘OFF’ periods
While it is always good to challenge existing concepts of the complexity of PD, it is also necessary to provide directions for future investigation. This review has questioned the current view that ‘OFF’ periods are purely dopaminergic in nature, but does not rule out some manifestations of ‘OFF’ such as ‘wearing OFF’ being due to altered pharmacokinetic or pharmacodynamic responses to levodopa—although whether this would apply to dopamine agonists as well is unknown. But what it does raise is the question of the pathophysiology of ‘OFF’ periods in general, which is an area that needs more basic research. While much emphasis has been placed on understanding dyskinesia at the cellular and molecular level [171, 172], the same degree of investigation has not been given to ‘OFF’ periods even though they are at least an equally common clinical problem and an area of unmet pharmacological need. It is plausible to argue that abnormal signalling within the striato-thalamo-cortical loop contributes to ‘OFF’ periods in the same way as postulated for dyskinesia [171], but this has not been examined. A significant problem is that there are not sufficiently adequate experimental models of ‘OFF’ periods in the manner available for dyskinesia [173, 174]. This may tell us that using ‘simple’ dopaminergic denervation to model PD is not sufficient to induce those changes that lead to ‘OFF’ periods. But there has been so far, relatively little interest in exploring more widespread pathological changes in animal models as a way of understanding ‘OFF’ periods. It is quite possible that an imbalance between monoaminergic transmitters (dopamine, noradrenaline and serotonin) could be at the heart of neural network disruption leading to ‘OFF’ periods as all are known to be involved in the control of motor function and probably dyskinesia [164, 165, 175]. What is clear from the use of a range of non-dopaminergic drugs, including some that alter serotoninergic, noradrenergic, glutamatergic, cholinergic and adenosine transmission (as detailed earlier), is that while these can decrease ‘OFF’ time, no single pharmacological manipulation has yet been shown to eliminate ‘OFF’ periods. Perhaps we need to revert to the use of ‘dirty’ drugs that would influence multiple neurotransmitters affected by PD if ‘OFF’ periods are to be controlled.
A final but highly relevant question that requires investigation is the meaning of ‘OFF’ periods, whether a redefinition is in order. First, ‘OFF’ periods are a catch-all term that is routinely employed to cover unexplained immobility. We have highlighted the difference between ‘predictable’ and ‘unpredictable’ ‘OFF’ but there seems to be virtually no detailed clinical investigation of the characteristics and temporal components of ‘OFF’ periods in recent times. For example, we have highlighted the effects of CDD on ‘OFF’ periods, but despite the increasingly common use of advanced therapies, there seems no study investigating the changes of pattern, intensity, and temporal occurrence of ‘OFF’ periods in individual patients before versus during CDD. This investigation is relatively easy to undertake and would produce meaningful outcomes to advance our understanding of those ‘OFF’ periods unresponsive to dopaminergic medication; however, the requirement of a levodopa pharmacokinetic profile creates logistical and funding difficulties. Second and perhaps most provocatively, is what we regard as ‘OFF’: is it a pure manifestation of a failure of voluntary movement due to altered basal ganglia function or is it something else? Is it some other dysregulation of movement, a loss of cortical command, a manifestation of ‘freezing’? It could be that the ‘OFF’ periods relate to the occurrence of specific non-motor symptoms of PD—apathy, lethargy, depression, impaired cognition—and we may miss important clues to the cause or causes of one of the most troublesome deficits in current treatment of PD. Lastly, it is important to acknowledge that ‘OFF’ and ‘ON’ are arbitrarily dichotomized aspects of the experience of PD patients who rarely endorse switch-like changes between these theoretical states. Instead, they experience “shades” of ‘OFF’ and ‘ON’ along a continuum whose artificial borders were created as endpoints in clinical trials. Future integration of technologies to measure motor and non-motor fluctuations in the patients’ own environment may well replace the rigid construct of ‘OFF’ and ‘ON’ and prompt the revisitation of the issues noted here from a patient-centric perspective.
Conclusions
‘OFF’ periods during CDD remain one of the biggest challenges in the care and treatment of patients with PD. More studies are needed to better characterize and understand this phenomenon, whose pathogenesis seems complex and beyond the simple dopaminergic dysfunction hypothesis [176].
Availability of data and materials
Not applicable.
Abbreviations
- PD:
-
Parkinson’s disease
- CDD:
-
Continuous drug delivery
- CDS:
-
Continuous dopaminergic stimulation
- CSAI:
-
Continuous subcutaneous apomorphine infusion
- LCIG:
-
Levodopa-carbidopa intestinal gel
- SIBO:
-
Small intestinal bacteria overgrowth
References
Chaudhuri KRRA, Sethi KD. Motor and nonmotor complications in Parkinson’s disease: an argument for continuous drug delivery? J Neural Transm (Vienna). 2013;120:1305–20.
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–508.
Fahn S, Parkinson Study G. Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol. 2005;252:IV37-42.
Stocchi F, Jenner P, Obeso JA. When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol. 2010;63(5):257–66.
Vijiaratnam N, Foltynie T. Therapeutic strategies to treat or prevent off episodes in adults with Parkinson’s disease. Drugs. 2020;80(8):775–96.
Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord. 2008;23(10):1428–34.
Kerr C, Lloyd EJ, Kosmas CE, Smith HT, Cooper JA, Johnston K, et al. Health-related quality of life in Parkinson’s: impact of “off” time and stated treatment preferences. Qual Life Res. 2016;25(6):1505–15.
Haahr A, Kirkevold M, Hall EO, Ostergaard K. Living with advanced Parkinson’s disease: a constant struggle with unpredictability. J Adv Nurs. 2011;67(2):408–17.
