Abstract
Background
Sedative premedication with benzodiazepines has been linked with prolonged recovery and inadequate emergence during the immediate postoperative period. We aimed to analyze the association between postanesthesia care unit (PACU) delirium and sedative premedication with oral midazolam.
Methods
We performed a secondary analysis of prospectively collected data before (midazolam cohort) and after (non-midazolam cohort) implementation of a restrictive strategy for oral premedication with midazolam. From March 2015 until July 2018, we included patients 60 years and older, who underwent elective radical prostatectomy for prostate cancer. Exclusion criteria were contraindications to premedication with midazolam, preoperative anxiety, and a history of neurological disorders. Patients, who were scheduled for postoperative admission to the intensive care unit, were excluded. Between 2015 and 2016, patients received 7.5 mg oral midazolam preoperatively (midazolam cohort). Patients included between 2017 and 2018 did not receive any sedative medication preoperatively (non-midazolam cohort). The primary endpoint was the incidence of PACU delirium.
Results
PACU delirium rates were 49% in the midazolam cohort (n = 214) and 33% in the non-midazolam cohort (n = 218). This difference was not statistically significant on multivariable logistic regression analysis (OR 0.847 [95% CI 0.164; 4.367]; P = 0.842). Age (OR 1.102 [95% CI 1.050; 1.156]; P < 0.001), the cumulative dose of sufentanil (OR 1.014 [95% CI 1.005; 1.024]; P = 0.005), and propofol-sufentanil for anesthesia maintenance (OR 2.805 [95% CI 1.497; 5.256]; P = 0.001) were significantly associated with PACU delirium.
Conclusion
Midazolam for sedative premedication was not significantly associated with PACU delirium. The reduction in the incidence of PACU delirium throughout the study period may be attributable to improvements in perioperative management other than a more restrictive preoperative benzodiazepine administration.
Similar content being viewed by others
Background
Postoperative delirium (POD) is a common complication after surgery and anesthesia that particularly affects patients older than 60 years (Inouye et al. 2014; Vlisides and Avidan 2019). It is defined as an acute cerebral dysfunction characterized by disturbance of consciousness, change in cognition or perception with fluctuating symptoms (American Psychiatric Association 2013). Postoperative delirium has been linked with subsequent cognitive impairment, increased institutionalization at hospital discharge, increased morbidity, mortality, and higher health care cost (Gottesman et al. 2010; Leslie et al. 2008; Sprung et al. 2017; Witlox et al. 2010).
Postanesthesia care unit (PACU) delirium is one subtype of POD that occurs early after surgery in the PACU (Card et al. 2015; Hernandez et al. 2017). Evidence from longitudinal studies suggests that PACU delirium is associated with the subsequent development of POD during hospital stay (Neufeld et al. 2013a; Sharma et al. 2005; Stukenberg et al. 2016; Zhang et al. 2020).
To reduce preoperative anxiety and sympathetic activation, sedative premedication with benzodiazepines has been routinely used in periprocedural management (Bucx et al. 2016; Kain et al. 1997). However, accumulating evidence suggests that premedication with benzodiazepines may be a potential trigger for delayed time to extubation, prolonged postoperative recovery, and agitated emergence without improving self-reported patient experience after surgery (Lepousé et al. 2006; Maurice-Szamburski et al. 2015; Radtke et al. 2010). Owing to their neurocognitive adverse effects the American Geriatrics Society included benzodiazepines in the Beers list for potentially inappropriate medication use in older adults (American Geriatrics Society 2012). Importantly, the European Society of Anaesthesiology guidelines on POD published in 2017 recommend a restrictive use of sedative premedication with benzodiazepines (Aldecoa et al. 2017).
In accordance with the novel recommendations, the liberal use of premedication with benzodiazepines has been restricted at our institution in 2017. The aim of this before-after secondary analysis was to compare the incidence of PACU delirium between a liberal and a restrictive benzodiazepine strategy in patients scheduled for radical prostatectomy. We hypothesized that premedication with midazolam would be associated with a higher incidence of PACU delirium.
