Abstract
In this systematic review, we highlight the differences between the male and female zebrafish brains to understand their differentiation and their use in studying sex-specific neurological diseases. Male and female brains display subtle differences at the cellular level which may be important in driving sex-specific signaling. Sex differences in the brain have been observed in humans as well as in non-human species. However, the molecular mechanisms of brain sex differentiation remain unclear. The classical model of brain sex differentiation suggests that the steroid hormones derived from the gonads are the primary determinants in establishing male and female neural networks. Recent studies indicate that the developing brain shows sex-specific differences in gene expression prior to gonadal hormone action. Hence, genetic differences may also be responsible for differentiating the brain into male and female types. Understanding the signaling mechanisms involved in brain sex differentiation could help further elucidate the sex-specific incidences of certain neurological diseases. The zebrafish model could be appropriate for enhancing our understanding of brain sex differentiation and the signaling involved in neurological diseases. Zebrafish brains show sex-specific differences at the hormonal level, and recent advances in RNA sequencing have highlighted critical sex-specific differences at the transcript level. The differences are also evident at the cellular and metabolite levels, which could be important in organizing sex-specific neuronal signaling. Furthermore, in addition to having one ortholog for 70% of the human gene, zebrafish also shares brain structural similarities with other higher eukaryotes, including mammals. Hence, deciphering brain sex differentiation in zebrafish will help further enhance the diagnostic and pharmacological intervention of neurological diseases.
Similar content being viewed by others
Introduction
In mammals, the development of the gonads begins with an undifferentiated gonad (bipotential gonad) that can develop into either a testis or ovary [1]. The decision to follow the testis or ovary pathway is primarily governed by the chromosome constitution (XX/XY), which was established while studying human sex-related diseases [2, 3]. In males, the presence of the sex determining region Y (SRY) gene on the Y chromosome drives testis differentiation [1, 4,5,6]. In the absence of Sry, ovarian development continues [1, 4] and consequently, ovarian differentiation is suggested to be the default pathway [7]. However, as indicated by Yao [8], it is premature to suggest that the ovary is a default pathway, as it could have its own active genetic or signaling pathway to regulate development. In addition, the bipotential gonad has also been observed in several fish species before sex differentiation, and several sex determination genes have also been identified, including dmY [9], gsdfY [10], sox3Y [11], amhy [12, 13], amhr2 [14], gdf6Y [15], dmrt1 [16, 17], and sdY [18, 19]. Other sex-differentiation mechanisms further improve our understanding of the gonadal sex differentiation process. It has been argued that the downstream signaling cascades and genes in zebrafish and other fish species show a pattern similar to that observed in mammals (reviewed in [20,21,22,23]). Many studies using exogenous compounds (hormones and chemicals) [24,25,26,27,28], morpholinos [29,30,31,32], and gene editing [33,34,35,36,37,38] have identified important genes, pathways and critical periods of gonadal development in fish species. Hence, the absence of a strong master regulator gene such as Sry in fish species allows for the easy manipulation of the sex differentiation outcome and in-depth study of the process.
The developing gonads secrete the steroid hormones testosterone (T) and estradiol (E2), which further differentiate the sex organs. The secreted T and E2 also organize the brain neuronal networks in a male- or female-specific manner. Similar to the ovary, the female brain has been suggested to be the default developmental pathway [39]. On the other hand, in males, testosterone masculinizes the developing brain, either directly through the activation of the androgen receptor (AR) or indirectly via the stimulation of estrogen receptors (ER) following the conversion of T into E2 [3, 40].
Recent findings suggest that human male and female brains display differential connectomic, methylomic and transcriptomic profiles [41, 42]. Despite extensive advances in neuroscience, the molecular mechanisms underlying these differences remain unclear. Pfeiffer in the 1930s and Phoenix et al. in the 1950s provided important information on brain sex differentiation. Pfeiffer transplanted ovaries into adult mice and noted cyclical activity only in mice that were castrated in the neonatal stage and not in the adult stage [43]. Phoenix et al. [3] working with guinea pigs, demonstrated that treating prenatal female guinea pigs with testosterone resulted in typical male behavior in adults, but this effect was not observed when testosterone was administered during the perinatal or adult stages. These and other studies have resulted in the formulation of the classical model of brain sex differentiation, which suggests that the gonadal steroid hormones (androgens and estrogens) are the main drivers for establishing male and female neural networks [3, 40]. The classical concept of brain sexual differentiation states that the chromosomal constitution (XX or XY) determines the type of gonad, and the hormones secreted by these organs program the brain neural network differently [3, 40]. In males, T directly or indirectly following conversion to E2 by aromatase results in the masculinization of the brain. In females comparatively high levels of alpha-fetoprotein bind to estrogen and protect the brain from masculinizing events [44,45,46] (Fig. 1).
