Abstract
Key message
We elucidated the age trends of narrow-sense heritability and phenotypic/genetic correlations and the age–age genetic correlation of growth ring components of Cryptomeria japonica D. Don by investigating progenies of controlled crossings by soft X-ray densitometry analysis. Wood density in the C. japonica breeding program can be efficiently improved by selecting trees with a higher earlywood density and latewood percentage as early as forest stand ages of 5–9 years.
Context
Wood density within the trunk is affected by the intra-ring wood density profile and its age trend from pith to bark. Wood density can be efficiently improved by clarifying whether wood density and highly correlated intra-ring components are under additive genetic control.
Aims
The aim of this study was to elucidate the age trends of narrow-sense heritability and phenotypic/genetic correlations of growth ring components and investigate the possibility of early selection for improving wood density in Cryptomeria japonica.
Methods
We quantified seven growth ring components (width, density, earlywood/latewood width, average earlywood/latewood density, and latewood percentage) for 5112 annual rings of 342 18-year-old trees derived from 24 controlled pollinated full-sib families of C. japonica plus tree clones by soft X-ray densitometry analysis. Genetic parameters and correlations among the seven growth ring components were analyzed using a linear mixed model and the breedR package.
Results
Earlywood density and latewood percentage exhibited a higher phenotypic and genetic correlation with ring density than the other ring components at almost all ages. Earlywood density and latewood percentage exhibited a lower correlation with ring width than the other ring components after a stand age of 5 years. The age–age genetic correlation of earlywood density and latewood percentage was 0.70 for a stand age of 17 years and was strong at stand ages of 5 and 9 years.
Conclusion
Efforts focusing on improving earlywood density and latewood percentage may contribute to improving wood density efficiently in tree breeding programs for C. japonica. Traits related to the ring density of C. japonica trees can possibly be selected as early as stand ages of 5–9 years, and the required period for progeny selection may be substantially shortened when selecting trees with high wood density.
Similar content being viewed by others
1 Introduction
Wood density is an important trait influencing the quality of wood and the quantity of wood biomass produced. Wood density is considered the basic trait when selecting wood as a material (Zobel and van Buijtenen 1989) and is an essential variable for the estimation of carbon sequestration potential in forests and trees (Roxburgh et al. 2006; Tamura et al. 2006). The amount of carbon sequestered as xylem in a tree trunk is expressed as the product of trunk volume, basic density, and carbon content. Since the carbon content varies little in C. japonica, it is possible that wood density and trunk volume alone control carbon sequestration at the tree trunk level (Tamura et al. 2006). Therefore, the importance of wood density has been recognized for a long time in most forest tree breeding programs (Zobel 1961), and there has also been extensive research on the genetic control of wood density (Zobel and Jett 1995). Furthermore, the relationships between growth characteristics and wood density have been intensively examined, and it is important to understand them (Zobel and Jett 1995). Previous studies have shown cases of negative genetic correlations between growth characteristics and wood density in some coniferous species (e.g., Picea mariana, − 0.35; Pinus sylvestris, − 0.42 ~ − 0.49; Larix gmelinii × L. kaempferi, − 0.57), indicating the difficulty of achieving compatibility (Zhang 1998; Fries and Ericsson 2006; Fujimoto et al. 2008; Hong et al. 2014). On the other hand, the strength of the negative relationship varies and sometimes weakens with age in some tree species when focusing on the genetic correlation between growth traits and wood density at the annual ring level (Zhang 1998; Fukatsu et al. 2013).
Wood density within the trunk is affected by the intra-ring wood density profile, from earlywood to latewood. The relationship between the intra-ring wood density profile and wood density is species-specific. For instance, latewood percentage (LWP) in the genus Larix (e.g., Fujimoto et al. 2008) or earlywood density (ED) in Pinus sylvestris (Hong et al. 2014) has a large influence on wood density, whereas both ED and LWP have such an influence in Picea mariana (Zhang 1998) and Pinus pinaster (Louzada 2003) (Table 1). Wood density can be efficiently improved by clarifying whether wood density and highly correlated intra-growth ring components are under additive genetic control for each tree species.
Age trends of the intra-ring wood density profile also modulate wood density (Hylen 1999; Kumar and Lee 2002; Li and Wu 2005). Previous studies on age trends (radial change from pith to bark) of the intra-ring wood density profile have shown that those of Larix kaempferi and Pinus radiata are weaker near the pith and stronger towards bark (Li and Wu 2005; Fukatsu et al. 2013), while that of Picea mariana is stronger near the pith and weaker towards the bark (Zhang 1998). Understanding the age trends of intra-growth ring components, which are under additive genetic control, may allow for the early selection of trees with superior trunk wood density (Kumar and Lee 2002).
