Abstract
Background
We aimed to conduct a systematic review and meta-analysis of randomized controlled trials (RCTs) to investigate the effects of rice bran supplementation on serum lipid profile levels.
Methods
We searched PubMed/Medline, Scopus, ISI Web of Science, and Google Scholar using related keywords. Published RCTs exploring the effects of rice bran consumption on lipid profile were searched up to June 2022. Evidence certainty was assessed on the basis of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. The data were pooled using a random-effects model and reported as weighted mean difference (WMD) and 95% confidence interval (CI) for each outcome.
Results
Meta-analysis of eight RCTs (with 11 effect sizes) showed no significant effect of rice bran supplementation on serum levels of triglyceride (WMD: -11.38 mg/dl; 95% CI: -27.73, 4.96; P = 0.17), total cholesterol (WMD: -0.68 mg/dl; 95% CI: -7.25, 5.88; P = 0.834), low-density lipoprotein cholesterol (WMD: -1.68 mg/dl; 95% CI: -8.46, 5.09; P = 0.627) and high-density lipoprotein cholesterol (WMD: 0.16 mg/dl; 95% CI: -1.52, 1.85; P = 0.848) compared to control group.
Conclusion
Our meta-analysis suggests that rice bran supplementation has no significant effects on serum levels of lipid profile components. However, larger studies with longer durations and improved methodological quality are needed before firm conclusions can be reached.
Similar content being viewed by others
Introduction
Dyslipidemia is a multifactorial disorder characterized by a combination (two or more) of increased serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG) levels and decreased serum high-density lipoprotein cholesterol (HDL-C) concentrations [1, 2]. The global prevalence of dyslipidemia has increased dramatically over the past 30 years and is among the chronic disease with high mortality rate [3]. Dyslipidemia, as a metabolic abnormality, is recognized as one of the most important risk factors of cardiovascular disease, which accounts for most deaths caused by non-communicable diseases (NCDs) [4].
Pharmacotherapy and lifestyle modifications, especially dietary interventions, are the two main components in optimizing plasma lipid profiles and subsequently reducing the risk of cardiovascular disease [5, 6]. Due to the side effects of drugs, the first line of treatment in dyslipidemia is dietary interventions [6]. Dietary recommendations emphasize the replacement of animal fats with vegetable fats as well as increasing fiber intake. Rice bran (RB) is known as a nutraceutical due to its high fiber content (20–51%), plant sterols and a composition of fatty acids such as oleic acid (38.4%) and linoleic acid (34.4%) [7, 8]. RB also contains a great variety of bioactive phytochemicals, such as γ oryzanol which has a well-defined cholesterol-lowering function [8, 9]. Rice bran contains a wide variety of bioactive compounds with health properties, including amino acids, vitamins and cofactors, and secondary metabolites [10].
The beneficial effects of rice bran, and its derivatives such as rice bran oil, in improving glycemic control [11,12,13], optimizing lipid profile [14,15,16], lowering blood pressure [17,18,19] and weight management [14, 20, 21] have been shown in several studies. An animal study by Zhang et al. [22] showed that fresh rice bran protein can modulate cholesterol metabolism and reduce serum levels of very-low density lipoprotein cholesterol (VLDL-C), LDL-C, TG and hepatic total cholesterol. Qureshi et al. [23,24,25] also showed the lipid-lowering effects of vitamin E analogues of rice bran in several studies. The lipid-lowering effects of rice bran have also been partly attributed to γ oryzanol, an exclusive rice bran polyphenol [26, 27]. Nevertheless, the results of studies on the effects of rice bran and its components on lipid profile are contradictory and not conclusive. Although two meta-analyses have been published on the effects of rice bran oil on lipid profile [28, 29], meta-analysis has not yet investigated the effects of whole rice bran on lipid profile. Accordingly, we decided to conduct a systematic review and meta-analysis to investigate the effects of RB supplementation on the lipid profile.
Materials and methods
This systematic review and meta-analysis was registered in PROSPERO before the start of the literature search with registration number CRD42022337982. This study was reported based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement in terms of processing, analyzing, and reporting of the data [30].
Data sources and search strategies
A systematic literature search was performed in PubMed, Scopus, Web of Science and Google Scholar without specific time frames and language limits, up to June 2022. The purpose of our search was to identify published clinical trials that examined the effects of RB supplementation on lipid profiles (TC, TG, LDL-C, and HDL-C) of adult human.
The following Medical subject headings (MeSH) and non-MeSH terms were used (supplementary): ("rice bran" OR "rice bran powder" OR "rice bran supplement" OR "stabilized rice bran") AND (cholesterol OR "low density lipoprotein" OR LDL OR TC OR "total cholesterol" OR "high density lipoprotein" OR HDL OR "triglyceride" OR TG OR "lipoprotein" OR "lipid profile" OR lipid OR "cardiovascular disease" OR "heart disease" OR "hypercholesterolemia"). In order to complete the search process, a manual screening was performed in article references and review articles, so that no randomized controlled trials (RCTs) were missed.
Eligibility criteria and study selection
The screening of the titles and abstracts and the further assessment of the full-texts was performed by two independent investigators (Z.H. & F.A.). Studies with the following criteria were included in this meta-analysis: (a) clinical trials (with either parallel or cross-over design), (b) studies which investigated whole rice bran, stabilized rice bran or rice bran powder (c) having a control group (placebo or oral powder similar to rice bran powder) (d) performed in individuals over 18 years old, (e) reported at least one of the following measures: TC and/or TG and/or LDL-C and/or HDL-C.