Methods
Study design
We performed a secondary analysis following an uncontrolled before-after design to compare the incidence of PACU delirium between patients who received midazolam preoperatively (study 1: midazolam cohort) and patients who did not receive any sedative medication preoperatively (study 2: non-midazolam cohort). The midazolam cohort represents a convenience sample of 347 patients who had been enrolled in a prospective observational study (study 1) between January 2015 and March 2016 to compare the incidence of PACU delirium between open retropubic and robot-assisted radical prostatectomy (Beck et al. 2020b). Between November 2017 and October 2018, 222 patients had been enrolled in a prospective observational study (study 2) to assess the association between PACU delirium and patient-reported outcomes representing the non-midazolam cohort (Kainz et al. 2022).
Setting and participants
This study was performed at a high-volume prostate cancer center in Hamburg, Germany. Throughout the study period, approximately 2,500 radical prostatectomies were performed annually. The flow of participants is presented in Fig. 1. For the initial prospective cohort studies, patients were included, if they were scheduled for elective radical prostatectomy either by open retropubic or robot-assisted technique for treatment of prostate cancer and if they were fluent in German in order to undergo psychometric assessments (Beck et al. 2020a; Kainz et al. 2022). Study 1 included adult patients of any age; for study 2 we included patients of 60 years or older. Exclusion criteria were preexisting neurological disorders including cognitive impairment and dementia, or a history of cerebrovascular disease. Patients that were prescheduled for postoperative admission to the intensive care unit were excluded from study participation, since all postoperative assessments were performed during the PACU stay.
Participants from the two prospective cohort studies 1 and 2 were included in the current analysis, if they were 60 years or older and had completed the assessment for PACU delirium. Patients with contraindications to sedative premedication with midazolam such as obstructive sleep apnea and patients with preoperative anxiety were excluded.
Premedication with midazolam
The midazolam cohort routinely received 7.5 mg oral midazolam for sedative premedication in the absence of contraindications. Premedication with midazolam was administered 30 to 60 min preoperatively. Since 2017, the liberal administration of midazolam prior to anesthesia and surgery has been restricted at our institution. Anesthetic premedication with midazolam has been administered in patients with preoperative anxiety and a score of 11 or more on the Amsterdam Preoperative Anxiety and Information Scale (Berth et al. 2007; Moerman et al. 1996).
Anesthesiologic management
General anesthesia was induced with sufentanil (0.3–0.7 μg kg−1), propofol (2–3 mg kg−1), and rocuronium (0.6 mg kg−1). Anesthesia was maintained with sufentanil and sevoflurane (MAC target 0.8–1.2) or total intravenous anesthesia with sufentanil and propofol (4–7 mg kg−1 h−1). The choice of the anesthetic maintenance agent was at the discretion of the attending anesthesiologist. Anesthesia depth was monitored targeting a bispectral index (BIS™, Medtronic, MN) between 30 and 40. In the PACU, monitoring was continued (electrocardiogram, arterial blood pressure, peripheral oxygen saturation, urine output, arterial blood gas) and supplementary oxygen was administered as needed to maintain a peripheral oxygen saturation ≥ 94% (88–92% in patients with chronic obstructive pulmonary disease). If the numeric rating scale was 3 or higher, metamizole and/or piritramide were administered (Knipper et al. 2020).
Assessment of delirium
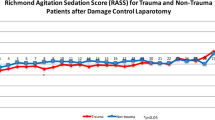
The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) was used to determine the presence of PACU delirium. The CAM-ICU was performed 15, 30, 45, and 60 min after extubation by six members of the study team, who were trained in CAM-ICU assessment. The Richmond Agitation-Sedation Scale (RASS) was applied at the same time points to evaluate the severity of agitation or sedation. The RASS is a 10-point scale ranging from − 5 (unarousable) to + 4 (combative). The CAM-ICU was only applied in patients with a RASS score of − 3 or higher. We defined PACU delirium as a positive CAM-ICU at any one time point between 15 and 60 min after extubation.
Data collection
On the day before surgery, patients were screened for depressive disorders with the Patient Health Questionnaire (PHQ-9). To screen for preexisting cognitive impairment, the Mini-mental Status Examination was administered preoperatively. Information on baseline demographic and clinical variables (age, body mass index, medical history, American Society of Anesthesiologists (ASA) physical status, and education), were obtained during baseline assessment on the day before surgery. Variables related to anesthesia and surgery were collected from the anesthesia protocol on the day of surgery. Patients with one or more missing variables were excluded from analysis (Fig. 1).