Cryptomeria japonica D. Don is a major forest tree species in Japan, planted over 4.4 million ha, accounting for approximately 44% of the artificial forest area (Forestry Agency 2019). C. japonica lumber is often used in construction as a structural material. The simultaneous improvement of growth characteristics and wood density of C. japonica is especially important for the breeding program of this species. The selection of trees with excellent wood density and growth performance requires information on the narrow-sense heritability and genetic correlation of growth characteristics and wood density and the age trend of these genetic parameters. Several previous studies have quantified the radial variation of phenotypic values and heritability for basic density at the tree trunk level based on block samples by dividing every 5-year ring in C. japonica (Tamura et al. 2006; Fukatsu et al. 2011; Yasuda et al. 2021). However, age-related trends in the phenotypic values and heritability in more detailed growth ring components, such as earlywood and latewood, have not been assessed in C. japonica. Clarifying which ring component and which age could be targeted to improve wood density would help in applying early selection to C. japonica.
In the present study, we evaluated the radial variation and narrow-sense heritability of growth ring components and the phenotypic/genetic correlations of the growth ring components to improve both growth characteristics and wood density using full-sib families of C. japonica plus tree clones. Moreover, we evaluated the relations for the early selection of growth ring components related to wood density. Using age–age genetic correlation analysis, we aimed to examine whether traits at the maturity stage can be predicted at a juvenile stage to shorten the rotation period.
2 Materials and methods
2.1 Sample trees
In this study, tree samples were collected from 342 full-sib progenies consisting of 24 full-sib families derived from four sets of 4 × 4 half-diallel crosses using 16 C. japonica plus tree clones (Table 2). The progeny test site used in this study was established in 1995 at the Forest Tree Breeding Center (36.69°N, 140.69°E), Forestry and Forest Products Research Institute, Hitachi City, Ibaraki Prefecture. The progeny test was designed to include randomized blocks of six replicates with an inter-tree distance of 1.8 × 1.8 m (3000 trees/ha), mainly consisting of 45 full-sib families derived from eight sets of 4 × 4 half-diallel crosses using 32 C. japonica plus tree clones (Yasuda et al. 2021). The number of trees in the experimental design was 2046 before thinning. Thinning was implemented in 2012 with an orthorhombic lattice pattern without regard to tree size (forest stand age was 18 years). During the process of preparing thin sections for densitometric analysis, samples for which it was difficult to measure and calculate each parameter were excluded, resulting in differences in the number of samples among the parameters (Table 2).
2.2 Quantifying growth ring components
A 10-cm-thick disk at breast height was collected from each tree stem, and two 5-mm-thick strip samples with different radial directions including the pith were prepared from each disk. The strip samples were placed in a room with a constant temperature (20 °C) and relative humidity (60%) for air-drying for 8 months. Then, the strip samples were placed on an X-ray-sensitive film (Industrial X-ray film Enveropak IXFR; Fujifilm Co., Tokyo, Japan) and irradiated using a soft X-ray transmission device (Softex Co., Tokyo, Japan). The developed film was digitized at a resolution of 2400 dpi using a film scanner (GT-X900; Seiko Epson Co., Suwa, Japan).
Growth ring components in each growth ring were measured by soft X-ray densitometry techniques (Mothe et al. 1998; Jacquin et al. 2017). From the acquired image data, seven growth ring components, i.e., growth ring width (RW), RD, earlywood/latewood width (EW, LW), average ED and latewood density (LD), and LWP (percentage of latewood width to growth RW [LWP]), were quantified using a software package (WinDENDRO, Regent Instruments Inc., Quebec City, Canada) with a path width of 2.0 mm in the tangential direction and a resolution of approximately 10 μm in the radial direction. The demarcation between earlywood and latewood was defined as the point where the density value was 0.55 g/cm3 within each annual ring (Ohta 1970; Polge 1970; Mothe et al. 1998; Jacquin et al. 2017). In this study, the analysis of each growth ring component was based on stand age (Fukatsu et al. 2013) rather than cambial age because the numbers of growth rings in the collected discs were different due to variation in the tree age at which the sample trees reached breast height. This “stand age-based analysis” had the advantage of eliminating the effects of annual weather variations (Fukatsu et al. 2013). Regarding the data for ages of 1, 2, and 18 years, we found ambiguity (xylem formation was not completely ceased at the ring of age 18 years, the outermost growth ring, in some samples), and these data were excluded from the statistical analysis.