Brain sex differentiation. The steroid hormones synthesized in the gonads act on the brains to differentiate it into male and female types. In mammals, it is also indicated that the gene expression in developing brains is sexually dimorphic prior to the action of gonadal hormones. This suggests that the early brain could signal the gonads, or it could pre-condition the brain so that testosterone (T) and estradiol (E2) act differentially in male and female brains. The brain sex differentiation process in fish species, including zebrafish is largely unknown. Unlike mammals, fish are born with a developed brain, which helps them to forage and escape predators. This could indicate that the developing fish brain signals the gonad to develop into either a testis or ovary and later the steroid hormones synthesized by the gonads further enhances the sex-specific differences in the brain
As indicated by Arnold and Breedlove [47], the organization and activation effect of steroid hormones (the classical theory) needs to be reevaluated as many elements are not taken into account. The classical model does not provide a complete picture, as the genetic differences are not considered; more importantly, hormones may not be the early determinant of sexual differentiation. There is evidence that prenatal brains can show sex-specific differences [48, 49]. Studies on mice and chickens indicate that the developing brains display differential gene expression prior to gonadal hormone action in the brain [48, 49]. This indicates that there are genetic differences in developing brains and that these differences result in the differential development of male and female brains. Sry is a master regulator gene that determines testis fate [4]. Sry expression can be detected at approximately 10.5 days post coitum (dpc) and peaks at 11.5 dpc in mouse gonads to initiate testis differentiation [50]. In the brain, Sry expression co-localizes with tyrosine hydroxylase, an enzyme involved in dopamine synthesis in the substantia nigra. The downregulation of Sry in the brain leads to reduced expression of tyrosine hydroxylase, suggesting a positive regulation by Sry [51]. The sex chromosomal constitution (XX or XY) of brain cells could also contribute to sexual dimorphism, as it has been reported that XXY and XYY boys develop testes, but show different social behaviors than XY boys [52]. A study on zebra finches showed that a hormone treatment in females could not fully masculinize the song center [53]. An aromatase inhibitor treatment induced testicular tissue in the genetic female zebra finches; however, the song system remained completely feminine [54]. The gynandromorphic zebra finch has one-half of its brain and body genetically male, and the other half is genetically female. Despite a bilateral body where the brain is under the influence of the same gonadal hormone, the finch still develops a histologically identifiable song center on the male side of the brain, whereas the female side of the brain remains feminine [55]. These studies further support the notion that genetic differences also play an essential role in orchestrating sex-specific neuronal networks apart from gonadal steroid hormones.
In contrast to mammals, where the gonads develop first and the secreted steroid hormones organize the brain, the opposite could be true in teleosts. This indicates that brain development prior to gonadal development is important for teleosts as they are independent right from the start and this could mean that the brain will determine the fate of the gonadal development [56]. However, recent studies on mouse and chicken brains indicate that the developing brains have sex-specific differences prior to the action of gonadal steroids on the brain [48, 49]. In zebrafish, depending on the expression of brain-specific aromatase (cyp19a1b), larvae can be segregated into high- and low-expression groups prior to gonadal differentiation [57,58,59]. However, these studies did not determine the sex of these two populations, and used the whole embryo for gene analysis, which could confound the analysis owing to signals from other tissues. Nonetheless, these studies indicate that the zebrafish brain exhibits sex-specific differences before gonadal development.
The Sry gene is the master regulator that drives testis differentiation [4]. An interesting question that remains unanswered is whether there is a similar master regulator gene that regulates brain sex differentiation. It has been argued that Sry originated from the brain determining gene Sry-type HMG box 3 (SOX3) [60]. Mutations in SOX3 cause an abnormal hypothalamic–pituitary–gonadal axis leading to male hypogonadism [61]. This suggests that the brain may harbor other important genes that regulate gonadal function and sex differentiation in a sex-specific manner.
The zebrafish is an ideal model system that holds promise to dissect the sex-specific differences in the brain as it offers several advantages over other models, including small size, short generation time, and easy gene manipulation. With recent advances in omics and gene editing technology, it has become possible to elucidate the signaling mechanisms in the zebrafish brain. Moreover, the zebrafish genome is well conserved with humans, and approximately 82% of the human disease genes are present in the zebrafish genome [62]. In some cases, zebrafish show higher conservation with humans than mice [62] and there is a high similarity between zebrafish and human brain organization [63]. Consequently, zebrafish has emerged as an outstanding model and its recent experimental use in neurological studies is significantly outnumbering those conducted in other model organisms [64].
There are many reviews on gonadal sex differentiation of zebrafish [20, 65, 66] and other teleosts [23, 67,68,69]; however, no systemic studies have summarized all available information on zebrafish brain sex differentiation. Hence, this study provides comprehensive information to further understand zebrafish brain sex differentiation and its associated challenges.