Freitas ME, Hess CW, Fox SH. Motor complications of dopaminergic medications in Parkinson’s disease. Semin Neurol. 2017;37(2):147–57.
Metman LV, Konitsiotis S, Chase TN. Pathophysiology of motor response complications in Parkinson’s disease: hypotheses on the why, where, and what. Mov Disord. 2000;15(1):3–8.
de la Fuente-Fernandez R, Schulzer M, Mak E, Calne DB, Stoessl AJ. Presynaptic mechanisms of motor fluctuations in Parkinson’s disease: a probabilistic model. Brain. 2004;127(Pt 4):888–99.
Cenci MA. Presynaptic mechanisms of l-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front Neurol. 2014;5:242.
Holloway RG, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol. 2004;61(7):1044–53.
Verhagen Metman L, Locatelli ER, Bravi D, Mouradian MM, Chase TN. Apomorphine responses in Parkinson’s disease and the pathogenesis of motor complications. Neurology. 1997;48(2):369–72.
Engber TM, Susel Z, Kuo S, Gerfen CR, Chase TN. Levodopa replacement therapy alters enzyme activities in striatum and neuropeptide content in striatal output regions of 6-hydroxydopamine lesioned rats. Brain Res. 1991;552(1):113–8.
Calabresi P, Ghiglieri V, Mazzocchetti P, Corbelli I, Picconi B. Levodopa-induced plasticity: a double-edged sword in Parkinson’s disease? Phil Trans R Soc B Biol Sci. 2015;370(1672):20140184. https://doi.org/10.1098/rstb.2014.0184.
Bravi D, Mouradian MM, Roberts JW, Davis TL, Sohn YH, Chase TN. Wearing-off fluctuations in Parkinson’s disease: contribution of postsynaptic mechanisms. Ann Neurol. 1994;36(1):27–31.
Barbato L, Stocchi F, Monge A, Vacca L, Ruggieri S, Nordera G, et al. The long-duration action of levodopa may be due to a postsynaptic effect. Clin Neuropharmacol. 1997;20(5):394–401.
Stocchi F, Vacca L, Berardelli A, De Pandis F, Ruggieri S. Long-duration effect and the postsynaptic compartment: study using a dopamine agonist with a short half-life. Mov Disord. 2001;16(2):301–5.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–303.
Nutt JG, Woodward WR, Hammerstad JP, Carter JH, Anderson JL. The “on–off” phenomenon in parkinson’s disease: relation to levodopa absorption and transport. N Engl J Med. 1984;310(8):483–8.
Tanner CM. Exploring the clinical burden of OFF periods in Parkinson disease. Am J Manag Care. 2020;26(12 Suppl):S255–64.
Leta V, Jenner P, Chaudhuri KR, Antonini A. Can therapeutic strategies prevent and manage dyskinesia in Parkinson’s disease? An update Expert Opin Drug Saf. 2019;18(12):1203–18.
Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5(8):677–87.
van Wamelen DJ, Grigoriou S, Chaudhuri KR, Odin P. Continuous drug delivery aiming continuous dopaminergic stimulation in Parkinson’s disease. J Parkinsons Dis. 2018;8(s1):S65–72.
Senek M, Nielsen EI, Nyholm D. Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: a randomized crossover study. Mov Disord. 2017;32(2):283–6.
Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–73.
Poewe W, Stocchi F, Arkadir D, Ebersbach G, Ellenbogen AL, Giladi N, et al. Subcutaneous levodopa infusion for Parkinson’s disease: 1-year data from the open-label BeyoND study. Mov Disord. 2021;36(11):2687–92.
Ray Chaudhuri K, Qamar MA, Rajah T, Loehrer P, Sauerbier A, Odin P, et al. Non-oral dopaminergic therapies for Parkinson’s disease: current treatments and the future. NPJ Parkinsons Dis. 2016;2:16023.
Giladi N, Boroojerdi B, Korczyn AD, Burn DJ, Clarke CE, Schapira AH, et al. Rotigotine transdermal patch in early Parkinson’s disease: a randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord. 2007;22(16):2398–404.
Trenkwalder C, Kies B, Rudzinska M, Fine J, Nikl J, Honczarenko K, et al. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord. 2011;26(1):90–9.
Watts RL, Jankovic J, Waters C, Rajput A, Boroojerdi B, Rao J. Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology. 2007;68(4):272–6.
Nicholas AP, Borgohain R, Chana P, Surmann E, Thompson EL, Bauer L, et al. A randomized study of rotigotine dose response on “off” time in advanced Parkinson’s disease. J Parkinsons Dis. 2014;4(3):361–73.
Antonini A, Nitu B. Apomorphine and levodopa infusion for motor fluctuations and dyskinesia in advanced Parkinson disease. J Neural Transm (Vienna). 2018;125(8):1131–5.
Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtosek Z, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord. 2017;45:13–20.
Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749–59.
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–9.
Olanow CW, Poewe W, Rascol O, Stocchi F. On-demand therapy for OFF episodes in Parkinson’s disease. Mov Disord. 2021;36(10):2244–53.
Jankovic J, Watts RL, Martin W, Boroojerdi B. Transdermal rotigotine: double-blind, placebo-controlled trial in Parkinson disease. Arch Neurol. 2007;64(5):676–82.
Parkinson Study G. A controlled trial of rotigotine monotherapy in early Parkinson’s disease. Arch Neurol. 2003;60(12):1721–8.
LeWitt PA, Lyons KE, Pahwa R, Group SPS. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER study. Neurology. 2007;68(16):1262–7.