2.3 Statistical analysis
Phenotypic correlations between growth ring components were analyzed by calculating Pearson’s correlation coefficients. The variance component for each trait was estimated using a linear mixed model (Eq. 1) with the restricted maximum likelihood method. Preliminary analyses for variance components indicated that block × non-additive genetic and block × additive genetic interactions (those corresponding to the between-block error) were not selected for most stand ages for all ring components in the model selection based on the Akaike information criterion (Akaike 1974). Therefore, in this study, all statistics (narrow-sense heritabilities and genetic correlations) were calculated using the following equation:
Here, Yijkl is the phenotypic value, μ is the general mean, bi is the fixed effect of block i, ajkl is the random additive genetic effect of individual j for parents k and l, skl is the random non-additive genetic family effect of the parent k and l combination, and eijkl is the random residual effect. The variances of the abovementioned parameters were estimated using the restricted maximum likelihood method. The contributions of the random factors were calculated by applying the animal model (White et al. 2007) of best linear unbiased prediction using the “add_animal” option of the R package breedR (Munoz and Sanchez 2020).
The random effects were assumed to be distributed as follows in Eq. (2):
where G, S, and R are the variance‒covariance matrices of a, s, and e, respectively. However, G = A* σ2a. Here, A is an additive relation matrix that is calculated from the pedigree information.
The random effects are estimated by best linear unbiased prediction (BLUP), which is calculated by solving the mixed model equations given by Eq. (3):
where X and Z are the design matrixes.
The narrow-sense heritability (h2) of each trait was estimated using Eq. (4).
Here, σ2a, σ2s, and σ2e are the variance components of the additive genetic, non-additive genetic, and residual portions, respectively.
The genetic correlation between trait x and trait y was estimated using Eq. (5).
Here, r is the genetic correlation coefficient of trait x and trait y, COV is the covariance parameter of additive genetic effect between trait x and trait y, and σ2g(x) and σ2g(y) are the variance components of additive genetic effects for trait x and trait y, respectively. Age–age genetic correlation analyses were conducted between the stand age of 17 years and younger ages using Eq. 5. Genetic parameters were evaluated using breedR in R 4.0.2. (R Core Team 2020). The approximate standard errors of heritability and genetic correlations were calculated according to Falconer and Mackay (1996, pages 180 and 316).
3 Results
3.1 Basic statistics and age trends of seven growth ring components
The results are summarized in Table 3. The coefficients of variation (CVs) for growth components (RW, EW, and LW) (70.7–96.2%) were greater than those for density components (RD, ED, and LD) (10.0–17.5%). The CV for LW was the largest (96.2%) among the growth components. Within the density components, the CV for ED was the largest (17.5%). The CV of LWP was 47.6%, which was intermediate among the CVs of all growth ring components.
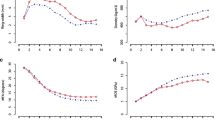
The values of RD and ED decreased as the age increased (RD was 0.364 g/cm3 and ED was 0.290 g/cm3 at a stand age of 17 years; Fig. 1a). However, LD increased towards the bark side (0.747 g/cm3 at the stand age of 17 years) and showed a variation pattern different from that of RD and ED (Fig. 1a). RW, EW, and LW were high at stand ages of 3–6 years (Fig. 1b) but decreased beginning at 7 years (Fig. 1b). LWP reached its maximum at 3 years (29.3%) and gradually decreased after 6 years, showing low values (between 14.9 and 20.0%) at ages of 7–17 years.
Age trends of the mean value for each ring component in all sample trees. Each figure shows changes in a density-related traits, b growth-related traits, and c latewood percentage. The abbreviations are the same as those in Table 3. Vertical lines indicate standard deviations
3.2 Phenotypic and genetic correlations between growth ring components
3.2.1 Relationships between growth ring components at the individual tree level
Estimates of phenotypic and genetic correlations between the growth ring components at the individual tree level are shown in Table 4. RD displayed strong and positive phenotypic (0.84–0.85) and genetic (0.83–0.89) correlations with ED and LWP. RW had negative correlations with RD (phenotypic, − 0.30; genetic, − 0.45) and ED (phenotypic, − 0.35; genetic, − 0.48) and positive correlations with EW (phenotypic, 0.97; genetic, 0.92).
3.2.2 Age trends in the relationships between RD and its components
ED and LWP showed a stronger positive phenotypic correlation with RD than the other ring components at all ages, except at 3 years (ED, 0.69–0.88; LWP, 0.68–0.86; Fig. 2b, f). Negative phenotypic correlations with RD were observed for RW at the ages of 3–6 years and for EW at the ages of 3–7 years (RW − 0.46 to − 0.80, EW − 0.48 to − 0.80), but they were weak at the subsequent ages (RW − 0.08 to − 0.26, EW − 0.26 to − 0.41) (Fig. 2a, c). There were almost no phenotypic correlations between RD and LW at the ages of 3–7 years (0.04–0.30), but there was a weak positive phenotypic correlation after an age of 8 years (0.35–0.51) (Fig. 2e). LD showed a slight phenotypic correlation with RD at all ages (− 0.13 to 0.21) (Fig. 2d).