Statistical analysis
Data are presented as mean ± standard deviation (SD) or absolute numbers and percentages, according to the measurement level of the data. Demographic and clinical characteristics as well as the incidence of PACU delirium in the historical and the new midazolam policy were compared with Mann-Whitney U tests, χ2 tests, or Fisher’s exact tests, as appropriate. CAM-ICU positivity at different time points was compared with χ2 tests or Fisher’s exact tests and Bonferroni corrected for multiple comparisons. We analyzed the association between preoperative medication with midazolam and PACU delirium using binary logistic regression. The multivariable model included ‘PACU delirium’ as the dependent variable and ‘midazolam cohort’ as the independent variable of primary interest. Other clinically relevant variables were included in the model as possible confounders: anesthetic agent for anesthesia maintenance (propofol vs. sevoflurane), type of surgery (open vs. robot-assisted radical prostatectomy), age, ASA physical status, estimated blood loss (ml), duration of surgery (min), sufentanil (μg kg−1 h−1), and the year of enrollment. With exception of ‘midazolam cohort’ and ‘year of enrollment’, which remained in the model, variables were eliminated stepwise backwards. ‘Duration of surgery’ and ‘estimated blood loss’ were transformed to their binary logarithm (ln(x)/ln(2)).
We performed a subgroup analysis to assess the association between premedication with midazolam and PACU delirium in patients who had received inhalational anesthesia with sevoflurane in combination with sufentanil. The multivariable model included the same variables as the main analysis except for the variable ‘type of anesthesia’ (propofol vs. sevoflurane).
SPSS Statistics 24 (IBM Deutschland GmbH) was used for statistical analyses. Figures were designed with GraphPad Prism 8 (GraphPad Software, San Diego, CA). This manuscript adheres to the STROBE reporting guidelines for observational studies.
Results
Patients’ characteristics and clinical variables
A total of 569 patients had been enrolled. Of these, 454 completed the assessment for PACU delirium and 432 (midazolam cohort: n = 214; non-midazolam cohort: n = 218) were included in the final analysis. Details on the flow of participants throughout the study are presented in Fig. 1. The mean age of the study population was 67 ± 4 years. The majority of patients (n = 373/432, 86.3%) fulfilled the criteria for ASA physical status I–II with significantly more ASA I and III patients in the midazolam cohort (p < 0.001). The duration of anesthesia (p < 0.001) and surgery (p = 0.002) and the estimated blood loss (p = 0.015) were significantly higher in the midazolam cohort compared with the non-midazolam cohort. Compared with participants from the midazolam cohort, patients in the non-midazolam cohort received sevoflurane for anesthesia maintenance more frequently (p < 0.001) and received significantly higher doses of sufentanil (p = 0.003). Scores from the Mini-mental status examination differed significantly between cohorts, without being clinically relevant. Details on baseline demographic and clinical characteristics of the study population are listed in Table 1.
Incidence of postanesthesia care unit delirium
Of 214 patients, who had received midazolam, 105 (49.1%) tested positive for delirium at any one time point during the PACU stay. In the non-midazolam cohort 72/218 patients (33.0%) showed delirium signs in the PACU (Table 2). The incidence of PACU delirium differed significantly between the historical and the new midazolam policy (P < 0.001, Supplementary Figure 1). The incidence of PACU delirium was highest at 15 min following extubation and decreased during the PACU stay in both groups (Fig. 2). At 30 min, 45 min, and 60 min significantly more patients in the midazolam cohort were screened positive for PACU delirium compared with patients, who had not received midazolam for premedication (Fig. 2).
Association between premedication with midazolam and postanesthesia care unit delirium
Multivariable analysis did not show a significant association between premedication with midazolam and PACU delirium (OR 0.847 [95% CI 0.164; 4.367]; P = 0.842). Age (OR 1.102 [95% CI 1.050; 1.156]; P < 0.001), the cumulative dose of sufentanil (OR 1.014 [95% CI 1.005; 1.024]; P = 0.005), and total intravenous anesthesia (TIVA) for anesthesia maintenance (OR 2.805 [95% CI 1.497; 5.256]; P = 0.001) were significantly associated with PACU delirium (Table 3).