Mizuno Y, Nomoto M, Hasegawa K, Hattori N, Kondo T, Murata M, et al. Rotigotine vs ropinirole in advanced stage Parkinson’s disease: a double-blind study. Parkinsonism Relat Disord. 2014;20(12):1388–93.
Nomoto M, Mizuno Y, Kondo T, Hasegawa K, Murata M, Takeuchi M, et al. Transdermal rotigotine in advanced Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. J Neurol. 2014;261(10):1887–93.
Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, Martignoni E, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 2007;6(6):513–20.
Zhang ZX, Liu CF, Tao EX, Shao M, Liu YM, Wang J, et al. Rotigotine transdermal patch in Chinese patients with advanced Parkinson’s disease: a randomized, double-blind, placebo-controlled pivotal study. Parkinsonism Relat Disord. 2017;44:6–12.
Kehr J, Hu XJ, Goiny M, Scheller DK. Continuous delivery of rotigotine decreases extracellular dopamine suggesting continuous receptor stimulation. J Neural Transm. 2007;114(8):1027–31.
Elshoff JP, Cawello W, Andreas JO, Mathy FX, Braun M. An update on pharmacological, pharmacokinetic properties and drug-drug interactions of rotigotine transdermal system in Parkinson’s disease and restless legs syndrome. Drugs. 2015;75(5):487–501.
Rascol O, Zesiewicz T, Chaudhuri KR, Asgharnejad M, Surmann E, Dohin E, et al. A randomized controlled exploratory pilot study to evaluate the effect of rotigotine transdermal patch on Parkinson’s disease-associated chronic pain. J Clin Pharmacol. 2016;56(7):852–61.
Raeder V, Boura I, Leta V, Jenner P, Reichmann H, Trenkwalder C, et al. Rotigotine transdermal patch for motor and non-motor Parkinson’s disease: a review of 12 years’ clinical experience. CNS Drugs. 2021;35(2):215–31.
Rukavina K, Batzu L, Boogers A, Abundes-Corona A, Bruno V, Chaudhuri KR. Non-motor complications in late stage Parkinson’s disease: recognition, management and unmet needs. Expert Rev Neurother. 2021;21(3):335–52.
Ray Chaudhuri K, Poewe W, Brooks D. Motor and nonmotor complications of levodopa: phenomenology, risk factors, and imaging features. Mov Disord. 2018;33(6):909–19.
Leitt PA, Boroojerdi B, Surmann E, Poewe W, Group SPS. Rotigotine transdermal system for long-term treatment of patients with advanced Parkinson’s disease: results of two open-label extension studies, CLEOPATRA-PD and PREFER. J Neural Transm. 2013;120(7):1069–81.
FDA. Neupro® 2007 [Available from:https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021829lbl.pdf" https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021829lbl.pdf]
Baker WL, Silver D, White CM, Kluger J, Aberle J, Patel AA, et al. Dopamine agonists in the treatment of early Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2009;15(4):287–94.
Fahn S. Levodopa in the treatment of Parkinson’s disease. J Neural Transm Suppl. 2006;71:1–15.
Antonini A. Apomorphine and levodopa infusion therapies for advanced Parkinson’s disease. J Mov Disord. 2009;2(1):4–9.
Ruan X, Lin F, Wu D, Chen L, Weng H, Yu J, et al. Comparative efficacy and safety of dopamine agonists in advanced Parkinson’s disease with motor fluctuations: a systematic review and network meta-analysis of double-blind randomized controlled trials. Front Neurosci. 2021;15:728083.
Boyle A, Ondo W. Role of apomorphine in the treatment of Parkinson’s disease. CNS Drugs. 2015;29(2):83–9.
Antoniya Todorova K, Chaudhuri R. Subcutaneous, intranasal and transdermal dopamine agonists in the management of Parkinson’s disease. In: Galvez-Jimenez N, Fernandez HH, Espay AJ, Fox SH, editors. Parkinson’s Disease: Current and Future Therapeutics and Clinical Trials. Cambridge University Press; 2016. p. 48–62.
Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, et al. Long-term safety and efficacy of apomorphine infusion in Parkinson’s disease patients with persistent motor fluctuations: results of the open-label phase of the TOLEDO study. Parkinsonism Relat Disord. 2021;83:79–85.
Carbone F, Djamshidian A, Seppi K, Poewe W. Apomorphine for Parkinson’s disease: efficacy and safety of current and new formulations. CNS Drugs. 2019;33(9):905–18.
Martinez-Martin P, Reddy P, Katzenschlager R, Antonini A, Todorova A, Odin P, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord. 2015;30(4):510–6.
Dafsari HS, Martinez-Martin P, Rizos A, Trost M, Dos Santos Ghilardi MG, Reddy P, et al. EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34(3):353–65.
LeWitt PA. At last, a randomised controlled trial of apomorphine infusion. Lancet Neurol. 2018;17(9):732–3.
Busk KJA, Nyholm D. Long-term efficacy and safety with continuous dopaminergic stimulation pump treatments in Parkinson’s disease. Eur Neurol Rev. 2011;6:156–60.
Nicolle E, Pollak P, Serre-Debeauvais F, Richard P, Gervason CL, Broussolle E, et al. Pharmacokinetics of apomorphine in parkinsonian patients. Fundam Clin Pharmacol. 1993;7(5):245–52.
Acland KM, Leslie T, Dowd PM. Panniculitis associated with subcutaneous apomorphine. Hosp Med. 1998;59(5):413–4.
Stibe CM, Lees AJ, Kempster PA, Stern GM. Subcutaneous apomorphine in parkinsonian on–off oscillations. Lancet. 1988;331:403–6.