Age trends of correlation coefficients between RD and other growth ring components. a Phenotypic correlation coefficients and b genetic correlation coefficients. The abbreviations are the same as those in Table 3. Vertical lines indicate standard errors
ED and LWP also showed a stronger positive genetic correlation with RD than the other ring components at all ages, except at 3 years (ED, 0.77–0.87; LWP, 0.70–0.94; Fig. 2h, l). A negative genetic correlation was observed between RW and RD at the ages of 3–4 years (− 0.52 to − 0.84), but it became weak or was almost absent at subsequent ages (− 0.39 to 0.12) (Fig. 2g). EW showed a negative genetic correlation with RD at the ages of 3–14 years (− 0.41 to − 0.84) (Fig. 2i). LW showed a positive genetic correlation with RD at the ages of 3–17 years (0.44–0.77) (Fig. 2k). LD showed positive and negative genetic correlations with RD at the ages of 3 and 5–7 years (− 0.47, 0.47–0.48), but the genetic correlation was absent at other ages (− 0.33 to 0.15) (Fig. 2j).
3.2.3 Age trends in the relationships between RW and other ring components
EW exhibited a strong positive phenotypic correlation with RW at all ages (0.93–0.99, Fig. 3b). LW exhibited the second strongest positive phenotypic correlation with RW after EW at 6–17 years (0.51–0.73, Fig. 3d). RW was correlated with ED, LD, and LWP depending on age, but the phenotypic correlation coefficients were lower than those for EW and LW overall (Fig. 3a, c, e).
Age trends of correlation coefficients between RW and other growth ring components. a Phenotypic correlation coefficients and b genetic correlation coefficients. The abbreviations are the same as those in Table 3. Vertical lines indicate standard errors
EW exhibited a strong positive genetic correlation with RW at all ages (0.85–0.98, Fig. 3g). LW and LD showed a large amount of variation in the genetic correlation with RW due to age (LW, − 0.55 to 0.64; LD, − 0.12 to 0.68) (Fig. 3h, i). Although ED and LWP showed a strong negative genetic correlation with RW at the age of 3 years (ED, − 0.70; LWP, − 0.80), this correlation was weak or almost absent after the age of 4 years (ED, − 0.44 to 0.10; LWP, − 0.42 to 0.12) (Fig. 3f, j).
3.3 Age trends of narrow-sense heritability and variance components for the seven growth ring components
The narrow-sense heritability (h2) values for RD, ED, and LWP were higher than those for the other ring components at almost all stand ages (Fig. 4a, c, g). The h2 values for RD and ED were 0.43 and 0.42 at the age of 8 years, respectively, and remained the same or even increased (0.30–0.49 and 0.37–0.49, respectively) at subsequent ages (Fig. 4a, c). The h2 value for LD was higher (0.24) at the age of 9 years but lower at all other ages (h2 = 0.00–0.18; Fig. 4e). The h2 values for RW, EW, and LW were low at all ages (0.01–0.24 for RW, 0.02–0.27 for EW, and 0.00–0.26 for LW; Fig. 4b, d, f). The h2 value for LWP decreased until the age of 5 years but increased and remained at 0.30 (0.20–0.40) after the age of 6 years (Fig. 4g).
Age trends in the narrow-sense heritability of each growth ring component. The abbreviations are the same as those in Table 3. Vertical lines indicate standard errors
At most stand ages, the ratio of additive genetic variance was larger than that of non-additive genetic variance for all growth ring components (Fig. 6 in Appendix). In some stand age and growth ring component combinations, such as LW at the ages of 5 and 6 years, the non-additive genetic variance proportions were higher than the additive genetic variance proportions.
3.4 Age–age genetic correlations of growth ring components
We conducted age–age genetic correlation analyses between the stand age of 17 years and younger ages for RD, ED, and LWP because ED and LWP showed strong positive correlations with RD and exhibited higher narrow-sense heritability at almost all stand ages. RD and ED showed a strong positive age–age correlation at a stand age of 5 years (0.68 and 0.70, respectively, Fig. 5a, b), and the correlation became stronger at subsequent ages. LWP exhibited a strong positive age–age correlation at 9 years and above (0.70, Fig. 5c). ED exhibited a stronger age–age correlation at younger ages than LWP.