The exclusion criteria were: (a) animal and in vitro studies (b) studies which investigated the effects of RB concurrently with other interventions, (c) studies which examined only certain components of the RB, such as RB oil, γ oryzanol, ferulic acid, tocols (tocopherol and tocotrienol) or specific amino acids (d) studies which examined defatted RB or RB extract (e) studies without complete information about the outcomes of interest, (f) with less than one-week follow-up, (g) studies without control or placebo group, and (h) studies with other designs except for a clinical trial design.
Data extraction
The initial screening of articles was done by Z.H. based on the inclusion and exclusion criteria and then double checked by F.A. Any discrepancies in the results were resolved by a third researcher (Z.Y.). In cases where article information was not available, an email was sent to the corresponding author to access the full text of the article. After achieving eligible articles, following data were extracted from each: first author’s name, year of publication, study location, trial duration, gender, mean age and mean body mass index (BMI) of participants, RCT design, the health status of the study population, sample sizes in each group, dose of RB supplementation, and TG (mg/dl), TC (mg/dl), LDL-C (mg/dl) and HDL-C (mg/dl) levels before and after the intervention. This information is presented in Table 1.
Quality assessment
We classified all studies into 4 groups according to the GRADE guidelines (Grading of Recommendations Assessment, Development, and Evaluation): high, moderate, low, and very low [37].
To evaluate the risk of bias, the Cochrane risk of bias 2.0 tool (RoB 2) per protocol for parallel group randomized trials [38] was used. In this method, risk of bias is evaluated using seven indicators: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Two researchers independently assessed the risk of bias. Risk of bias was divided into 3 levels: unclear risk (U), low risk (L) and high risk (H) (Additional file 1: Table S1).
Data synthesis and analysis
In order to evaluate the effects of RB, the mean differences in TG, TC, LDL-C and HDL-C between the intervention and control groups with their standard deviations (SDs) were calculated. The mean change was calculated by following formula: (measure at the end of follow-up in the intervention group—measure at baseline in the intervention group)—(measure at the end of follow-up in the control group—measure at baseline in the control group) [39]. Also, their SDs were calculated as follows: SD = square root [(SD pre-treatment) 2 + (SD post-treatment) 2 -(2R × SD pre-treatment × SD post-treatment)] [39]. In case a study reported the mean change or SD, we considered the same. When standard error of the mean (SEM) was reported instead of SD, we used the following formula to convert it to SD: SEM × √n (n = number of participants in each group). The random-effects model was applied to evaluate the pooled weighted mean difference (WMD) with 95% confidence intervals (CIs). The presence of between-study heterogeneity was assessed by Cochrane’s Q test and I2 statistic. I2 value > 40% or P < 0.05 for the Q-test was characterized as significant between-study heterogeneity [40]. To detect heterogeneity among subgroups, we performed a pre-defined subgroup analysis based on baseline TG, TC, LDL-C and HDL-C, country (USA or non-USA), study design (parallel or cross-over), age (≥ 50 years or less), study duration (≤ 4 weeks or more), RB dose (≥ 60 g/day or less), health status (hypercholesterolemic, non hypercholesterolemic), gender (male, female, or both) and baseline BMI (Normal (18.5–24.9 kg/m2), Overweight (25–29.9 kg/m2) or Obese (> 30 kg/m2)). The potential non-linear effects of RB dose (g/day) and treatment duration (weeks) were investigated using fractional polynomial modeling [41]. Meta-regression analysis was executed to evaluate the association between pooled effect size and RB dose (g/day) and follow-up length (trial duration). A bubble plot was obtained with the size of the "bubble" proportional to the accuracy of the estimate for each of the four factors separately. We also performed the sensitivity analysis method to assess the effect of each study on the overall result, by removing one by one of the studies. Probable publication bias was evaluated by Begg's test [42] (significance point at P < 0.05), Egger's test [43] (and visual funnel plots. All statistical analyzes were performed by STATA software (version 17.0; StatCorp, College Station, TX, USA). In this review P < 0.05 was indicated statistically significant.
Results
Study selection
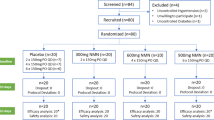
Out of 2893 of articles from the database searching and one additional article from reference list checking, 1073 duplicates were removed. Of the remaining 1821 studies, after screening the title and abstract, 1769 were excluded due to lack of relevance (1127) owing to being review articles (189) and being animal studies (453). Fifty-two papers were subjected for thorough full text assessment. Out of that, 44 studies were excluded due to the following reasons: (a) RB oil but not rice bran was examined (n = 26), (b) The RB components have been investigated (n = 7) [14, 15, 19, 25, 44,45,46], (c) Full text paper was not found (n = 4) [47,48,49,50], (d) Short trial duration (postprandial assessment) (n = 3) [51,52,53], (e) Lack of control group (n = 1) [54], (f) Conducted on children (n = 1) [55], and (g) Defatted RB was examined (n = 1) [56]. Finally, eight eligible RCTs were included in this systematic review and meta-analysis. The PRISMA flow diagram for study selection is shown in Fig. 1.
Study characteristics
Table 1 summarizes the characteristics of all the included studies. In total, 472 participants (286 cases and 186 controls) with age range between 37 and 62 years old and BMI range between 24.43 and 28.7 kg/m2 were recruited.