Zebrafish brain sex differentiation
Similar to mammals, the molecular mechanism of brain sex differentiation in teleost fish is not fully understood. Among vertebrates, fish exhibit the most diverse sex-determination systems. Some fishes are reported to have XX/XY system, while others have ZW/ZZ system for sex differentiation [9, 70]. In zebrafish, the presence of sex chromosomes was debatable, as many studies have failed to find a sex chromosomes [71,72,73,74] and only two studies showed the presence of sex chromosomes in zebrafish, with females being heterogametic (ZW/ZZ system) [75, 76]. A study by Anderson et al. [77] revealed that this could be because sex chromosomal analysis has mostly been performed in domesticated strains. The authors working with the wild zebrafish collected from nature showed that there is polymorphism in chromosome 4 with females being heterogametic, suggesting a ZW/ZZ system [77]. The same polymorphism has not been found in the domesticated strains [77]. Consequently, the domesticated strain relies on multiple genes for gonadal differentiation and a polygenic sex determination system has been proposed [20, 78]. The administration of exogenous androgens and estrogens from the early developmental stages is known to skew the sex ratio in zebrafish [24, 25, 28]. However, sex reversal has also been reported in adult zebrafish, where an aromatase inhibitor treatment for 5 months in adult female zebrafish resulted in the retraction of the ovaries and formation of testis-like structures filled with spermatozoa-like cells [79]. In foxl2, cyp17a1, and cyp19a1a (gonadal aromatase) knockout zebrafish, defects in ovarian differentiation were observed, and an estradiol treatment could rescue the phenotype [38, 80, 81]. More specifically, cyp19a1a knockout in zebrafish resulted in all-male populations, and an estradiol treatment could rescue the phenotype by promoting ovarian development [80]. This suggests that zebrafish gonads are plastic even after sexual maturation, and steroid hormones or their genetic regulators are necessary to maintain the internal sex organs. Similar to gonads, the zebrafish brain also shows plasticity. For instance, exposure to 11-ketotestosterone (11-KT) in adult female fish can change the sexual behavior to that of male fish [82]. Using cyp17a1 (which is involved in androgen synthesis) knockout zebrafish, we showed that a lack of androgen could lead to the development of an all-male population, and the mating behavior of the knockout males was also altered. A testosterone or 11-KT treatment on the cyp17a1-deficient fish at the adult stage restored the male-typical mating behaviors [83]. Germ cell numbers are also involved in brain sex differentiation, as the morpholino-mediated depletion of germ cells leads to an all-male population, but the brain transcript profile resembles that of the female brain transcript [29]. To date, steroid hormones have emerged as the main regulators of brain sex differentiation. However, the precise role of steroid hormones in organizing sex-specific neuronal networks remains unclear. Knockout studies of steroid hormone synthesizing genes have resulted in sex reversal; therefore, the direct effect of steroid hormones on the brain is unclear. The generation of brain-specific knockouts could help unravel the molecular mechanisms and key players involved in zebrafish brain sex differentiation.
The zebrafish is excellent for the study of different aspects of human physiology and has potential in unravelling the molecular mechanisms and etiology of human neurological diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), etc. [62,63,64, 84]. Zebrafish can help predict the outcome as well as the mechanisms of action following gene and pharmacological intervention in the zebrafish brain. The molecular mechanism of zebrafish brain sex differentiation and sex-specific differences are essential to better understanding these neurological issues. There are some limitations with zebrafish as a neurodegenerative disease model has certain limitations; for instance, in contrast to mammals, adult zebrafish display extensive neurogenesis capability and can regenerate their brain following traumatic injury [85,86,87,88]. This could present an obstacle in understanding the progression of neurodegenerative diseases.
Differences at the cellular level
Each brain cell type plays a specific role in maintaining brain homeostasis. For instance, microglia are known to regulate immune function, and sexual behavior, and they are also implicated in neurological diseases [89, 90]. Males and females are known to show sex-related differences in brain cell types: for instance, the rodent medial preoptic area that controls male sexual behavior has 2- to 3-fold more dendritic spines [91]. Microglia, the innate immune cells of the central nervous system, have been implicated to be involved in the shaping of neuronal networks. Female neonatal rat hippocampi show a higher number of microglia and phagocytic activity than the male hippocampi [92]. An analysis of the deposited RNA sequencing data showed that the microglia of mice have sex-specific gene expression [93]. Sex-specific differences at the cellular level have started to emerge in mammalian systems; however, similar information is lacking for the zebrafish model.