Chaudhuri KR, Critchley P, Abbott RJ, Pye IF, Millac PAH. Subcutaneous apomorphine for on–off oscillations in Parkinson’s disease. Lancet. 1988;332:1260.
Frankel JP, Lees AJ, Kempster PA, Stern GM. Subcutaneous apomorphine in the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1990;53:96–101.
Pollak P, Champay AS, Gaio JM, Hommel M, Benabid AL, Perret J. Subcutaneous administration of apomorphine in motor fluctuations in Parkinson’s disease. Rev Neurol (Paris). 1990;146:116–22 (in French).
Hughes AJ, Bishop S, Kleedorfer B, Turjanski N, Fernandez W, Lees AJ, et al. Subcutaneous apomorphine in Parkinson’s disease: response to chronic administration for up to five years. Mov Disord. 1993;8:165–70.
Stocchi F, Bramante L, Monge A, Viselli F, Baronti F, Stefano E, et al. Apomorphine and lisuride infusion. A comparative long-term study. Adv Neurol. 1993;60:653–5.
Poewe W, Kleedorfer B, Wagner M, Bosch S, Schelosky L. Continuous subcutaneous apomorphine infusions for fluctuating Parkinson’s disease. Long-term follow-up in 18 patients. Adv Neurol. 1993;60:656–9.
Kreczy-Kleedorfer B, Wagner M, Bösch S. Poewe W Long-term results of continuous subcutaneous apomorphine pump therapy in patients with advanced Parkinson disease. Nervenarzt. 1993;64:221–5 (in German).
Gancher ST, Nutt JG, Woodward WR. Apomorphine infusional therapy in Parkinson’s disease: clinical utility and lack of tolerance. Mov Disord. 1995;10:37–43.
Colzi A, Turner K, Lees AJ. Continuous subcutaneous waking day apomorphine in the long term treatment of levodopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:573–6.
Pietz K, Hagell P, Odin P. Subcutaneous apomorphine in late stage Parkinson’s disease: a long-term follow-up. J Neurol Neurosurg Psychiatry. 1998;65:709–16.
Wenning GK, Bösch S, Luginger E, Wagner M, Poewe W. Effects of long-term, continuous subcutaneous apomorphine infusions on motor complications in advanced Parkinson’s disease. Adv Neurol. 1999;80:545–8.
Kanovsky P, Kubova D, Bares M, ortová H, Streitová H, Rektor I, et al. Levodopa-induced dyskinesias and continuous subcutaneous infusions of apomorphine: results of a two-year, prospective follow-up. Mov Disord. 2002;17:188–91.
Manson AJ, Turner K, Lees AJ. Apomorphine monotherapy in the treatment of refractory motor complications of Parkinson’s disease: long-term follow-up study of 64 patients. Mov Disord. 2002;17:1235–41.
Di Rosa AE, Epifanio A, Antonini A, Stocchi F, Martino G, Di Blasi L, et al. Continuous apomorphine infusion and neuropsychiatric disorders: a controlled study in patients with advanced Parkinson’s disease. Neurol Sci. 2003;24:174–5.
Morgante L, Basile G, Epifanio A, Spina E, Antonini A, Stocchi F, et al. Continuous apomorphine infusion (CAI) and neuropsychiatric disorders in patients with advanced Parkinson’s disease: a follow-up of two years. Arch Gerontol Geriatr Suppl. 2004;9:291–6.
Katzenschlager R, Hughes A, Evans A, Manson AJ, Hoffman M, Swinn L, et al. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: a prospective study using single-dose challenges. Mov Disord. 2005;20:151–7.
De Gaspari D, Siri C, Landi A, Cilia R, Bonetti A, Natuzzi F, et al. Clinical and neuropsychological follow up at 12 months in patients with complicated Parkinson’s disease treated with subcutaneous apomorphine infusion or deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 2006;77:450–3.
Garcia Ruiz PJ, Sesar Ignacio A, Ares Pensado B, Castro García A, Alonso Frech F, Alvarez López M, et al. Efficacy of long-term continuous subcutaneous apomorphine infusion in advanced Parkinson’s disease with motor fluctuations: a multicenter study. Mov Disord. 2008;23:1130–6.
Martinez-Martin P, Reddy P, Antonini A, Henriksen T, Katzenschlager R, Odin P, et al. Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson’s disease compared to conventional therapy: a real-life study of non-motor effect. J Parkinson’s Dis. 2011;1:197–203.
Antonini A, Isaias IU, Rodolfi G, Landi A, Natuzzi F, Siri C, et al. A 5-year prospective assessment of advanced Parkinson disease patients treated with subcutaneous apomorphine infusion or deep brain stimulation. J Neurol. 2011;258:579–85.
Drapier S, Gillioz AS, Leray E, Péron J, Rouaud T, Marchand A, et al. Apomorphine infusion in advanced Parkinson’s patients with subthalamic stimulation contraindications. Parkinsonism Relat Disord. 2012;18:40–4.
Borgemeester RWK, van Laar T. Continuous subcutaneous apomorphine infusion in Parkinson’s disease patients with cognitive dysfunction: A retrospective long-term follow-up study. Parkinsonism Relat Disord. 2017;45:33–8.
Sesar A, Fernández-Pajarín G, Ares B, Rivas MT, Castro A. Con- tinuous subcutaneous apomorphine infusion inadvanced Parkin- son’s disease: 10-year experience with 230 patients. J Neurol. 2017;264(5):946–54.
Sesar Á, Fernández-Pajarín G, Ares B, Relova JL, Arán E, Rivas MT, Gelabert-González M, Castro A. Continuous subcutaneous apomorphine in advanced Parkinson’s disease patients treated with deep brain stimulation. J Neurol. 2019;266(3):659–66.