These RCTs were conducted in Australia [31], United States [32, 33, 36], Iran [12, 34], Taiwan [13] and Italy [35]. Studies were published between 1990 and 2016 and their duration ranged from 3 to 12 weeks. Three of the selected RCTs were designed crossover [31, 32, 35] and the remaining were parallel. The study subjects were patients with hypercholesterolemia [31,32,33,34,35], patients with diabetes [12, 13], and colorectal cancer survivors [36]. Two studies exclusively enrolled men [31, 35], one enrolled only women [34] and the remaining enrolled both sexes [12, 13, 32, 33, 36]. Four studies applied a double blind design [12, 13, 31, 33], one was single-blinded [36] and blindness was not mentioned in the rest [32, 34, 35]. Participants did not take lipid-lowering drugs in none of the studies, although in three studies this was not mentioned [31, 32, 36]. The control group of the studies were as follows: Wheat bran (Kestin et al. (A) [31] and Matani et al. [34]), Oat bran (Kestin et al. (B) [31], Hegsted et al. [32] and Gerhardt (A) [33]), rice starch (Gerhardt et al. (B)[33]), white flour without fiber (Tazakori et al. [12]), milled rice flour (Cheng et al. [13]), beta-glucan enriched foods (Rondanelli et al. [35]), navy bean powder (Borresen et al. (A) [36]) and same ingredients as the intervention foods but not include RB (Borresen et al. (B) [36]).
Quality assessment
In terms of random sequence generation, two studies had low risk of bias [31, 35], five studies had unclear risk [13, 32,33,34, 36] and one had high risk [12]. Allocation concealment was unclear in all studies, with the exception of two studies which had a low risk of bias [33, 36]. All the trials had a low risk of bias regarding selective outcome reporting. Three studies were performed double blinded so considered low risk [13, 31, 33], one was single blinded (high risk) [36] and the others did not mention blinding (unclear risk) [34, 35]. Outcome assessor blinding was ruled out in two studies (high risk) [13, 36], but was unclear in the other studies (unclear risk). None of the studies had incomplete outcome data or other sources of bias, so they were considered low risk in both aspects. After evaluation of studies based on the above seven criteria, if a study met more than two high-risk indicators, it was considered generally high-risk, if it met two indicators, it was considered moderate risk and if it met less than two indicators, it was considered low risk.
As a whole, all studies had a low risk of general bias, except for two studies that had a moderate risk of general bias [34, 36].
Meta-analysis results
The effects of RB supplementation on TG, LDL-C, HDL-C and TC were evaluated by eight studies (with 11 effect sizes) involving 369 participants (186 cases and 183 controls).
Effect of RB supplementation on TG concentrations
Eleven effect sizes, including a total of 369 participants (186 intervention and 183 control subjects) assessed the effect of RB supplementation on circulating TG levels. The overall meta-analysis reported that RB supplementation does not significantly change serum TG levels (WMD: -11.38 mg/dl; 95% CI: -27.73, 4.96; P = 0.17) (Additional file 1: Figure S2.A). Also a significant degree of heterogeneity was found (I2 = 79.5%, P < 0.001). Subgroup analysis showed a significant decrease in TG in studies involving both sexes, overweight and obese individuals, and 50 years’ old participants or younger (Table 2).
Effect of RB supplementation on TC concentrations
Overall, 11 arms of included clinical trials (186 intervention and 183 control subjects) investigated the effect of RB supplementation on TC concentration, and pooled effect size showed a non-significant decreased serum TC concentration (WMD: -0.68 mg/dl; 95% CI: -7.25, 5.88; P = 0.834) with a significant heterogeneity between studies (I2 = 79.5%, P < 0.001) (Additional file 1: Figure S2.B). Furthermore, performing subgroup analyses, we did not find any significant effect of RB intake on TC levels among all the subgroups (Table 2).
Effect of RB supplementation on LDL-C concentrations
In total, 11 effect sizes with a sample size of 369 participants were included in the analysis. Combining these effect sizes, a significant reduction was not seen in serum concentrations of LDL-C following RB supplementation (WMD: -1.68 mg/dl; 95% CI: -8.46, 5.09; P = 0.627) (Additional file 1: Figure S2.C). In addition, the degree of heterogeneity was significant (I2 = 81.2%, P < 0.001). In addition, the subgroup analysis revealed that in the study which exclusively enrolled obese women, the reduction in LDL-C following RB supplementation was significant (Table 2).
Effect of RB supplementation on HDL-C concentrations
The meta-analysis of 11 effect sizes involving 369 individuals revealed no significant change in HDL-C levels after RB intervention (WMD: 0.16 mg/dl; 95% CI: -1.52, 1.85; P = 0.848) compared with control group (Additional file 1: Figure S2.D). The amount of heterogeneity was also notable among the studies (I2 = 60.3%, P = 0.005). Based on the analysis, in studies conducted in the United States, in cross-over studies and those with patients with hypercholesterolemia the intervention group experienced a lower increase or even a decrease in HDL-C compared to the control group (Table 2).
Sensitivity analysis
In order to evaluate the contribution of each study to the final result of this meta-analysis, we removed each study in turn and assessed the results without them. As a result of this analysis, the overall effect size was not influenced by a single study except for HDL-C that the overall effect was changed significantly with the omission of Tazakori et al. study [12] (WMD: -1.46 mg/dl, 95%CI: -1.85, -1.07).
Publication bias
According to Eager's test, Begg's test and visual inspection of funnel plots, no publication bias was detected in studies evaluating the effect of RB supplementation on TG (P = 0.204, SE: 1.02, CI: -0.91—3.73, Egger’s test) (P = 0.276, Begg's test), TC (P = 0.844, SE: 0.94, CI: -1.94—2.32, Egger’s test) (P = 0.436 Begg's test), LDL-C (P = 0.981, SE: 0.88, CI: -1.99–2.03, Egger’s test) (p = 0.276 Begg's test) or HDL-C (P = 0.081, SE: 0.50, CI: -0.15–2.13, Egger’s test) (P = 0.436 Begg's test) (Additional file 1: Figure S3 A-D).