The adult zebrafish forebrain shows a sex-specific cell proliferation pattern; however, it is not known if these differences lead to differential neural organization [94]. It is also unknown at what stage this differential proliferation pattern is evident in zebrafish. Ampatzis et al. [94] used 5-bromo-2′-deoxyuridine (BrdU) and TUNEL assays to examine proliferative and apoptotic cells, and showed that the cell renewal properties in female zebrafish were higher in the medial zone of the telencephalic area, periventricular nucleus of the posterior tuberculum and the ventral part of the periventricular pretectal nucleus. In males, the dorsal zone of the periventricular hypothalamus showed higher activity [94]. The adult neurogenesis capability dictates neural plasticity; it has been suggested that fish species have higher plasticity than mammals. Apart from their high neurogenic potential, fish brains also have a high expression of aromatase (an enzyme that converts androgen to estrogens), which has been linked to neurogenesis [95]. In zebrafish, aromatase is localized in the radial glial cells of the forebrain, the pallial and subpallial regions, the preoptic area and the hypothalamus [96]. Radial glial cells are suggested to play a critical role in supporting the newly generated neurons by providing a scaffold for cell migration [97]. The radial cells are also shown to be the precursors of neuronal cells [98, 99]. Single-cell transcriptomics of male and female brains can further reveal critical sex-specific differences.
Hormonal differences
Sex steroids are strong neuromodulators that can regulate behavior; in turn, altered behavior can change steroid levels. In teleosts, including zebrafish, 11-KT is a potent androgen [100,101,102]. In teleosts, sexual behavior is primarily regulated by gonadal steroid hormones [82], however, prostaglandins are also known to affect sexual behavior [103]. It was indicated that the sex steroids released by the female fish act as pheromones to attract males; however, it has also been shown that males can release pheromones as well to attract females for mating [104, 105]. In the protogynous grouper, the expression of genes involved in steroidogenesis can be detected in the brain during gonadal differentiation [106]. In rainbow trout, the expression of cyp19a1b (the gene that codes for brain aromatase) and cyp11a1 (the gene that codes for the enzyme involved in converting cholesterol to steroids) is higher in male brains prior to gonad differentiation [107]. This suggests that steroid hormones in the brain can be produced prior to gonadal steroids in teleosts.
Hormonal analysis of the zebrafish brain showed that the E2 level is moderately high in the female brain, whereas, 11-KT is almost 2.5 times higher in males [29]. In another study, the testosterone levels were not significantly different between the male and female brains, but 11-KT was (average male level = 4725 pg/g and average female level = 91 pg/g) [83]. The brain can synthesize steroid hormones (also referred to as neurosteroids); however, how much of the measured steroid hormones are of the brain and gonadal origin is unclear. Different groups working on neurosteroids have shown that steroid hormones, including pregnenolone, dehydroepiandrosterone, and their sulfates are present in the mammalian brain [108,109,110]. Interestingly, these levels were maintained in the brain even after removing the adrenal and testis tissues. Using in vitro cell culture, Ruiz-Palmero et al. [111] demonstrated that neuronal cells can synthesize neurosteroids. Neurosteroid synthesis in the brain has also been observed in non-mammalian species, including birds and fish. Biochemical analysis revealed that zebrafish brain slices could convert pregnenolone to progesterone, suggesting a 3β-hydroxysteroid dehydrogenase isomerase activity [112].
Another sex hormone, progesterone, could also be important for brain functions, as in mammalian species; it acts as a pheromone [113] and promotes male and female sexual behavior [114,115,116]. The progesterone receptor (pgr) expression is sex-specific in the ventromedial nucleus of the hypothalamus (VMN) in rats [114]. The impact of progesterone on the sex-specific regulation of brain function has not yet been identified. However, progesterone exposure has been shown to negatively affect zebrafish reproduction and lower estradiol levels in females and testosterone and 11-KT levels in males [117]. Similar effects following exposure to synthetic progesterone, norethindrone, were also observed in other fish species, including fathead minnows and Japanese medaka [118]. In another study, a higher concentration of synthetic progesterone resulted in the masculinization of female fathead minnows [119]. This suggests that progesterone may also play an important role in the sex-specific regulation of zebrafish brain functions.
Using zebra finches (Taeniopygia guttata), Remage-Healey et al. [120] demonstrated that steroid levels in the forebrain fluctuate during social interactions. The fluctuation in the brain steroid levels was not related to the circulating levels, as the authors noted that when the male birds were given social stimuli, the circulating steroid level did not change but the estradiol level changed [120]. Recently, Nishiike et al. [121] reported that estrogen receptor 2b (esr2b) is the major determinant of sex-typical mating behavior and sexual preference in medaka, as the female medaka deficient in Esr2b are not receptive to males, but rather court females, despite retaining normal ovarian function with an unaltered sex steroid milieu. This emphasizes the role of the estradiol/Esr2b signaling pathway in determining sex-typical mating behaviors and sexual preference in teleosts. This is inconsistent with the esr2b-deficient female zebrafish, which showed normal mating and breeding behaviors [122]. The different observations in the esr2b-deficient medaka and zebrafish may be due to the divergent role of estrogen receptors in different species.