Subgroup analysis
Of 337 patients with sevoflurane-sufentanil for anesthesia maintenance, 121 patients had received midazolam and 216 patients had not. In the midazolam cohort, 49/337 (40.5%) patients tested positive for PACU delirium. A total of 71/337 (32.9%) patients in the non-midazolam cohort showed signs of PACU delirium. Multivariable analysis did not reveal a significant association between midazolam and PACU delirium (OR 1.240 [95% CI 0.241; 6.386]; P = 0.797). Age (OR 1.100 [95%CI 1.043; 1.161]; P < 0.001) and duration of surgery (OR 4.123 [95% CI 1.284; 13.241]; P = 0.017) were associated with the development of PACU delirium (Table 4).
Discussion
The aim of this study was to compare the incidence of PACU delirium between patients who had received oral midazolam preoperatively and patients who had not been administered any sedative medication prior to anesthesia induction. Compared with the midazolam cohort, the incidence of PACU delirium was significantly lower in patients under the new policy without sedative premedication. Contrary to our hypothesis, we did not find a significant association between premedication with midazolam and PACU delirium in multivariable analysis. Age, sufentanil, and TIVA for anesthesia maintenance were significantly associated with PACU delirium.
Prevention of delirium removes a tremendous burden from patients, their families, and caregivers and may help to substantially reduce morbidity, mortality, and health care cost. It is thus of utmost importance to identify and eliminate factors that trigger delirium. There is a strong association between benzodiazepines, administered postoperatively or prolonged benzodiazepine use, and delirium status that has been demonstrated in numerous studies (Fraser et al. 2013; Maldonado et al. 2009; Pandharipande et al. 2006; Zaal et al. 2015). However, the role of premedication with benzodiazepines in the development of PACU delirium is not well understood. Importantly, the single use of benzodiazepines to reduce preoperative anxiety has not been linked with POD so far (Wang et al. 2021). In a prospective study, Radtke et al. observed that premedication with benzodiazepines was a risk factor for inadequate emergence after anesthesia (Radtke et al. 2010). Similarly, data from an observational study showed an association between benzodiazepines and agitation during the immediate postoperative period (Lepousé et al. 2006). By contrast, Card and colleagues did not observe a statistical association between midazolam equivalents and delirium features during the PACU stay (Card et al. 2015). In line with these results, we did not find a significant association between premedication with midazolam and PACU delirium in multivariable analysis. It is plausible that preoperative benzodiazepines may contribute to inadequate emergence and prolonged recovery after anesthesia without necessarily causing subsequent POD (Lepousé et al. 2006; Radtke et al. 2010). A randomized controlled trial that compares liberal and restrictive premedication with midazolam with respect to PACU delirium is urgently needed.
We found a significant association between TIVA for anesthesia maintenance and a higher incidence of PACU delirium. Data on the influence of anesthetic substances on perioperative neurocognitive disorders is contradictory. Single studies report an association between inhalational anesthetics and PACU delirium (Ishii et al. 2016; Munk et al. 2016). In a recent meta-analysis, the incidence of POD was compared between propofol-based TIVA and inhalational agents for anesthesia maintenance. Interestingly, the authors did not find a beneficial effect of either substance on the development of POD (Miller et al. 2018). In line with our results, several studies report on the association between propofol for anesthesia maintenance and perioperative neurocognitive disorders (Hesse et al. 2019; Schoen et al. 2011). Interestingly, data from an in vitro study suggest strong anticholinergic properties including epigenetic modifications under the influence of propofol that may provide an explanation for the positive association between propofol use and delirium (Holtkamp et al. 2019). It is important to note that our study was not primarily designed to detect a difference in delirium signs between two different anesthetic regimens. Therefore, our findings on the association between TIVA and PACU delirium should be interpreted with caution.
We performed a subgroup analysis to assess whether a potential association between midazolam and PACU delirium may have been confounded by the strong association between propofol and PACU delirium. However, we did not find a significant association between midazolam and signs of delirium in patients, who had received inhalational anesthesia.