Age–age genetic correlation between a stand age of 17 years and younger ages. The abbreviations are the same as those in Table 3. Vertical lines indicate standard errors
4 Discussion
4.1 Age trend of growth ring density
In general, wood density fluctuates in the radial direction from the pith to the bark side. The growth ring density (RD) of C. japonica was high near the pith and tended to decrease towards the bark in this study (Fig. 1a). This pattern was consistent with that in previous reports for C. japonica (Fujisawa et al. 1993; Fujiwara 2007). The LW around the pith (ages 3–6 years) was higher, and therefore, RD around the pith (ages 3–6 years) was higher (Fig. 1b). Similar radial changes in RD were found in Picea species (Zhang 1998; Hylen 1999). However, different age trends of RD were also reported in other coniferous species. In Larix (Fukatsu et al. 2013; Fujimoto et al. 2006, 2008) and Pinus (Fries and Ericsson 2006, 2009; Li and Wu 2005) species, the RD near the pith was lower and tended to increase towards the bark. These age trends of RD were different from those observed for C. japonica in this study. This difference in the age trends of RD between tree species can be attributed to the pattern of shoot formation or branching pattern at the species level. For example, the timing of peaks in shoot elongation differs between conifer species grown in the same forest area (Cuny et al. 2012). The species-specific characteristics of shoot formation or branching patterns may influence the amount of cell wall formation per annual ring area as a result of differences in the load on the trunk (the above-ground part that supports the entire tree body) at the juvenile and mature stages.
4.2 Inheritance of each growth ring component in C. japonica
Regarding wood density traits measured at the tree trunk level, reports showed the high heritabilities, including high broad- and narrow-sense heritabilities, of Pilodyn penetration depth in standing trees (h2 = 0.43 ~ 0.71) and basic density in disks (h2 = 0.50 ~ 0.78) (Tamura et al. 2006; Fukatsu et al. 2011; Yasuda et al. 2021). However, there are no reports on narrow-sense heritability for traits related to wood density at a finer scale, i.e., growth ring components, of C. japonica. The present study showed that the RD of individuals of C. japonica plus tree clones has high additive genetic variance, low non-additive genetic variance (Fig. 6 in Appendix), and high narrow-sense heritability at each stand age (Fig. 4a). The high narrow-sense heritability of RD is believed to be the basis of the high heritability of basic density and air-dried density, which are the average values of the densities, including multiple annual rings, in the progeny trials created by a half-diallel cross design in C. japonica (Takahashi et al. 2020; Yasuda et al. 2021). These results suggest that the genetically improved planting materials supplied through mass selection and seed orchards is promising for increasing the wood density of C. japonica.
At each age, RD exhibited higher narrow-sense heritability than RW. In earlywood, ED showed higher narrow-sense heritability than EW in each growth ring, and in latewood, the narrow-sense heritability was low for both LD and LW in each growth ring. Basic density and air-dried density show higher narrow-sense heritability than growth characteristics in C. japonica (Takahashi et al. 2020; Yasuda et al. 2021). Fujisawa et al. (1993) demonstrated that RD and ED have higher broad-sense heritability in each ring than other growth ring components, whereas LW and LD showed lower broad-sense heritability in each growth ring than other ring components in C. japonica. In general, wood density has higher heritability than growth characteristics in conifers such as P. radiata and P. pinaster (Zobel and Jett 1995; Louzada and Fonseca 2002; Wu et al. 2008). Similar results were observed in this study; therefore, in C. japonica, wood density would be under stronger genetic control than growth characteristics, as reported previously for other conifers. In contrast, LWP showed higher narrow-sense heritability than RW, EW, and LW (growth characteristics) after a stand age of 6 years. The heritability of LWP varies considerably depending on the coniferous species, for example, a heritability for P. taeda ranging from 0.25 to 0.92, because the range of variation in the response of latewood formation to environmental factors differs according to the species (Zobel and Buijtenen 1989). RD, ED, and LWP exhibited higher narrow-sense heritability than the other ring components, indicating that RD, ED, and LWP are more genetically controlled than the other components in C. japonica.
4.3 Relationships between RW, RD, and other growth ring components in C. japonica
EW exhibited a stronger correlation with RW than LW at all stand ages. EW is a trait that largely influences RW, that is, the radial growth rate (Fig. 3b, g). In C. japonica, Hirano et al. (2021) reported that EW showed a significant positive correlation with the increase in trunk biomass, but no significant positive correlation was found with LW. In this study, RW and RD showed a strong phenotypic/genetic correlation at stand ages of 3–4 years, but they showed a weak correlation at stand ages of 5 years and above (Fig. 2a, g). Li and Wu (2005) examined the relationships between annual ring traits using Pinus radiata and reported that a strong negative phenotypic/genetic correlation between RW and RD may lead to decreased wood density when trees are bred with an emphasis on growth traits. The results of this study suggest that RW and RD in C. japonica are almost independent at older ages, and both growth characteristics and wood density can be improved at the same time.