Papuć E, Trzciniecka O, Rejdak K. Continuous subcutaneous apomorphine monotherapy in Parkinson’s disease. Ann Agric Environ Med. 2019;26(1):133–7.
Isaacson S, Espay A, Pahwa R, Clinch T, LeWitt P. Safety and efficacy of continuous apomorphine infusion in patients with Parkinson’s disease: results from a phase 3, open-label study 1771. Neurology. 2020;94:15.
Nyholm D, Odin P, Johansson A, Chatamra K, Locke C, Dutta S, et al. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J. 2013;15(2):316–23.
Othman AA, Rosebraugh M, Chatamra K, Locke C, Dutta S. Levodopa-carbidopa intestinal gel pharmacokinetics: lower variability than oral levodopa-carbidopa. J Parkinsons Dis. 2017;7(2):275–8.
Wang L, Li J, Chen J. Levodopa-carbidopa intestinal gel in Parkinson’s disease: a systematic review and meta-analysis. Front Neurol. 2018;9:620.
Antonini A, Odin P, Pahwa R, Aldred J, Alobaidi A, Jalundhwala YJ, et al. The long-term impact of levodopa/carbidopa intestinal gel on ’Off’-time in patients with advanced Parkinson’s disease: a systematic review. Adv Ther. 2021;38(6):2854–90.
Odin P, Ray Chaudhuri K, Slevin JT, Volkmann J, Dietrichs E, Martinez-Martin P, et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord. 2015;21(10):1133–44.
Yamashita K, Yube Y, Yamazaki Y, Fukuchi T, Kato M, Koike T, et al. The impact of tube replacement timing during LCIG therapy on PEG-J associated adverse events: a retrospective multicenter observational study. BMC Neurol. 2021;21(1):242.
Metta V, Leta V, Mrudula KR, Prashanth LK, Goyal V, Borgohain R, et al. Gastrointestinal dysfunction in Parkinson’s disease: molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J Neurol. 2022;269(3):1154–63.
Pfeiffer RF, Isaacson SH, Pahwa R. Clinical implications of gastric complications on levodopa treatment in Parkinson’s disease. Parkinsonism Relat Disord. 2020;76:63–71.
van Kessel SP, El Aidy S. Contributions of gut bacteria and diet to drug pharmacokinetics in the treatment of Parkinson’s disease. Front Neurol. 2019;10:1087.
Stocchi F, Torti M. Constipation in Parkinson’s disease. Int Rev Neurobiol. 2017;134:811–26.
Frankel JP, Kempster PA, Bovingdon M, Webster R, Lees AJ, Stern GM. The effects of oral protein on the absorption of intraduodenal levodopa and motor performance. J Neurol Neurosurg Psychiatry. 1989;52(9):1063–7.
Miyaue N, Hosokawa Y, Yoshida A, Yamanishi Y, Tada S, Ando R, et al. Fasting state is one of the factors associated with plasma levodopa fluctuations during levodopacarbidopa intestinal gel treatment. Parkinsonism Relat Disord. 2021;91:55–8.
Thomas I, Memedi M, Westin J, Nyholm D. The effect of continuous levodopa treatment during the afternoon hours. Acta Neurol Scand. 2019;139(1):70–5.
Antonini A, Bondiolotti G, Natuzzi F, Bareggi SR. Levodopa and 3-OMD levels in Parkinson patients treated with Duodopa. Eur Neuropsychopharmacol. 2010;20(10):683–7.
Olanow CW, Espay AJ, Stocchi F, Ellenbogen AL, Leinonen M, Adar L, et al. Continuous subcutaneous levodopa delivery for Parkinson’s disease: a randomized study. J Parkinsons Dis. 2021;11(1):177–86.
Giladi N, Gurevich T, Djaldetti R, Adar L, Case R, Leibman-Barak S, Sasson N, Caraco Y. ND0612 (levodopa/carbidopa for subcutaneous infusion) in patients with Parkinson’s disease and motor response fluctuations: a randomized, placebo-controlled phase 2 study. Parkinsonism Relat Disord. 2021;91:139–45.
Nilsson D, Nyholm D, Aquilonius SM. Duodenal levodopa infusion in Parkinson’s disease–long-term experience. Acta Neurol Scand. 2001;104(6):343–8.
Eggert K, Schrader C, Hahn M, Stamelou M, Rüssmann A, Dengler R, et al. Continuous jejunal levodopa infusion in patients with advanced Parkinson disease: practical aspects and outcome of motor and non-motor complications. Clin Neuropharmacol. 2008;31(3):151–66.
Antonini A, Mancini F, Canesi M, Zangaglia R, Isaias IU, Manfredi L, et al. Duodenal levodopa infusion improves quality of life in advanced Parkinson’s disease. Neurodegener Dis. 2008;5(3–4):244–6.
Santos-García D, Macías M, Llaneza M, Fuster-Sanjurjo L, Echarri-Piudo A, Belmonte S, et al. Experiencia con la infusión continua de levodopa intraduodenal (Duodopa(®)) en pacientes con enfermedad de Parkinson avanzada en un hospital de segundo nivel asistencial [Experience with continuous levodopa enteral infusion (Duodopa(®)) in patients with advanced Parkinson’s disease in a secondary level hospital]. Neurologia. 2010;25(9):536–43.
Merola A, Zibetti M, Angrisano S, Rizzi L, Lanotte M, Lopiano L. Comparison of subthalamic nucleus deep brain stimulation and Duodopa in the treatment of advanced Parkinson’s disease. Mov Disord. 2011;26(4):664–70.