Strengths and limitations
Studies on the incidence of PACU delirium vary substantially with regard to screening instruments used and time points of assessment (Card et al. 2015; Hernandez et al. 2017; Hesse et al. 2019; Saller et al. 2020). Methodological heterogeneity among trials on PACU delirium may limit the comparability of study results. One strength of this study is the repetitive assessment at four predefined time points during the PACU stay (15, 30, 45, and 60 min postoperatively). Moreover, all patients were screened by one team of six investigators. However, delirium screening in our study was limited to the postopeorative recovery period in the PACU and we cannot generalize our results to POD beyond the PACU stay. We used the CAM-ICU, which is a validated and widely used tool for the assessment of POD (Card et al. 2015; Hesse et al. 2019; Saller et al. 2020). Despite high specificity, sensitivity for delirium signs in the PACU may be limited (Neufeld et al. 2013b). Therefore, we may have even underestimated the incidence of PACU delirium. Screening instruments that are more suitable for the PACU environment have been developed after patients had been enrolled in this study (Hight et al. 2018; Olbert et al. 2019). Future studies on PACU delirium should use novel screening instruments that take into account the specific circumstances of the PACU setting.
This secondary analysis of prospectively collected data before and after implementation of a restrictive benzodiazepine policy was neither randomized nor blinded. Thus, the nature of the study design only allows for exploratory analysis. Participants were enrolled between 2015 and 2018. To reduce history bias, we included the year of patient enrollment as a potential confounding factor on midazolam and PACU delirium in the statistical analysis. It is important to note, however, that this might not sufficiently reflect all advances of perioperative management throughout the study period.
The lack of association between midazolam and PACU delirium might be attributable to the relatively low perioperative risk of our study population, the majority being categorized as ASA physical status I or II. Moreover, patients with a history of cerebrovascular or neurodegenerative disease including mild cognitive impairment or dementia had been excluded from study participation. Therefore, we may have missed the patients most susceptible to neurocognitive adverse effects of benzodiazepines. It is possible, that the role of benzodiazepines as a risk factor for delirium becomes more relevant in patients with pre-existing neurocognitive impairment who have a high baseline vulnerability (Marcantonio et al. 1994). We only included patients undergoing prostatectomy. While the homogeneity of the baseline characteristics among cohorts is certainly one strength of our study, generalizability of the results may be limited.
We administered oral midazolam in the midazolam cohort. Oral midazolam is absorbed reliably and reaches peak plasma concentrations after around 60 min (Smith et al. 1981). Its major disadvantage is reduced bioavailability due to the hepatic first-pass effect. However, the prolonged time to reach peak plasma concentrations and to exert clinical effects allows for application before transfer to the surgical area. Despite a difference in absorption and reduced bio-availability of oral midazolam between 35 and 44%, clearance and terminal half-life values do not differ substantially between intravenous and oral midazolam application (Smith et al. 1981). Therefore, the route of midazolam administration should not affect the occurrence of delirium.
Conclusion
Over a 4-year period we observed a significant decrease in PACU delirium from 49% to 33% in elderly patients, who underwent radical prostatectomy. Of note, midazolam for sedative premedication was not significantly associated with PACU delirium. The reduction in the incidence of PACU delirium throughout the study period between 2015 and 2018 may be attributable to unobserved variables, such as improvements in perioperative management, other than a more restrictive preoperative benzodiazepine administration.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- BIS:
-
Bispectral index (BIS™)
- CAM-ICU:
-
Confusion Assessment Method for the Intensive Care Unit
- PHQ-9:
-
Patient Health Questionnaire
- PACU:
-
Postanesthesia care unit
- POD:
-
Postoperative delirium
- RASS:
-
Richmond Agitation-Sedation Scale
- TIVA:
-
Total intravenous anesthesia
References
Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192–214.
American Geriatrics Society. American Geriatrics Society 2012 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–31.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders [Internet]. Fifth Edition. American Psychiatric Association; 2013. Available from: https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. Cited 2020 Sep 24.
Beck S, Hoop D, Ragab H, Rademacher C, Meßner-Schmitt A, von Breunig F, et al. Postanesthesia care unit delirium following robot-assisted vs open retropubic radical prostatectomy: A prospective observational study. Int J Med Robot Comput Assist Surg. 2020a;16(3):e2094.