The results of phenotypic and genetic correlation analyses showed that ED and LWP were strongly related to RD in C. japonica (Fig. 2b, f, h, l). Furthermore, ED and LWP showed higher narrow-sense heritability than the other ring components at most stand ages (Fig. 4c, g). Hirakawa et al. (2003) also analyzed the phenotypic correlation coefficient of each growth ring component of first-generation C. japonica plus tree clones and reported that RD and LWP showed a strong correlation (no genetic correlation was shown). Therefore, improving ED and LWP would be effective for improving RD. For instance, in Pinus radiata, ED and LD were more related to the outermost RD than LWP in an analysis of open-pollinated families plus tree clones (Kumar and Lee 2002). In another study on Larix kaempferi in a 29-year-old stand, LD and LWP exhibited stronger correlations with RD after a stand age of 15 years than the other ring components (Fukatsu et al. 2013). Correlations between RD and other components in previous studies and in this study show that which parts (earlywood, latewood, or the proportions of the two) of the annual ring are more strongly affected by RD values vary depending on the species. These results indicate that the influence of each growth ring component on wood density varies significantly among species of conifers, indicating the need to determine more influential growth ring components for each tree species.
4.4 Selection of trees with better growth characteristics and wood density
Clarifying the genetic relationships between growth characteristics and wood density is a major objective in most forest tree breeding programs in several countries (Zhang and Morgenstern 1995; Hylen 1999; Louzada 2003; Li and Wu 2005; Hong et al. 2014). This study showed that RW, which is an indicator of radial growth amount, correlated weakly with RD, ED, and LWP at most stand ages in the individuals of C. japonica plus tree clones (Fig. 2a, g; Fig. 3a, e, f, j). On the other hand, the results clarified that the fluctuation of EW was important for the radial growth of C. japonica (Fig. 3b, g). Since earlywood is related to both growth characteristics and wood density in C. japonica, improving the earlywood part may enable the production of progeny with both superior radial growth and superior wood density. Furthermore, the higher narrow-sense heritability observed for ED than for the other growth ring components suggests that the progeny of C. japonica with high wood density can be selected by choosing parents with superior ED. Earlywood formation is closely linked with shoot formation (primary growth) in conifers (Huang et al. 2014), including C. japonica. The fact that earlywood density was found to be a heritable trait in C. japonica in this experiment could indicate that shoot formation phenology is also a genetically influenced trait if the timing of cell wall thickening in the earlywood is linked to shoot formation patterns or phenology. In other words, the phenology of shoot formation for C. japonica may vary between families (intraspecific variation), and further investigations of inter-family differences in the relationship between earlywood formation and shoot formation phenology are needed in the future.
4.5 Early selection for wood density improvement
In this study, we examined the age–age genetic correlation between younger stand ages and the stand age of 17 years for RD, ED, and LWP. As shown in Fig. 5, early selection for improving the wood density of C. japonica can be carried out at a stand age of approximately 5 years or above for RD and ED and at a stand age of approximately 9 years for LWP. Early selection on the basis of growth ring components related to the wood density at these stand ages would have an evident impact on shortening the breeding period in the selection of trees with higher wood density. In P. radiata, RD and ED showed high family mean age–age genetic correlations (> 0.80) at the age of 2–4 years, whereas LWP showed the lowest correlation coefficient among the traits at all ages (Kumar and Lee 2002). Studies on Pinus pinaster and Picea abies demonstrated that RD, ED, LD, and LWP were already high around the third year, counting from the pith (Hylen 1999; Louzada 2003; Gaspar et al. 2008). Although the age at which the wood density of C. japonica can be selected differs from that of other coniferous tree species by approximately 1–3 years, our results indicated that using ED as a selection criterion for wood density breeding may shorten the rotation time for progeny selection in C. japonica.
5 Conclusions
Age trends in narrow-sense heritability, phenotypic and genetic correlations between growth ring density and other growth ring components, and ring width and other growth ring components were clarified for the seven growth ring components using soft X-ray densitometry analysis based on full-sib families derived from half-diallel crosses of C. japonica plus tree clones in this study. Average ring density, earlywood density, and latewood percentage are indicated to be highly heritable traits in the juvenile wood of C. japonica. Regarding the correlations of the growth ring components, average ring density presented similar strong correlations with earlywood density and latewood percentage. As a prospect for future research, it will be important to know which kind of cell formation patterns occur in families with high earlywood density values as a possible application in forest tree breeding programs. Efforts focusing on improving earlywood density and latewood percentage could contribute to improving wood density efficiently in the forest tree breeding of C. japonica.
We also found that an emphasis on the earlywood was important for the genetic improvement of both annual ring width and density in C. japonica. In the future, it will be essential to investigate the relationship between the phenology of shoot formation and the earlywood cell formation pattern for genetic improvement of the earlywood parts and hence total ring growth rate and density.