Fasano A, Ricciardi L, Lena F, Bentivoglio AR, Modugno N. Intrajejunal levodopa infusion in advanced Parkinson’s disease: long-term effects on motor and non-motor symptoms and impact on patient’s and caregiver’s quality of life. Eur Rev Med Pharmacol Sci. 2012;16(1):79–89.
Fernandez HH, Vanagunas A, Odin P, Espay AJ, Hauser RA, Standaert DG, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease open-label study: interim results. Parkinsonism Relat Disord. 2013;19(3):339–45.
Foltynie T, Magee C, James C, Webster GJ, Lees AJ, Limousin P. Impact of duodopa on quality of life in advanced Parkinson’s disease: a UK case series. Parkinsons Dis. 2013;2013:362908.
Zibetti M, Merola A, Ricchi V, Marchisio A, Artusi CA, Rizzi L, et al. Long-term duodenal levodopa infusion in Parkinson’s disease: a 3-year motor and cognitive follow-up study. J Neurol. 2013;260(1):105–14.
Antonini A, Odin P, Opiano L, Tomantschger V, Pacchetti C, Pickut B, et al. Effect and safety of duodenal levodopa infusion in advanced Parkinson’s disease: a retrospective multicenter outcome assessment in patient routine care. J Neural Transm (Vienna). 2013;120(11):1553–8.
Zibetti M, Merola A, Artusi CA, Rizzi L, Angrisano S, Reggio D, et al. Levodopa/carbidopa intestinal gel infusion in advanced Parkinson’s disease: a 7-year experience. Eur J Neurol. 2014;21(2):312–8.
Cáceres-Redondo MT, Carrillo F, Lama MJ, Huertas-Fernández I, Vargas-González L, Carballo M, et al. Long-term levodopa/carbidopa intestinal gel in advanced Parkinson’s disease. J Neurol. 2014;261(3):561–9.
Sensi M, Preda F, Trevisani L, Contini E, Gragnaniello D, Capone JG, et al. Emerging issues on selection criteria of levodopa carbidopa infusion therapy: considerations on outcome of 28 consecutive patients. J Neural Transm (Vienna). 2014;121:633–42.
Lundqvist C, Beiske AG, Reiertsen O, Kristiansen IS. Real life cost and quality of life associated with continuous intraduodenal levodopa infusion compared with oral treatment in Parkinson patients. J Neurol. 2014;261(12):2438–45.
Fernandez HH, Standaert DG, Hauser RA, Lang AE, Fung VS, Klostermann F, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord. 2015;30(4):500–9.
Calandrella D, Romito LM, Elia AE, Del Sorbo F, Bagella CF, Falsitta M, et al. Causes of withdrawal of duodenal levodopa infusion in advanced Parkinson disease. Neurology. 2015;84(16):1669–72.
Buongiorno M, Antonelli F, Cámara A, Puente V, de Fabregues-Nebot O, Hernandez-Vara J, et al. Long-term response to continuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: the Barcelona registry. Parkinsonism Relat Disord. 2015;21(8):871–6.
Slevin JT, Fernandez HH, Zadikoff C, Hall C, Eaton S, Dubow J, et al. Long-term safety and maintenance of efficacy of levodopa-carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis. 2015;5(1):165–74.
Lopiano L, Modugno N, Marano P, Sensi M, Meco G, Cannas A, et al. Motor outcomes in patients with advanced Parkinson’s disease treated with levodopa/carbidopa intestinal gel in Italy: an interim analysis from the GREENFIELD observational study. Neurol Sci. 2016;37(11):1785–92.
Valldeoriola F, Grandas F, Santos-García D, Regidor I, Catalán MJ, Arbelo JM, et al. Long-term effectiveness of levodopa-carbidopa intestinal gel in 177 Spanish patients with advanced Parkinson’s disease. Neurodegener Dis Manag. 2016;6(4):289–98.
Merola A, Espay AJ, Romagnolo A, Bernardini A, Rizzi L, Rosso M, et al. Advanced therapies in Parkinson’s disease: long-term retrospective study. Parkinsonism Relat Disord. 2016;29:104–8.
Chang FC, Kwan V, van der Poorten D, Mahant N, Wolfe N, Ha AD, et al. Intraduodenal levodopa-carbidopa intestinal gel infusion improves both motor performance and quality of life in advanced Parkinson’s disease. J Clin Neurosci. 2016;25:41–5.
De Fabregues O, Dot J, Abu-Suboh M, Hernández-Vara J, Ferré A, Romero O, et al. Long-term safety and effectiveness of levodopa-carbidopa intestinal gel infusion. Brain and behavior. 2017;7(8):e00758.
Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtošek Z, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Parkinsonism Relat Disord. 2017;45:13–20.
Standaert DG, Rodriguez RL, Slevin JT, Lobatz M, Eaton S, Chatamra K, et al. Effect of levodopa-carbidopa intestinal gel on non-motor symptoms in patients with advanced Parkinson’s disease. Mov Disord Clin Pract. 2017;4(6):829–37.
Juhász A, Aschermann Z, Ács P, Janszky J, Kovács M, Makkos A, et al. Levodopa/carbidopa intestinal gel can improve both motor and non-motor experiences of daily living in Parkinson’s disease: an open-label study. Parkinsonism Relat Disord. 2017;37:79–86.
Zibetti M, Angrisano S, Dematteis F, Artusi CA, Romagnolo A, Merola A, et al. Effects of intestinal levodopa infusion on freezing of gait in Parkinson disease. J Neurol Sci. 2018;385:105.
Fernandez HH, Boyd JT, Fung VSC, Lew MF, Rodriguez RL, Slevin JT, et al. Long-term safety and efficacy of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease. Mov Disord. 2018;33(6):928–36.