In our study of cyp17a1 knockout zebrafish, we observed that androgen is important for gonadal sex differentiation as all the knockouts developed into males [38]. Although, the knockouts developed into males, the behavior and brain transcript analyses suggested that proper androgen levels are critical to fully masculinize the male brain [83]. We further performed transcriptomic analysis on the brain samples to determine whether the mutant male brain was similar to the wild-type female brain. We found 218 differentially expressed genes (DEGs) common between cyp17a1 KO and male brains. Of these, oat (downregulated in the presence of testosterone) [123], mos and syt13 (female enriched genes) [124] were significantly downregulated in both cyp17a1 KO and male brains. Meanwhile, 75 DEGs were shared by female and cyp17a1 KO brains (for the complete list of DEGs, refer Additional file 1: Table S1). Interestingly, we observed that genes, including dio2 and igf1 were significantly downregulated in female and cyp17a1 KO brains compared to male brains. We further performed a principal component analysis to investigate whether there is a specific clustering among female, male and cyp17a1 KO brain transcriptional profiles. We found that male and cyp17a1 KO brains clustered together, whereas female brains showed a different clustering pattern (Fig. 2). Taken together, these findings indicate that the depletion of androgen impacts the brain transcriptomics; however, the overall transcript profile of the KO male fish is close to that of wild-type males. We anticipated that the profile would be very similar to that of females. We suggest that this may be due to the compensatory effect of another cyp17a gene (cyp17a2) in zebrafish.
Comparison of female, male and cyp17a1 KO brain transcriptomics. Zebrafish were maintained in a recirculating system in the Wuhan lab. Zebrafish at 90 dpf were anesthetized, and brain samples from wild-type females, wild-type males, and cyp17a1 KO males were isolated. The sex was determined by examining the gonadal samples under the microscope. The brain samples were sent to NanJing Personal Gene Technology Co., Ltd., for transcriptomic analysis. Raw data were assessed for quality control using FastQC (v0.11.5). Adapter sequences were removed using Trim Galore (v0.4.3). The zebrafish reference genome (GRCz11/v104.11, Apr.2018) and the reference Index (the GTF file) were downloaded from Ensembl. First, hisat2-build was used to index the reference genome, and then HISAT2 (version 2.2.4) was used to map the reads to the reference genome. Finally, the gene counts were summarized with feature Counts (Subread software, v 2.03). The differential expression analysis was performed with the DESeq2 package (v1.30.1) using a fold change of 2 and a p value cutoff of 0.05. All the differentially expressed genes are presented in Additional file 1: Table S1. Hierarchical clustering of DEGs was performed in R (version 4.1.2) using the heatmap package. Venn diagram (A), heatmap (B), PCA (C), and dendrogram (D). Three independent biological replicates were used and for each biological replicate, four brain samples were pooled. F, female, M, male, K, cyp17a1 KO
Zebrafish also show sex-specific differences in the expression of steroidogenic genes during the day–night cycle. The expression of cyp19a1b (neural aromatase) and cyp11b peaks at night only in males [125]. This suggests that the hormonal levels have different peak periods, which should be considered during hormonal or gene expression analysis.
Gene-level differences
The transcript analysis has revealed important sex-specific differences in the zebrafish brain. With the advancement of RNA sequencing and microarray techniques, it has become possible to screen for differences in almost the entire genome. Our lab has identified important genes that are expressed in a sex-specific manner in different regions of the male and female brains [82]. Among the 32 genes analyzed, 13 genes showed differential expression. The genes, including cyp19a1b (brain aromatase), esr1, esr2b, mtf, ptgds, ptgs2b, sirt1 and sod1 were highly expressed in females while cfos, dio2, gabbr1a, gabbr1b and igf1 were highly expressed in males [82]. The sex-specific expression of dio2 (deiodinase type 2) gene has been consistent in different studies [25, 29, 82, 83, 126]. The Dio2 enzyme is involved in the synthesis of thyroid hormone, it converts prohormone, thyroxine (T4) to the biologically potent hormone, triiodothyronine (T3). Thyroid hormone is involved in different aspects of brain development, as it regulates neurogenesis, neuronal migration, neuronal and glial differentiation, myelination and synaptogenesis [127, 128]. Thyroid hormone signaling has been found to be sex-specific in mouse brain [93, 129], but it is not known whether thyroid hormone is involved in induction of sex-specific signaling in the developing brain. The role of thyroid hormone in zebrafish is not entirely clear. In zebrafish, thyroid hormone disruption using goitrogen (methimazole) drives ovarian differentiation, whereas an exogenous treatment with T4 results in a male-biased population (sexing based on histological analysis) [130]. In a study by Houbrechts et al. [131] using whole-body dio2 knockout zebrafish, it was observed that reduced thyroid hormone levels lowered the steroid hormones (T, E2, and 11-KT) in the gonads and delayed reproduction. Interestingly, our study showed that in the steroid hormone synthesizing gene cytochrome P450, family 17, subfamily A1 (cyp17a1) knockout zebrafish, the dio2 expression in the brain was downregulated threefold in both RNA sequencing and qPCR, while a rescue experiment with androgens upregulated dio2 levels by twofold compared to the non-rescued fish [83]. This suggests that steroid and thyroid hormones are interlinked and that they are involved in the regulation of zebrafish brain sex differentiation. In contrast, Dio2 knockout mice had normal T3 levels and unimpaired reproductive capacity, despite the disruption of thyroid stimulating hormone (TSH) feedback regulation, [132]. Dio1 knockout mice also did not experience any effects on general health or reproductive capacity [133]. This suggests that in mice, Dio1 and Dio2 could compensate for each other’s loss.