Earlywood density and latewood percentage at a stand age of 17 years in the individuals of C. japonica plus tree clones can possibly be selected as early as stand ages of 5–9 years, and the required period for progeny selection may be substantially shortened. Moreover, the weak phenotypic and genetic correlations between ring width and average ring density suggested that the simultaneous improvement of growth characteristics and wood density would be possible in C. japonica breeding programs with the selection of families with superior wood biomass productivity and quality.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CV:
-
Coefficient of variation
- ED:
-
Earlywood density
- EW:
-
Earlywood width
- LD:
-
Latewood density
- LW:
-
Latewood width
- LWP:
-
Latewood percentage
- RD:
-
Ring density
- RW:
-
Ring width
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723
Cuny HE, Rathgeber CBK, Lebourgeois F, Fortin M, Fournier M (2012) Life strategies in intra-annual dynamics of wood formation: example of three conifer species in a temperate forest in north-east France. Tree Physiol 32:612–625
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th editon. Prentice Hall, Harlow
Forestry Agency (2019) Annual Report on Forest and Forestry in Japan. https://www.rinya.maff.go.jp/j/kikaku/hakusyo/. Accessed 7 Dec 2021
Fries A, Ericsson T (2006) Estimating genetic parameters for wood density of Scots pine (Pinus sylvestris L.). Silvae Genet 55:84–92
Fries A, Ericsson T (2009) Genetic parameters for earlywood and latewood densities and development with increasing age in Scots pine. Ann for Sci 66:1–8
Fujimoto T, Kita K, Uchiyama K, Kuromaru M, Akutsu H, Oda K (2006) Age trends in the genetic parameters of wood density and the relationship with growth rates in hybrid larch (Larix gmelinii var. japonica x L. kaempferi) F1. J for Res 11:157–163
Fujimoto T, Kita K, Kuromaru M (2008) Genetic control of intra-ring wood density variation in hybrid larch (Larix gmelinii var. japonica × L. kaempferi) F1. Wood Sci Technol 42:227–240
Fujisawa Y, Ohta S, Tajima M (1993) Wood characteristics and genetic variations in Sugi (Cryptomeria japonica) II. Variation in growth ring components among plus-trees clones and test stands. Mokuzai Gakkaishi 39:875–882
Fujiwara T (2007) Estimation of annual increase of oven-dried weight of Sugi (Cryptomeria japonica) trunks. Mokuzai Gakkaishi 53:99–103
Fukatsu E, Tamura A, Takahashi M, Fukuda Y, Nakada R, Kubota M, Kurinobu S (2011) Efficiency of the indirect selection and the evaluation of the genotype by environment interaction using Pilodyn for the genetic improvement of wood density in Cryptomeria japonica. J for Res 16:128–135
Fukatsu E, Tsubomura M, Fujisawa Y, Nakada R (2013) Genetic improvement of wood density and radial growth in Larix kaempferi: results from a diallel mating test. Ann for Sci 70:451–459
Gaspar MJ, Louzada JL, Aguiar A, Almeida MH (2008) Genetic correlations between wood quality traits of Pinus pinaster Ait. Ann for Sci 65:1
Hirakawa Y, Fujisawa Y, Nakada R, Yamashita K (2003) Wood properties of sugi clones selected from plus trees in Kanto breeding region. Bull FFPRI 2:31–41
Hirano Y, Saitoh TM, Fukatsu E, Kobayashi H, Muraoka H, Shen Y, Yasue K (2021) Relationships among radial growth of Cryptomeria japonica, carbon budget of a forest ecosystem, and climate factors in a cool temperate zone. Mokuzai Gakkaishi 67:117–128
Hong Z, Fries A, Wu HX (2014) High negative genetic correlations between growth traits and wood properties suggest incorporating multiple traits selection including economic weights for the future Scots pine breeding programs. Ann for Sci 71:463–472
Huang J, Deslauriers A, Rossi S (2014) Xylem formation can be modeled statistically as a function of primary growth and cambium activity. New Phytol 203:831–841
Hylen G (1999) Age trends in genetic parameters of wood density in young Norway spruce. Can J for Res 29:135–143
Jacquin P, Longuetaud F, Leban J-M, Mothe F (2017) X-ray microdensitometry of wood: a review of existing principles and devices. Dendrochronologia 42:42–50
Koga S, Zhang SY (2002) Relationships between wood density and annual growth rate components in balsam fir (Abies balsamea). Wood Fiber Sci 34:146–157
Kumar S, Lee J (2002) Age-age correlations and early selection for end-of-rotation wood density in radiata pine. For Genet 9:323–330
Li L, Wu HX (2005) Efficiency of early selection for rotatin-aged growth and wood density traits in Pinus radiata. Can J Res 35:2019–2029
Louzada JLPC (2003) Genetic correlations between wood density components in Pinus pinaster Ait. Ann for Sci 60:285–294
Louzada JLPC, Fonseca FMA (2002) The heritability of wood density components in Pinus pinaster Ait. and the implications for tree breeding. Ann for Sci 59:867–873