Lopiano L, Modugno N, Marano P, Sensi M, Meco G, Solla P, et al. Motor and non-motor outcomes in patients with advanced Parkinson’s disease treated with levodopa/carbidopa intestinal gel: final results of the GREENFIELD observational study. J Neurol. 2019;266:2164–76.
Fabbri M, Zibetti M, Beccaria L, Merola A, Romagnolo A, Montanaro E, et al. Levodopa/carbidopa intestinal gel infusion and weight loss in Parkinson’s disease. Eur J Neurol. 2019;26(3):490–6.
Popa LC, Leucuta DC, Tohanean N, Popa SL, Perju-Dumbrava L. Intrajejunal vs oral levodopa-carbidopa therapy in Parkinson disease: a retrospective cohort study. Medicine. 2020;99(46):e23249.
Standaert DG, Aldred J, Anca-Herschkovitsch M, Bourgeois P, Cubo E, Davis TL, et al. DUOGLOBE: one-year outcomes in a real-world study of levodopa carbidopa intestinal gel for Parkinson’s disease. Mov Disord Clin Pract. 2021;8(7):1061–74.
Reimer J, Grabowski M, Lindvall O, Hagell P. Use and interpretation of on/off diaries in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(3):396–400.
Sundgren M, Andréasson M, Svenningsson P, Noori R-M, Johansson A. Does information from the Parkinson KinetiGraph (PKG) influence the neurologist’s treatment decisions?—An observational study in routine clinical care of people with Parkinson’s disease. J Pers Medicine. 2021;11(6):519. https://doi.org/10.3390/jpm11060519.
Heldman DA, Espay AJ, LeWitt PA, Giuffrida JP. Clinician versus machine: reliability and responsiveness of motor endpoints in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(6):590–5.
Isaacson SH, Boroojerdi B, Waln O, McGraw M, Kreitzman DL, Klos K, et al. Effect of using a wearable device on clinical decision-making and motor symptoms in patients with Parkinson’s disease starting transdermal rotigotine patch: a pilot study. Parkinsonism Relat Disord. 2019;64:132–7.
Fahn S. “On-off” phenomenon with levodopa therapy in parkinsonism: clinical and pharmacologic correlations and the effect of intramuscular pyridoxine. Neurology. 1974;24(5):431–431. https://doi.org/10.1212/WNL.24.5.431.
Kempster PA, Frankel JP, Bovingdon M, Webster R, Lees AJ, Stern GM. Levodopa peripheral pharmacokinetics and duration of motor response in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1989;52(6):718–23.
Nutt JG. On-off phenomenon: relation to levodopa pharmacokinetics and pharmacodynamics. Ann Neurol. 1987;22(4):535–40.
Jenner P, Katzenschlager R. Apomorphine - pharmacological properties and clinical trials in Parkinson’s disease. Parkinsonism Relat Disord. 2016;33(Suppl 1):S13–21.
Frampton JE. Rotigotine transdermal patch: a review in Parkinson’s disease. CNS Drugs. 2019;33(7):707–18.
Lopez A, Munoz A, Guerra MJ, Labandeira-Garcia JL. Mechanisms of the effects of exogenous levodopa on the dopamine-denervated striatum. Neuroscience. 2001;103(3):639–51.
Stansley BJ, Yamamoto BK. L-dopa and brain serotonin system dysfunction. Toxics. 2015;3(1):75–88.
Walters JR, Ruskin DN, Allers KA, Bergstrom DA. Pre- and postsynaptic aspects of dopamine-mediated transmission. Trends Neurosci. 2000;23(10 Suppl):S41–7.
Quinn N, Parkes JD, Marsden CD. Control of on/off phenomenon by continuous intravenous infusion of levodopa. Neurology. 1984;34(9):1131–6.
Hurley MJ, Jenner P. What has been learnt from study of dopamine receptors in Parkinson’s disease? Pharmacol Ther. 2006;111(3):715–28.
Monge A, Viselli F, Stocchi F, Barbato L, Bolner A, Modugno N, et al. Variation in the dopaminergic response during the day in Parkinson disease. Clin Neuropharmacol. 2004;27(3):116–8.
Nutt JG, Carter JH, Van Houten L, Woodward WR. Short- and long-duration responses to levodopa during the first year of levodopa therapy. Ann Neurol. 1997;42(3):349–55.
Stewart J, Bachman G, Cooper C, Liu L, Ancoli-Israel S, Alibiglou L. Circadian dysfunction and fluctuations in gait initiation impairment in Parkinson’s disease. Exp Brain Res. 2018;236(3):655–64.
van Hilten JJ, Hoogland G, van der Velde EA, Middelkoop HA, Kerkhof GA, Roos RA. Diurnal effects of motor activity and fatigue in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1993;56(8):874–7.
van Hilten JJ, Kabel JF, Middelkoop HA, Kramer CG, Kerkhof GA, Roos RA. Assessment of response fluctuations in Parkinson’s disease by ambulatory wrist activity monitoring. Acta Neurol Scand. 1993;87(3):171–7.
van Wamelen DJ, Urso D, Ray CK. How time rules: diurnal motor patterns in de novo Parkinson’s disease. J Parkinsons Dis. 2021;11(2):695–702.
Urso D, Chaudhuri KR, Qamar MA, Jenner P. Improving the delivery of levodopa in Parkinson’s disease: a review of approved and emerging therapies. CNS Drugs. 2020;34(11):1149–63.
Brotchie JM. Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov Disord. 2005;20(8):919–31.