Non-linear dose–response analysis between dose and duration of RB supplementation and lipid profile
According to dose–response analysis, RB dose variety could significantly alter TG (r = -911.19, SE: 359.22, CI: -1739.55 – 82.82, P nonlinearity = 0.035), TC (r = 265.27, SE:55.22, CI: 137.92– 392.63, P nonlinearity = 0.001), and LDL-C (r = -547.14, SE:55.22, CI: 137.92– 392.63, P nonlinearity = 0.023) but did not alter HDL-C (r = 34.70, SE:35.64, CI: -47.48 – 116.89, P nonlinearity = 0.359) significantly (Additional file 1: Figure S4 A-D). Furthermore, dose–response analysis based on duration showed that although supplementation with RB significantly altered LDL-C (r = 1573.83, SE: 415.94, CI: 614.66–2532.99, P nonlinearity = 0.005), it did not significantly change TG (r = 70.85, SE: 57.90, CI: -62.68 – 204.38, P nonlinearity = 0.256), and HDL-C (r = -216.14, SE: 163.16, CI: -592.41– 160.12, P nonlinearity = 0.222). The changes in TC levels were close to the significant level (r = 1019.33, SE: 452.79, CI: -24.75– 2063.42, P nonlinearity = 0.054) (Additional file 1: Figure 5 A-D).
Meta-regression analysis
Meta-regression analysis was performed to investigate the possible relationship between RB dose, study duration and lipid profile changes. Based on this analysis, no significant association was detected between RB supplementation dose and changes in TG (Slope = 0.17, Intercept = 53.82, SE: 0.310, CI: -0.53 – 0.87, P linearity = 0.595), TC (Slope = 0.16, Intercept = 51.12, SE:0.60, CI: -1.21 – 1.53, P linearity = 0.798), LDL-C (Slope = 0.15, Intercept = 51.39, SE:0.50, CI: -0.98 – 1.29, P linearity = 0.763) or HDL-C (Slope = -0.44, Intercept = 50.90, SE:3.03 CI: -7.31 – 6.42, P linearity = 0.887) levels (Additional file 1: Figure 6 A-D). Similarly, the relationship between study duration and changes in TG (Slope = 0.00, Intercept = 3.66, SE:0.14 CI: -0.32 – 0.32, P linearity = 0.981), TC (Slope = -0.05, Intercept: 3.27, SE:0.14 CI = -0.37 – 0.27, P linearity = 0.721), LDL-C (Slope = -0.08, Intercept = 3.03, SE:0.13 CI: -0.39 – 0.22, P linearity = 0.533), and HDL-C (Slope = -0.17, Intercept = 5.13, SE:0.35 CI: -0.97 – 0.63, P linearity = 0.640) levels were not significant (Additional file 1: Figure 7 A-D).
Grading of evidence
The grading of evidence is presented in Additional file 1: Table S2. The quality of studies evaluating the effect of RB supplementation on TG, TC, LDL-C and HDL-C were deemed to be low due to their heterogeneity percentage between studies and their insignificancy.
Discussion
For the first time in this systematic review and meta-analysis, we assessed the effects of RB supplementation on lipid profile changes among human adults. After analyzing the eight studies (with 11 effect sizes), we concluded that RB supplementation has no statistically significant effect on improving the serum levels of TG, TC, LDL-C, and HDL-C compared to control group. However, based on the subgroup analysis, the effect of RB on TG levels was significant in studies involving both men and women, as well as in studies in which participants were older than 50 years old. This significance was mainly attributed to the study of Hegsted et al. [32]. In this study, the effect of 100 g of stabilized RB supplementation in patients with hypercholesterolemia was investigated and since 100 g was the highest dose of RB supplementation among the studies, this significant difference can be attributed to the high dose of RB in this study [32]. Furthermore, based on a non-linear dose–response analysis, RB dose variety could significantly change TG, TC and LDL-C levels, but the variation of the study duration only caused a significant change in LDL-C concentration. Meta-regression analysis was performed as well to detect the possible linear association between dose and duration and changes in lipid profile, but did not show a significant association.
The results of this meta-analysis are inconsistent with the results of some of the RCTs included in this review. Tazkari et al. investigated the effectiveness of RB supplementation on lipid profile changes in patients with diabetes and reported that RB supplementation could significantly reduce TG levels and increase HDL-C levels [12]. In another study in patients with diabetes, it was found that supplementation with stabilized RB was able to significantly reduce TC and LDL-C concentrations [13].
Notably, two meta-analyses have been investigated the effect of RB oil on lipid profile changes [28, 29]. Jolfaie et al. which included 11 RCTs found that RB oil supplementation could reduce the risk of cardiovascular disease through reducing TC and LDL-C levels [28]. In another meta-analysis, Pourrajab et al. showed a significant effect of RB oil on TG reduction in addition to TC and LDL-C [29].
In addition to oil, rice bran also contains fiber and protein, which is considered in the present study. The results of previous study indicated that the effects of rice bran fiber concentrates on lowering TC and LDL-C in patients with diabetes was significantly higher than rice bran water soluble concentrates and stabilized rice bran [11]. There are about 21 g of dietary fiber per 100 g of RB, while the same amount of oat bran contains 15.4 g of fiber [57, 58]. 90% of RB dietary fiber is insoluble which includes cellulose, hemicellulose and arabinoxylans, and 10% is soluble fiber, which is mainly pectin and β-glucan [59,60,61]. Soluble fiber can lower blood cholesterol through following mechanisms: (a) binding to bile acids thus acting as bile acid sequestrate, (b) increasing short chain fatty acids (SCFAs) production and decreasing hepatic cholesterol production, (c) slowing down the absorption of carbohydrates, (d) reducing insulin secretion and thus reducing cholesterol production [62]. It has also been shown that RB soluble fiber could down-regulate the expression of genes involved in lipogenesis and significantly reduce TG, TL and LDL-C levels [63].