Mothe F, Duchanois G, Zannier B, Leban J-M (1998) Analyse microdensitométrique appliquée au bois : méthode de traitement des données utilisée à l’Inra-ERQB (programme Cerd). Annales Des Sciences Forestieres 55:301–313
Munoz F, Sanchez L (2020) breedR: Statistical methods for forest genetic resources analysts. R package version 0.12–5. https://github.com/famuvie/breedR. Accessed 15 Mar 2022
Ohta S (1970) Measurement of the wood density by the soft X-ray and densitometric technique. Wood Ind 25:27–29 ((In Japanese))
Polge H (1970) The use of x-ray densitometric methods in dendrochonology. Tree-Ring Bull 30:1–10
R Core Team (2020) A language and environment for statistical computing. R version 4.0.2. In: R Found Stat Comput Vienna, Austria. https://cran.r-project.org/bin/windows/base/old/3.6.1/. Accessed 22 Jun 2020
Roxburgh SH, Wood SW, Mackey BG, Woldendorp G, Gibbons P (2006) Assessing the carbon sequestration potential of managed forests: a case study from temperate Australia. J Appl Ecol 43:1149–1159
Takahashi Y, Ishiguri F, Aiso H, Takashima Y, Hiraoka Y, Iki T, Ohshima J, Iizuka K, Yokota S (2020) Inheritance of static bending properties and classification of load-deflection curves in Cryptomeria japonica. Holzforschung 75:1–9
Tamura A, Kurinobu S, Fukatsu E, Iizuka K (2006) An Investigation on the allocation of selection weight on growth and wood basic density to maximize carbon storage in the stem of sugi (Cryptomeria japonica D. Don) plus-tree clones. J Jpn for Soc 88:15–20 ((In Japanese with English abstract))
White TL, Adams WT, Neale DB (2007) Forest genetics. Cabi, Wallingford
Wu HX, Ivković M, Gapare WJ, Matheson AC, Baltunis BS, Powell MB, McRAE TA (2008) Breeding for wood quality and profit in Pinus radiata: a review of genetic parameter estimates and implications for breeding and deployment. New Zeal J for Sci 38(1):56–87
Yasuda Y, Iki T, Takashima Y, Takahashi M, Hiraoka Y, Mishima K (2021) Genetic gains in wood property can be achieved by indirect selection and nondestructive measurements in full-sib families of Japanese cedar (Cryptomeria japonica. D. Don) plus tree clones. Ann For Sci 78:50. https://doi.org/10.1007/s13595-021-01064-1
Zhang SY (1998) Effect of age on the variation, correlations and inheritance of selected wood characteristics in black spruce (Picea mariana). Wood Sci Technol 32:197–204
Zhang SY, Morgenstern EK (1995) Genetic variation and inheritance of wood density in black spruce (Picea mariana) and its relationship with growth: implications for tree breeding. Wood Sci Technol 30:63–75
Zobel BJ (1961) Inheritance of wood properties in conifers. Silvae Genet 10:65–96
Zobel BJ, Jett JB (1995) Genetics of wood production. Series in Wood Science, Berlin
Zobel BJ, van Buijtenen JP (1989) Wood variation: its causes and control. Series in Wood Science, Berlin
Acknowledgements
We would like to express our appreciation to all the staff of the Forest Tree Breeding Center of the Forestry and Forest Products Research Institute for their assistance in conducting this study. We would also like to thank Dr. Takeshi Fujiwara and the staff of the Woodworking Office of the Forestry and Forest Products Research Institute for their assistance in processing samples for soft X-ray densitometry.
Code availability
Not applicable.
Funding
This study was conducted with the support of an Agriculture, Forestry and Fisheries Research Promotion Project “Efficient breeding project for afforested tree species with excellent carbon storage capacity,” financed by the Ministry of Agriculture, Forestry and Fisheries.
Author information
Authors and Affiliations
Contributions
Conceptualization: Yuko Yasuda, Taiichi Iki; Methodology: Yuko Yasuda, Taiichi Iki, and Yuya Takashima; Formal Analysis: Yuko Yasuda, Taiichi Iki, and Yuya Takashima; Investigation: Taiichi Iki, Yuya Takashima, and Yuichiro Hiraoka; Resources: Taiichi Iki, Yuya Takashima, and Yuichiro Hiraoka; Data curation: Yuko Yasuda, Taiichi Iki, and Yuya Takashima; Writing of the original draft: Yuko Yasuda; Visualization: Yuko Yasuda; Supervision: Taiichi Iki, Yuya Takashima, Makoto Takahashi, Yuichiro Hiraoka, and Kentaro Mishima. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All co-authors viewed the final version of the manuscript and approved publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling editor: Jean-Michel Leban.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Age trends in the variance components of each growth ring component. The abbreviations are the same as those in Table 3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yasuda, Y., Iki, T., Takashima, Y. et al. Inheritance of growth ring components and the possibility of early selection for higher wood density in Japanese cedar (Cryptomeria japonica D. Don). Annals of Forest Science 81, 5 (2024). https://doi.org/10.1186/s13595-023-01212-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13595-023-01212-9