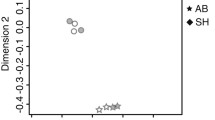
Depending on cyp19a1b expression, larvae can be segregated into high- and low-expression groups prior to gonadal differentiation [57,58,59], but these studies did not determine the sex of those two populations. Interestingly, some studies have shown high cyp19a1b expression in males [82, 126] and unbiased expression in the adult and juvenile zebrafish brains has also been reported [58, 134]. cyp19a1b expression was also not found to be sex-specific in our analysis using the deposited microarray data [135]. This suggests that cyp19a1b expression could be regulated by different factors and it could depend on the time of sample isolation, age, and strain. The microarray analysis of male and female brains of different age groups revealed that the sex-specific differences in the brain become more prominent as the fish mature [135]. In this study, the sex was determined by the visual observation of gonads, and fish without prominent eggs or testes were not used [135]. We analyzed the deposited microarray data [135], Gene Expression Omnibus (GSE53430) to further understand sex-specific differences. As indicated in the published study [135], we observed that male and female brains show distinct transcriptional profiles in both young (7.5- to 8.5-month-old fish) and adult (31- to 36-month-old fish) stages of life and start to be different from early on, but later in the adult stage, the differences are further noticeable (Fig. 3). We suggest that this could be due to the expression of different genes involved in steroid hormone biosynthesis in the male and female brains during the adult stage, as several genes including sult2st2 (sulfotransferase activity in steroid hormone biosynthesis), ugt1b5 (steroid hormone biosynthesis), cga (follicle hormone activity), fkbp5 and klf9 (glucocorticoid-responsive regulatory genes) [136,137,138] were differentially expressed in old female vs old male brains but not in young female vs young male brains. We further analyzed young female vs old female and young male vs old male transcriptomic data to understand whether steroid hormone biosynthesis-related genes differ during the adult stage in the same gender. We found that several steroid hormone biosynthesis-related genes such as hsd17b3 (synthesis of 11-KT), cyp39a1 (conversion of cholesterol to bile acid), apof (cholesterol transport and metabolic process), and rspo1 (ovarian germ cell differentiation) [137, 139,140,141] were differentially expressed in old male brains compared to young male brains. The expression of cyp27a1.4 (cholesterol metabolic process), cyp2aa7 (steroid hydroxylase activity), apoeb (lipid transport), cga and fkbp5 [136, 142, 143] were significantly different in old female brains compared to young female brains. Taken together, these data further confirm that male and female brains exhibit different transcriptomic patterns, and this difference becomes more distinctive during the adult stage.
The zebrafish brain shows sex- and age-dependent differences. Zebrafish microarray data from NCBI were downloaded and analyzed using the Partek Genomic Suit software. The data suggest that both the young and old brain zebrafish brains show sex-specific differences. Within the same sex there was age-dependent gene expression. The raw data were obtained from a previously published study [130]
The analysis of brain transcripts across four different strains revealed that only 61 genes showed sex-specific expression and out of these genes, 48 genes were highly expressed in males. A stronger effect was observed for genes involved in the steroid hormone pathway [126].
Apart from these molecular differences, a recent study indicated that epigenetic processes can also regulate masculinization and feminization processes in mammals [39]. DNA methylation patterns showed that new-born female rats had more methylated CpG sites than male rats. The DNA methyltransferase (Dnmt) activity was also lower in females, and the treatment of new-born females with estradiol resulted in decreased Dnmt activity. This suggests that the inhibition of masculinization through DNA methylation is important for brain feminization [39]. This indicates that epigenetic mechanisms could also be involved in prenatal brain for sex-specific gene expression.
In zebrafish, epigenetic mechanisms are known to alter gonadal sex differentiation. Ribas et al. [144] showed that exposure to the DNA methylation agent, 5-azacytidine can lead to the development of an all-female fish population. However, studies indicate that whether 5-azacytidine can drive the male or female pathway depends on the exposure concentration and time of exposure [145, 146]. Nevertheless, these studies show that the disruption of epigenetic mechanisms can alter the outcome of gonadal sex differentiation. The analysis of brain samples from the sex-changed zebrafish would have provided an important clue as to whether epigenetic changes also contributed to incomplete masculinization or feminization in sex-changed animals.