Although in general the cholesterol-lowering effect of soluble fiber is greater than that of insoluble fiber [64], mechanisms for improving lipid profile have also been described for insoluble fiber. By increasing the fecal bulk (bulking effect), insoluble fiber reduces the intestinal transit time and thus reduces fat absorption [65]. Another possible mechanism of insoluble fiber is to induce a long-term satiety [66]. It has also been shown that RB protein can exhibit lipid-lowering effects by preventing the binding of cholesterol to bile acids, thereby lowering serum cholesterol and increasing fat excretion [67].
A distinct feature of rice bran is its high oil content (≈ 20.8%) compared to other bran, including wheat (≈ 7.03%) and oat (≈ 4.25%) bran [58]. Despite the mentioned benefits for the defatted rice bran, a comparison of defatted RB and RB oil showed significant lipid lowering effects of RB oil [68]. Lipid-lowering effects of RB seem to be attributed to γ oryzanol, high amounts of vitamin E and excellent fatty acid profile [8, 57]. γ oryzanol can lower cholesterol through a variety of mechanisms, including: inhibition of cholesterol-esterase [69, 70] and increased fecal excretion of cholesterol and bile acids [27, 71]. Two mechanisms has been also proposed for lipid-lowering effects of tocopherol: antioxidant activity against cholesterol oxidation [72] and 3-hydroxy-3methylglutaryl-coenzyme A (HMG-CoA) reductase inhibiting [73,74,75,76].
It seems that the dose of RB oil supplemented in RCTs was higher than the oil content of rice bran. Therefore, studies investigating the effects of RB oil have reported a significant improvement in lipid profile changes.
There are some strengths in the present systematic review and meta-analysis. This is the first meta-analysis to evaluate the effects of RB supplementation on lipid profile in human adults. It has also relatively acceptable number of studies and large sample sizes. There was no time or language limitation while searching the databases. Moreover, to discover the cause of heterogeneity, a subgroup analysis was performed. All participants showed baseline TC > 200 mg/dl, which is considered as a borderline for hypercholesterolemia according to National Cholesterol Education Program (NCEP) III guidelines [77]. Sensitivity analysis was also performed for TG, TC and LDL-C parameters which showed that no study distinctly affected the overall result. No publication bias was discovered among studies according to Eager's test, Begg's test and visual inspection of funnel plots.
Despite these strengths, following limitations should be taken into account while interpreting the results. There was an insufficient number of RCTs, and most of the RCTs had relatively small sample sizes. The presence of only one female single-sex group in the subgroup analysis based on gender, reduces the significance. Some RCTs were open-labeled, which can affect the outcome. The degree of heterogeneity was also significant between studies, and this may be due to the limited number of studies and their small sample sizes. Besides, the dose of the study also ranged from 20 to 100 g/day, and this variation made the comparison more complicated. As a result, these limitations suggest that more placebo controlled randomized clinical trials with larger sample sizes are needed to determine the true effect of RB supplementation on lipid profile.
Conclusion
The present systematic review and meta-analysis disclosed that supplementation with rice bran did not show significant effects on serum levels of TG, TC, LDL-C and HDL-C. Given the existing contradictions, for more accurate and reliable conclusion on the effects of rice bran on serum lipid profiles, more clinical trials with larger sample sizes and different doses and durations are needed.
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript.
Abbreviations
- RCTs:
-
Randomized controlled trials
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- TG:
-
Triglycerides
- HDL-C:
-
High-density lipoprotein cholesterol
- NCDs:
-
Non-communicable diseases
- RB:
-
Rice bran
- VLDL-C:
-
Very-low density lipoprotein cholesterol
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- MeSH:
-
Medical subject headings
- BMI:
-
Body mass index
- GRADE:
-
Grading of Recommendations Assessment, Development, and Evaluation
- SDs:
-
Standard deviations
- SEM:
-
Standard error of the mean
- WMD:
-
Weighted mean difference
- CIs:
-
Confidence intervals
- SCFAs:
-
Short chain fatty acids
- HMG-CoA:
-
3-Hydroxy-3methylglutaryl-coenzyme A
- NCEP:
-
National Cholesterol Education Program
References
Klop B, Elte JWF, Cabezas MCJN. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–40.
Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40(1):195–211.
Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021;18(10):689–700.
Chou R, Dana T, Blazina I, Daeges M, Jeanne TLJJ. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US preventive services task force. JAMA. 2016;316(19):2008–24.
Hammad S, Pu S, Jones PJJL. Current evidence supporting the link between dietary fatty acids and cardiovascular disease. Lipids. 2016;51(5):507–17.
Zodda D, Giammona R, Schifilliti SJP. Treatment strategy for dyslipidemia in cardiovascular disease prevention: focus on old and new drugs. Pharmacy. 2018;6(1):10.
Eady S, Wallace A, Willis J, Scott R, Frampton C. Consumption of a plant sterol-based spread derived from rice bran oil is effective at reducing plasma lipid levels in mildly hypercholesterolaemic individuals. Br J Nutr. 2011;105(12):1808–18.