Beck S, Zins L, Holthusen C, Rademacher C, von Breunig F, Tennstedt P, et al. Comparison of cognitive function after robot-assisted prostatectomy and open retropubic radical prostatectomy: a prospective observational single-center study. Urology. 2020b;1(139):110–7.
Berth H, Petrowski K, Balck F. The Amsterdam Preoperative Anxiety and Information Scale (APAIS) - the first trial of a German version. Psychosoc Med. 20074:Doc01.
Bucx MJL, Krijtenburg P, Kox M. Preoperative use of anxiolytic-sedative agents; are we on the right track? J Clin Anesth. 2016;33:135–40.
Card E, Pandharipande P, Tomes C, Lee C, Wood J, Nelson D, et al. Emergence from general anaesthesia and evolution of delirium signs in the post-anaesthesia care unit. Br J Anaesth. 2015;115(3):411–7.
Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2013;41(9 Suppl 1):S30–8.
Gottesman RF, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67(3):338–44.
Hernandez BA, Lindroth H, Rowley P, Boncyk C, Raz A, Gaskell A, et al. Post-anaesthesia care unit delirium: incidence, risk factors and associated adverse outcomes. Br J Anaesth. 2017;119(2):288–90.
Hesse S, Kreuzer M, Hight D, Gaskell A, Devari P, Singh D, et al. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: an early sign of postoperative complications. Br J Anaesth. 2019;122(5):622–34.
Hight DF, Sleigh J, Winders JD, Voss LJ, Gaskell AL, Rodriguez AD, et al. Inattentive delirium vs. disorganized thinking: a new axis to subcategorize PACU Delirium. Front Syst Neurosci. 2018;12:22.
Holtkamp C, Koos B, Unterberg M, Rahmel T, Bergmann L, Bazzi Z, et al. A novel understanding of postoperative complications: in vitro study of the impact of propofol on epigenetic modifications in cholinergic genes. PLOS ONE. 2019;14(5):e0217269.
Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–22.
Ishii K, Makita T, Yamashita H, Matsunaga S, Akiyama D, Toba K, et al. Total intravenous anesthesia with propofol is associated with a lower rate of postoperative delirium in comparison with sevoflurane anesthesia in elderly patients. J Clin Anesth. 2016;33:428–31.
Kain ZN, Mayes LC, Bell C, Weisman S, Hofstadter MB, Rimar S. Premedication in the United States: a status report. Anesth Analg. 1997;84(2):427–32.
Kainz E, Stuff K, Kahl U, Wiessner C, Yu Y, Von Breunig F, et al. Impact of postanesthesia care unit delirium on self-reported cognitive function and health-related quality of life: a prospective observational cohort study. Qual Life Res. 2022. https://doi.org/10.1007/s11136-022-03087-1. Online ahead of print.
Knipper S, Hagedorn M, Sadat-Khonsari M, Tian Z, Karakiewicz PI, Tilki D, et al. Comparison of intra- and postoperative analgesia and pain perception in robot-assisted vs. open radical prostatectomy. World J Urol. 2020;38(6):1451–7.
Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747–53.
Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32.
Maldonado JR, Wysong A, van der Starre PJA, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50(3):206–17.
Marcantonio ER, Goldman L, Mangione CM, Ludwig LE, Muraca B, Haslauer CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–9.
Maurice-Szamburski A, Auquier P, Viarre-Oreal V, Cuvillon P, Carles M, Ripart J, et al. Effect of sedative premedication on patient experience after general anesthesia: a randomized clinical trial. JAMA. 2015;313(9):916–25.
Miller D, Lewis SR, Pritchard MW, Schofield-Robinson OJ, Shelton CL, Alderson P, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018;8(8):CD012317. https://doi.org/10.1002/14651858.CD012317.pub2.
Moerman N, van Dam FS, Muller MJ, Oosting H. The Amsterdam Preoperative Anxiety and Information Scale (APAIS). Anesth Analg. 1996;82(3):445–51.
Munk L, Andersen G, Møller AM. Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anaesthesiol Scand. 2016;60(8):1059–66.
Neufeld KJ, Leoutsakos J-MS, Sieber FE, Wanamaker BL, Gibson Chambers JJ, Rao V, et al. Outcomes of early delirium diagnosis after general anesthesia in the elderly. Anesth Analg. 2013a;117(2):471–8.