Jenner P. Pathophysiology and biochemistry of dyskinesia: clues for the development of non-dopaminergic treatments. J Neurol. 2000;247(Suppl 2):43–50.
Stayte S, Vissel B. Advances in non-dopaminergic treatments for Parkinson’s disease. Front Neurosci. 2014;8:113.
Hauser RA, Lytle J, Formella AE, Tanner CM. Amantadine delayed release/extended release capsules significantly reduce OFF time in Parkinson’s disease. NPJ Parkinsons Dis. 2022;8(1):29.
Li C, Xue L, Liu Y, Yang Z, Chi S, Xie A. Zonisamide for the treatment of Parkinson disease: a current update. Front Neurosci. 2020;14:574652.
Cummins L, Cates ME. Istradefylline: a novel agent in the treatment of “off” episodes associated with levodopa/carbidopa use in Parkinson disease. Ment Health Clin. 2022;12(1):32–6.
Stocchi F, Antonini A, Berg D, Bergmans B, Jost W, Katzenschlager R, et al. Safinamide in the treatment pathway of Parkinson’s disease: a European delphi consensus. NPJ Parkinsons Dis. 2022;8(1):17.
Bandopadhyay R, Mishra N, Rana R, Kaur G, Ghoneim MM, Alshehri S, et al. Molecular mechanisms and therapeutic strategies for levodopa-induced dyskinesia in Parkinson’s disease: a perspective through preclinical and clinical evidence. Front Pharmacol. 2022;13:805388.
Porras G, De Deurwaerdere P, Li Q, Marti M, Morgenstern R, Sohr R, et al. L-dopa-induced dyskinesia: beyond an excessive dopamine tone in the striatum. Sci Rep. 2014;4:3730.
Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2(8):577–88.
Morin N, Jourdain VA, Di Paolo T. Modeling dyskinesia in animal models of Parkinson disease. Exp Neurol. 2014;256:105–16.
Muller T. Current and investigational non-dopaminergic agents for management of motor symptoms (including motor complications) in Parkinson’s disease. Expert Opin Pharmacother. 2017;18(14):1457–65.
Udd M, Lyytinen J, Eerola-Rautio J, Kenttamies A, Lindstrom O, Kylanpaa L, et al. Problems related to levodopa-carbidopa intestinal gel treatment in advanced Parkinson’s disease. Brain Behav. 2017;7(7):e00737.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
PJ and KRC conceived the idea behind the review. SR (major contributor) and DU performed the review of the literature, drafted the manuscript, and critically revised it. DvW, VL, and IB participated in the manuscript’s drafting and the table preparation. PJ, KRC, PO, and AJE critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
SR has received speaker fees from Zambon. DU and IB do not have competing interests to disclose. DvW receives speaker fees from Bial and Britannia pharmaceuticals, consultancy fees from Britannia and Invisio, research grants from CHDI, NIHR BRC, and Britannia. VL has received grants from NIHR BRC, Parkinson’s UK, travel and congress grants from Bial UK Ltd, speaker-related activities fees from Britannia pharmaceuticals, Bial UK, Profile, and consultancy fees from Invisio Pharmaceuticals and Bial UK, outside the submitted work. PO has received honoraria for advisory boards from: AbbVie, Bial, Britannia, Kyowa, Zambon; honoraria for lectures from: Bial, AbbVie, Bial, Britannia, Kyowa, Nordic Infucare, Stada, Zambon; Grants (Investigator initiated): Abbvie; Academic grants: Lund Medical Faculty, Multipark, Parkinsonfonden, Southern Swedish Health Care Region, Swedish Parkinson Academy, Åhlens. AJE has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Neuroderm, Neurocrine, Amneal, Acadia, Acorda, Bexion, Kyowa Kirin, Sunovion, Supernus (formerly, USWorldMeds), Avion Pharmaceuticals, and Herantis Pharma; personal compensation as honoraria for speakership for Avion; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer. He cofounded REGAIN Therapeutics (a biotech start-up developing nonaggregating peptide analogues as replacement therapies for neurodegenerative diseases) and is co-owner of a patent that covers synthetic soluble nonaggregating peptide analogues as replacement treatments in proteinopathies. PJ receives honoraria for consultancies and advisory boards from Abbvie, BIAL, Britannia Pharmaceuticals, Celera, Eisai, FP Pharmaceuticals, Kyowa Kirin, Hoffman La Roche, Profile Pharma, UCB, Worldwide Clinical Trials, Zambon. KRC receives personal compensation as advisory board member from AbbVie, UCB, GKC, Bial, Cynapsus, Lobsor, Stada, Zambon, Profile Pharma, Synovion, Roche, Therevance, Scion, Britannia, Acadia, 4D Pharma (recent); Medtronic (2 years ago). Recent honoraria for lectures: AbbVie, Britannia, UCB, Zambon, Novartis, Boeringer Ingelheim, Bial, Kyowa Kirin, SK Pharma. Grants (Investigator Initiated): Bial (recent); Britannia Pharmaceuticals, AbbVie, UCB, GKC (over 2 years ago). Aacdemic grants: EU Horizon 2020, Parkinson's UK, NIHR, Parkinson’s Foundation, Wellcome Trust (recent); Kirby Laing Foundation, MRC (over 2 years ago). Royalties or licenses (ongoing): Oxford (book), Cambridge publishers (book), MAPI institute (KPPS, PDSS 2). Payment for expert testimony: GMC.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rota, S., Urso, D., van Wamelen, D.J. et al. Why do ‘OFF’ periods still occur during continuous drug delivery in Parkinson’s disease?. Transl Neurodegener 11, 43 (2022). https://doi.org/10.1186/s40035-022-00317-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40035-022-00317-x