Male and female zebrafish brains showed sex-specific methylome patterns at 914 sites. In males 435 CpG sites were hypermethylated and 479 hypomethylated compared to females [147]. Of the 914 differentially methylated CpGs, 708 were found to be associated with protein-coding genes [147]. This suggests that epigenetic mechanisms in the zebrafish brain regulate important signaling processes that may also dictate brain sex differentiation. Epigenetic processes, including DNA methylation in the brain, play essential roles and altered functions are linked to neurodegenerative diseases [148]. Differential epigenetic mechanisms in males and females may also be responsible for the sex-specific neurological disease susceptibility. Hence, the further understanding of zebrafish brain epigenetic regulation could help dissect neurodegenerative diseases.
Protein-level differences
It is indicated that around 50% of the human genome is expressed in the central nervous system, and the post-translational modifications and protein–protein interactions further increase the protein variants [149]. Alterations in protein structure, components and activity can lead to different neurodegenerative diseases, including amyotrophic sclerosis (ALS), Parkinson’s disease, and Alzheimer’s disease (AD) [150,151,152,153]. Understanding gene regulation at the protein level can help map signaling networks and neurodegenerative diseases. Many studies have identified sex-specific differences in zebrafish at the transcript level; however, there is limited information on these differences at the protein level. Recent advances in protein biochemistry have helped annotate proteins in zebrafish. Gabriel et al. [149] using two-dimensional electrophoresis and mass spectrophotometry (LC–ESI MS/MS) identified 95 different proteins in the adult zebrafish brain. The authors also showed that phosphorylation is a more common post-translational modification than glycosylation [149]. Sex-related differences at the protein level in the zebrafish brain were clearly shown in a recent study where the authors used hypoxia conditions to analyze changes in the brain proteome [154]. Among these proteins, H3k9 which is involved in epigenetic processes was significantly upregulated in male brain following hypoxia. Other proteins that were significantly altered were Eno1, Foxo1, Gp1, Hmox1, Nos2, Pkm, Ran, Vcp, Klf4, Nestin, Sox2, etc. Furthermore, pathway analysis showed clear sex-specific differences, especially in the disease and function categories [154].
Metabolite-level differences
Metabolomics can reveal crucial information on the signaling mechanisms in male and female brains. Metabolomics involves the measurement of the end-products of cellular processes that can help elucidate biochemical processes, including neurodegenerative diseases [155]. The brain is mainly composed of lipids, which are associated with maintaining brain homeostasis and common pathological conditions in the brain [156]. As there is an alteration in lipid metabolism signaling in certain neurological diseases, including AD, the lipidomics of brain samples can provide essential clues on signaling processes [156]. The metabolic profile of the brain has been shown to be unique in different brain regions [155, 157], and the metabolomics profiling of the mouse brain has revealed that the metabolites are expressed in a region-specific manner [155]. For example, glycerophosphoserine was found to be high in the cerebellum and low in the frontal cortex, and another metabolite, N-acetylaspartylglutamic acid was found to be high in the brain stem and thalamus midbrain [155]. Hence, further analyses could shed light on the region-specific homeostasis during disease onset and progression.
Different factors, including age, genetics, lifestyle, and sex, can influence lipid metabolism in the brain. Testosterone and thyroid hormone are crucial hormones known to affect brain development, while they can also influence lipid metabolism [158, 159].
There is no clear understanding on sex-related differences at the metabolite level. To understand whether the zebrafish brain shows sex-specific differences, we analyzed the lipidomics data from a previous study [160]. The analyzed data suggests that metabolomic profile (lipidomics) in male and female zebrafish was different (Fig. 4). The raw data were obtained from a previous study in which control male and female differences were not reported [160]. Raw data was analyzed using the software MetaboAnalyst 5.0. The analysis showed that 98 lipid molecules in total were differentially produced in male and female brains (Fig. 4A). Out of 98 lipid molecules, 18 were highly produced in females, while 80 were highly produced in males (Fig. 4A, B). Different metabolites, including ceramide, cholesteryl palmitate, triacylglycerol, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine and sphingomyelin showed differential expression. Gene expression analysis (at the transcript level) has been the preferred method to show the sex-specific differences in the brain. However, understanding the differences in metabolites could further provide vital clues for deciphering differences in neuronal signaling networks.
Metabolomics of male and female zebrafish brains. Control (WT; wild-type) male and female brains were analyzed for different lipid molecules. The fold change analysis indicated that there are 98 lipid molecules that are differentially produced (A). The heat map shows the overall difference (B) ANOVA, n = 4. The raw data were obtained from a previously published study [155]. In this study [155], the sex of the fish was determined by visual observation of the gonads
Conclusion
Zebrafish brains display sex-specific differences in terms of signaling and function. The differences are noticeable at the transcript, protein, hormonal, metabolite, and cellular levels. Although the primary determinant of brain sex differentiation has not been identified, the role of steroid hormones in the neuronal organization in a male and female typical manner is recognized. The zebrafish model can be an important tool to investigate how the sex-specific organization of the brain occurs and how different factors, including the genetic and environmental, influence the outcome. Deciphering the molecular mechanisms of brain sex differentiation could further help fill this knowledge gap and improve our understanding of neurological disease onset and progression. This can also help predict pharmacological interventions and bridge the gap between neurodegenerative drug discovery and clinical trials. This review discusses the essential differences that have been observed so far in male and female zebrafish brains. This information can be critical to target the candidate genes and signaling processes further to better understand the brain sex differentiation enigma.