Neufeld KJ, Leoutsakos JS, Sieber FE, Joshi D, Wanamaker BL, Rios-Robles J, et al. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth. 2013b;111(4):612–8.
Olbert M, Eckert S, Mörgeli R, Kruppa J, Spies CD. Validation of 3-minute diagnostic interview for CAM-defined Delirium to detect postoperative delirium in the recovery room: A prospective diagnostic study. Eur J Anaesthesiol. 2019;36(9):683–7.
Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–6.
Radtke FM, Franck M, Hagemann L, Seeling M. Risk factors for inadequate emergence after anesthesia: emergence delirium and hypoactive emergence. MINERVA Anestesiol. 2010;76(6):11.
Saller T, Hofmann-Kiefer KF, Saller I, Zwissler B, von Dossow V. Implementation of strategies to prevent and treat postoperative delirium in the post-anesthesia caring unit: a German survey of current practice. J Clin Monit Comput. 2021;35(3):599–605. https://doi.org/10.1007/s10877-020-00516-9. Epub 2020 May 9.
Schoen J, Husemann L, Tiemeyer C, Lueloh A, Sedemund-Adib B, Berger K-U, et al. Cognitive function after sevoflurane- vs propofol-based anaesthesia for on-pump cardiac surgery: a randomized controlled trial. Br J Anaesth. 2011;106(6):840–50.
Sharma PT, Sieber FE, Zakriya KJ, Pauldine RW, Gerold KB, Hang J, et al. Recovery room delirium predicts postoperative delirium after hip-fracture repair. Anesth Analg. 2005;101(4):1215–20.
Smith MT, Eadie MJ, Brophy TO. The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol. 1981;19(4):271–8.
Sprung J, Roberts RO, Weingarten TN, Nunes Cavalcante A, Knopman DS, Petersen RC, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316–23.
Stukenberg S, Franck M, Spies CD, Neuner B, Myers I, Radtke FM. How can postoperative delirium be predicted in advance? A secondary analysis comparing three methods of early assessment in elderly patients. Minerva Anestesiol. 2016;82(7):751–9.
Vlisides P, Avidan M. Recent advances in preventing and managing postoperative delirium. F1000Res. 2019;8:F1000.
Wang M-L, Min J, Sands LP, Leung JM, Perioperative medicine research group. Midazolam premedication immediately before surgery is not associated with early postoperative delirium. Anesth Analg. 2021.
Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–51.
Zaal IJ, Devlin JW, Hazelbag M, Klein Klouwenberg PMC, van der Kooi AW, Ong DSY, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130–7.
Zhang Y, He S-T, Nie B, Li X-Y, Wang D-X. Emergence delirium is associated with increased postoperative delirium in elderly: a prospective observational study. J Anesth. 2020. Available from: https://doi.org/10.1007/s00540-020-02805-8. Cited 2020 Sep 13.
Acknowledgements
Not applicable
Funding
The study was partly funded by the Else Kröner-Fresenius-Stiftung (2015_A33). MF receives financial support from the Johanna und Fritz Buch Gedächtnis-Stiftung. UK receives funding from the Clinician Scientist Program of the University of Hamburg. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MF conceived the study. KS and EK were responsible for data collection and patient contact. MF, UK and KS were involved in the analysis and interpretation of the data. HP provided consultation for statistics. KS wrote the first draft of the manuscript. MF and UK reviewed and contributed to writing the manuscript; SB, FB, RN, SF, and CZ critically reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study (PV 4782) was provided by the Ethical Committee at the Hamburg Chamber of Physicians (Vice-Chairperson Prof. Dr. M. Carstensen) on September 2nd, 2014. Written informed consent was obtained from all patients before initiation of study-related procedures.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Postanesthesia care unit (PACU) delirium in patients who received midazolam for sedative premedication (midazolam cohort) and patients without preoperative midazolam administration (non-midazolam cohort).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stuff, K., Kainz, E., Kahl, U. et al. Effect of sedative premedication with oral midazolam on postanesthesia care unit delirium in older adults: a secondary analysis following an uncontrolled before-after design. Perioper Med 11, 18 (2022). https://doi.org/10.1186/s13741-022-00253-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13741-022-00253-4