Sharif MK, Butt MS, Anjum FM, Khan SH. Rice bran: a novel functional ingredient. Crit Rev Food Sci Nutr. 2014;54(6):807–16.
Ju Y-H, Vali SR. Rice bran oil as a potential resource for biodiesel: a review. 2005.
Zarei I, Brown DG, Nealon NJ, Ryan EP. Rice bran metabolome contains amino acids, vitamins & cofactors, and phytochemicals with medicinal and nutritional properties. Rice. 2017;10(1):1–21.
Qureshi AA, Sami SA, Khan FA. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. J Nutr Biochem. 2002;13(3):175–87.
Tazakori Z, Dehghan MH, Iranparvar M, Zare M, Foladi N, Mohmmadi R. Effect of Rice Bran Powder on Blood Glucose Levels and Serum Lipid Parameters in Diabetes Patient II. Res J Biol Sci. 2007;2(3):252–5.
Cheng H-H, Huang H-Y, Chen Y-Y, Huang C-L, Chang C-J, Chen H-L, et al. Ameliorative effects of stabilized rice bran on type 2 diabetes patients. Ann Nutr Metab. 2010;56(1):45–51.
Ito Y, Nakashima Y, Matsuoka S. Rice bran extract containing acylated steryl glucoside fraction decreases elevated blood LDL cholesterol level in obese Japanese men. J Med Invest. 2015;62(1.2):80–4.
Nhung BT BT, Tuyen LD, Linh VA, Nguyen DV, Nga TT, Thuc VT, et al. Rice bran extract reduces the risk of atherosclerosis in post-menopausal Vietnamese women. J Nutr Sci Vitaminol. 2016;62(5):295–302.
Abd El-Wahab HM, Mohamed MA, El Sayed HH, Bauomy AE. Modulatory effects of rice bran and its oil on lipid metabolism in insulin resistance rats. J Food Biochem. 2017;41(1).
Boonloh K, Lee ES, Kim HM, Kwon MH, Kim YM, Pannangpetch P, et al. Rice bran protein hydrolysates attenuate diabetic nephropathy in diabetic animal model. Eur J Nutr. 2018;57(2):761–72.
Senaphan K, Sangartit W, Pakdeechote P, Kukongviriyapan V, Pannangpetch P, Thawornchinsombut S, et al. Rice bran protein hydrolysates reduce arterial stiffening, vascular remodeling and oxidative stress in rats fed a high-carbohydrate and high-fat diet. Eur J Nutr. 2018;57(1):219–30.
Ogawa Y, Shobako N, Fukuhara I, Satoh H, Kobayashi E,Kusakari T, et al. Rice bran supplement containing a functional substance, the novel peptide Leu-Arg-Ala, has anti-hypertensive effects: A double-blind, randomized, placebo-controlled study. Nutrients. 2019;11(4):726.
Justo ML, Rodriguez-Rodriguez R, Claro CM, Alvarez de Sotomayor M M, Parrado J J, Herrera MD MD. Water-soluble rice bran enzymatic extract attenuates dyslipidemia, hypertension and insulin resistance in obese Zucker rats. Eur J Nutr. 2013;52(2):789–97.
Hongu N, Kitts DD, Zawistowski J, Dossett CM, Kopeć A, Pope BT, et al. Pigmented rice bran and plant sterol combination reduces serum lipids in overweight and obese adults. J Am Coll Nutr. 2014;33(3):231–8.
Zhang H, Wang J, Liu Y, Gong L, Sun BJF. Rice bran proteins and their hydrolysates modulate cholesterol metabolism in mice on hypercholesterolemic diets. Food Funct. 2016;7(6):2747–53.
Qureshi AA, Bradlow BA, Salser WA, Brace LD. Novel tocotrienols of rice bran modulate cardiovascular disease risk parameters of hypercholesterolemic humans. J Nutri Biochem. 1997;8(5):290–8.
Qureshi AA, Sami SA, Salser WA, Khan FA. Synergistic effect of tocotrienol-rich fraction (TRF25) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J Nutr Biochem. 2001;12(6):318–29.
Qureshi AA, Sami SA, Salser WA, Khan FAJA. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002;161(1):199–207.
Berger A, Rein D, Schäfer A, Monnard I, Gremaud G, Lambelet P, et al. Similar cholesterol–lowering properties of rice bran oil, with varied γ–oryzanol, in mildly hypercholesterolemic men. Eur J Nutr. 2005;44(3):163–73.
Wilson TA, Nicolosi RJ, Woolfrey B, Kritchevsky DJ. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters. J Nutr Biochem. 2007;18(2):105–12.
Jolfaie N, Rouhani M, Surkan P, Siassi F, Azadbakht LJH, Research M. Rice bran oil decreases total and LDL cholesterol in humans: a systematic review and meta-analysis of randomized controlled clinical trials. Hormone Metabol Res. 2016;48(07):417–26.
Pourrajab B, Sohouli MH, Amirinejad A, Fatahi S, Găman M-A, Shidfar F. The impact of rice bran oil consumption on the serum lipid profile in adults: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2022;62(22):6005–15.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Kestin M, Moss R, Clifton PM, Nestel PJ PJ. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am J Clin Nutr. 1990;52(4):661–6.
Hegsted M, Windhauser MM, Morris SK, Lester SB. Stabilized rice bran and oat bran lower cholesterol in humans. Nutr Res. 1993;13(4):387–98.
Gerhardt AL, Gallo NB. Full-fat rice bran and oat bran similarly reduce hypercholesterolemia in humans. J Nutr. 1998;128(5):865–9.
Matani F, Aminpoor A, Tabibi H. Comparison of the effects of rice bran and wheat bran on serum lipid profiles in hypercholesterolemic women. Tehran Univ Med J TUMS Pub. 2006;64(7):17–27.
Rondanelli M, Opizzi A, Monteferrario F, Klersy C, Cazzola R, Cestaro B. Beta-glucan-or rice bran-enriched foods: a comparative crossover clinical trial on lipidic pattern in mildly hypercholesterolemic men. Eur J Clin Nutr. 2011;65(7):864–71.
Borresen EC, Brown DG, Harbison G, Taylor L, Fairbanks A, O’Malia J, et al. A randomized controlled trial to increase navy bean or rice bran consumption in colorectal cancer survivors. Nutr Cancer. 2016;68(8):1269–80.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2019;366: l4898.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):1–10.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Mitchell MN. Interpreting and visualizing regression models using Stata: Stata Press College Station, TX; 2012.
Begg CB, Mazumdar MJB. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088–101.
Egger M, Smith GD, Schneider M, Minder CJB. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Beg Z, Timani K, Iqbal J, Minhajuddin M, editors. Tocotrienols (vitamin E): Hypolipidemic impacts on cholesterol dynamics of a type II familial hypercholesterolemic patients with severe xanthomas. FASEB JOURNAL; 1997: FEDERATION AMER SOC EXP BIOL 9650 ROCKVILLE PIKE, BETHESDA, MD 20814–3998 USA.
Bhaskaragoud G, Rajath S, Mahendra V, Kumar GS, Krishna AG, Kumar GS, et al. Hypolipidemic mechanism of oryzanol components-ferulic acid and phytosterols. Biochem Biophys Res Commun. 2016;476(2):82–9.
Misawa K, Jokura H, Shimotoyodome AJF, Research N. Rice bran triterpenoids improve postprandial hyperglycemia in healthy male adults: a randomized, double-blind, placebo-controlled study. Food Nutr. 2018;62.
Tsuji H, Nomiyama K, Ikeda K, Kawatoko T, Cai J, Fujishima M, et al. Effects of rice bran fiber and cholestyramine on peripheral blood cells and biochemical parameters in Yusho. Fukuoka Igaku Zasshi. 1991;82(5):330–4.
Sanders T. Reddy S The influence of rice bran on plasma lipids and lipoproteins in human volunteers. Eur J Clin Nutr. 1992;46(3):167–72.
Chen C-W, Cheng H-H, editors. Rice bran improves glycemic control, increases insulin secretion, and lowers free fatty acids and LDL cholesterol levels in type 2 diabetics. ANNALS OF NUTRITION AND METABOLISM; 2007: KARGER ALLSCHWILERSTRASSE 10, CH-4009 BASEL, SWITZERLAND.
Dans AL, Florete O, Paz E, Tamesis B, Anonuevo J, Zarcilla F, et al. The Efficacy, Safety, and Acceptability of High-Fiber Rice-Bran Diet (Darak) In the Control of Diabetes Mellitus. Medicine. 2013;51(2):39–50.
Cara L, Dubois C, Borel P, Armand M, Senft M, Portugal H, et al. Effects of oat bran, rice bran, wheat fiber, and wheat germ on postprandial lipemia in healthy adults. Am J Clin Nutr. 1992;55(1):81–8.
Dubois C, Cara L, Borel P, Armand M, Senft M, Portugal H, et al. Cereal dietary fibers affect post-prandial lipoproteins in healthy human subjects. Carbohydrate Polymers. 1993;21(2–3):189–94.
Barkate M, Windhauser M, Schorle S, Richard S, Volaufova J, Lefevre H. The effect of chronic defatted rice bran consumption on postprandial lipemia. FASEB J. 1996;10(3):A256.
Kustiyah L, Dewi M, Damayanthi E, Dwiriani CM, Alamsah DJSICS. Lipid profile improvement of overweight-obese adults after high antioxidant tomato and rice bran drinks intervention. SCOPUS IJPHRD CITATION SCORE. 2019;10(6):569.
Borresen EC, Jenkins-Puccetti N, Schmitz K, Brown DG, Pollack A, Fairbanks A, et al. A pilot randomized controlled clinical trial to assess tolerance and efficacy of navy bean and rice bran supplementation for lowering cholesterol in children. Global Pediatr Health. 2017;4:2333794X17694231.
Saphyakhajorn W, Sapwarobol S, Hudthagosol C, Suttisansanee U, editors. Effect of Defatted Rice Bran Powder Supplementation on Blood Glucose and Lipid Profile in Overweight and Obese Adults with Hypercholesterolemia. ANNALS OF NUTRITION AND METABOLISM; 2019: KARGER ALLSCHWILERSTRASSE 10, CH-4009 BASEL, SWITZERLAND.
United States Department of Agriculture. Rice bran, crude. [US Department of Agriculture.]. Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169713/nutrients.
United States Department of Agriculture. Oat bran, raw. [US Department of Agriculture.]. Available from: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168872/nutrients.
Daou C. Zhang H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J Food Sci Technol. 2014;51(12):3878–85.
dos Santos OM, Feddern V, Kupski L, Cipolatti EP, Badiale-Furlong E. de Souza-Soares LA. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Biosource Technol. 2011;102(17):8335–8.
Rao RSP, Muralikrishna G. Non-starch polysaccharide–phenolic acid complexes from native and germinated cereals and millet. Food Chem. 2004;84(4):527–31.
Gunness P, Gidley MJ. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Function. 2010;1(2):149–55.
Nie Y, Luo F, Wang L, Yang T, Shi L, Li X, et al. Anti-hyperlipidemic effect of rice bran polysaccharide and its potential mechanism in high-fat diet mice. Food Function. 2017;8(11):4028–41.
Theuwissen E, Mensink RPJP. behavior. Water-soluble dietary fibers and cardiovascular disease. Physiol Behav. 2008;94(2):285–92.
Nie Y, Luo F. Dietary fiber: an opportunity for a global control of hyperlipidemia. Oxidative Med Cell Longevity. 2021;2021.
Lovegrove A, Edwards C, DeNoni I, Patel H, El S, Grassby T, et al. Role of polysaccharides in food, digestion, and health. Crit Rev Food Sci Nutr. 2017;57(2):237–53.
Wang J, Shimada M, Kato Y, Kusada M, Nagaoka SJB. Cholesterol-lowering effect of rice bran protein containing bile acid-binding proteins. Biosci Biotechnol Biochem. 2015;79(3):456–61.
Most MM, Tulley R, Morales S, Lefevre M. Rice bran oil, not fiber, lowers cholesterol in humans. Am J Clin Nutr. 2005;81(1):64–8.
Raghuram T, Rukmini C. Nutritional significance of rice bran oil. Indian J Med Res. 1995;102:241–4.
Sugano M, Tsuji E. Rice bran oil and cholesterol metabolism. J Nutr. 1997;127(3):521S-S524.
Seetharamaiah G, Krishnakantha T. Chandrasekhara N. Influence of oryzanol on platelet aggregation in rats. J Nutr Sci Vitaminol. 1990;36(3):291–7.
Xu Z, Hua N, Godber JS. Antioxidant activity of tocopherols, tocotrienols, and γ-oryzanol components from rice bran against cholesterol oxidation accelerated by 2, 2 ‘-azobis (2-methylpropionamidine) dihydrochloride. J Agric Food Chem. 2001;49(4):2077–81.
Khor H, Chieng D, Ong K. Tocotrienols inhibit liver HMG CoA reductase activity in the guinea pig. Nutr Res. 1995;15(4):537–44.
Thor H, Ng T. Effects of administration of α-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int J Food Sci Nutr. 2000;51(sup1):s3–11.
Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JK. Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem. 1992;35(20):3595–606.
Qureshi AA, Mo H, Packer L, Peterson DM. Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J Agric Food Chem. 2000;48(8):3130–40.
Aronow WS. Lipid Disorders in the Elderly. In: Martini L, editor. Encyclopedia of Endocrine Diseases. New York: Elsevier; 2004. p. 178–81.
Acknowledgements
Not applicable.
Deviations from the protocol
It should be noted that in order to improve the quality of this systematic review and meta-analysis, we made some corrections during the study, which caused deviations from the protocol registered in PROSPERO:
- In addition to the 4 mentioned databases (i.e. PubMed/Medline, Scopus, ISI Web of Science, and Google Scholar), we also performed a manual check to avoid missing eligible articles.
- Subgroup analysis based on country (USA or non-USA) was not performed due to the discretion of the journal reviewers to reduce the large number of subgroups.
- In order to analyze the impact of primary BMI more accurately, in the subgroup analysis, instead of two groups (< 30 kg/m2 or more), it was divided into three groups (18.5–24.9 kg/m2), (25–29.9 kg/m2) or (> 30 kg/m2).
Funding
This work received no specific external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization (ZH, GS); data curation (ZH, FA); formal analysis (ZH, FA, SS); investigation (ZH, FA, ZY); methodology (ZH, FA, ZY); project administration (ZH, FA); writing—original draft (ZH, SS, ZY); and writing—review & editing (ZY, SS). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not required as there was no individual patient data used.
Consent for publication
Not applicable.
Competing interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Risk of bias assessment. Table S2. GRADE profile of rice bran supplementation for lipid profile. Figure S2.A. Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effect of rice bran supplementation on triglycerides (TG). Figure S2.B. Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effect of rice bran supplementation on total cholesterol (TC). Figure S2.C. Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effect of rice bran supplementation on low density lipoprotein cholesterol (LDL-C). Figure S2.D. Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effect of rice bran supplementation on high density lipoprotein cholesterol (HDL-C). Figure S3. Funnel plot for the effect of rice bran supplementation on A) TG; B) TC; C) LDL-C and D) HDL-C. Abbreviations: TG: triglycerides; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol. Figure S4. Non-linear dose-response relations between dose of rice bran supplementation (g/day) and absolute mean differences in A) TG; B) TC; C) LDL-C and D) HDL-C. Abbreviations: TG: triglycerides; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol. Figure S5. Non-linear dose-response relations between duration of intervention (week) and absolute mean differences in A) TG; B) TC C) LDL-C and D) HDL-C. Abbreviations: TG: triglycerides; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol. Figure S6. Bubble plots of the association between dose of rice bran (g/day) and weighted mean difference of A) TG; B) TC; C) LDL-C and D) HDL-C. The size of the bubbles is proportional to the accuracy of the estimate. Abbreviations: TG: triglycerides; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol. Figure S7. Bubble plots of the association between duration of intervention and weighted mean difference of A) TG; B) TC C) LDL-C and B) HDL-C. The size of the bubbles is proportional to the accuracy of the estimate. Abbreviations: TG: triglycerides; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol. Search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hariri, Z., Afzalzade, F., Sohrab, G. et al. The effects of rice bran supplementation for management of blood lipids: A GRADE-assessed systematic review, dose–response meta-analysis, and meta-regression of randomized controlled trials. Syst Rev 12, 65 (2023). https://doi.org/10.1186/s13643-023-02228-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-023-02228-y