Availability of data and materials
Figure 2 transcriptomics raw data files are uploaded in NCBI database. Accession Number: SRP347573 and Bioproject: PRJNA783180.
Abbreviations
- 11-KT:
-
11-Ketotestosterone
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotropic sclerosis
- amhr2:
-
Anti-Mullerian hormone receptor type 2
- amhy:
-
Y chromosome-linked anti-Müllerian hormone
- apoeb:
-
Apolipoprotein Eb
- apof:
-
Apolipoprotein F
- AR:
-
Androgen receptor
- cga:
-
Glycoprotein hormones, alpha polypeptide
- cyp11a1:
-
Cytochrome P450, family 11, subfamily A, polypeptide 1
- Cyp17a1:
-
Cytochrome P450, family 17, subfamily A1
- cyp17a2:
-
Cytochrome P450, family 17, subfamily A, polypeptide 2
- cyp19a1a:
-
Cytochrome P450, family 19, subfamily A, polypeptide 1a
- cyp19a1b:
-
Cytochrome P450, family 19, subfamily A, polypeptide 1b
- cyp2aa7:
-
Cytochrome P450, family 2, subfamily AA, polypeptide 7
- cyp27a1.4:
-
Cytochrome P450, family 27, subfamily A, polypeptide 1, gene 4
- cyp39a1:
-
Cytochrome P450, family 39, subfamily A, polypeptide 1
- dio1:
-
Deiodinase type 1
- Dio2:
-
Deiodinase type 2
- dmrt1:
-
Doublesex- and mab-3-related transcription factor 1
- dmY:
-
DM-domain gene on the Y chromosome
- E2:
-
Estradiol
- Enp1:
-
Essential nuclear protein 1
- esr1:
-
Estrogen receptor 1
- esr2b:
-
Estrogen receptor 2b
- fkbp5:
-
FKBP prolyl isomerase 5
- foxl2:
-
Forkhead box L2
- foxo1:
-
Forkhead box O1
- gabbr1a:
-
Gamma-aminobutyric acid (GABA) B receptor, 1a
- gabbr1b:
-
Gamma-aminobutyric acid (GABA) B receptor, 1b
- gdf6Y:
-
Growth differentiation factor 6 on the Y chromosome
- Gp1:
-
Glycoprotein 1
- gsdfY:
-
Gonadal soma derived growth factor on the Y chromosome
- Hmox1:
-
Heme oxygenase 1
- hsd17b3:
-
Hydroxysteroid (17-beta) dehydrogenase 3
- igf1:
-
Insulin-like growth factor 1
- Klf4:
-
Kruppel-like factor 4
- KO:
-
Knockout
- mtf:
-
Metal transcription factor
- Nos2:
-
Nitric oxide synthase 2
- PD:
-
Parkinson’s disease
- Pkm:
-
Pyruvate kinase, muscle
- ptgds:
-
Prostaglandin D2 synthase
- ptgs2b:
-
Prostaglandin-endoperoxide synthase 2b
- Ran:
-
Ras-related nuclear protein
- rspo1:
-
R-spondin 1
- sdY:
-
Sexually dimorphic on the Y chromosome
- sirt1:
-
Sirtuin 1
- sod1:
-
Superoxide dismutase 1
- SOX:
-
Sry-type HMG box
- Sox2:
-
SRY-box transcription factor 2
- sox3Y:
-
Sox3 on the Y chromosome
- SRY:
-
Sex determining region Y
- T:
-
Testosterone
- T3:
-
Triiodothyronine
- T4:
-
Thyroxine
- TSH:
-
Thyroid stimulating hormone
- Vcn:
-
Valosin containing protein
- WT:
-
Wild type
References
Kashimada K, Koopman P. Sry: the master switch in mammalian sex determination. Development. 2010;137:3921–30.
Jacobs PA, Strong JA. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature. 1959;183:302–3.
Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82.
Koopman P. Sex determination: a tale of two Sox genes. Trends Genet. 2005;21:367–70.
Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–21.
Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, et al. A Gene-mapping to the sex-determining region of the mouse y-chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–50.
Ditewig AC, Yao HH. Organogenesis of the ovary: a comparative review on vertebrate ovary formation. Organogenesis. 2005;2:36–41.
Yao HH. The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol. 2005;230:87–93.
Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–63.
Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, et al. Tracing the Emergence of a Novel Sex-Determining Gene in Medaka. Oryzias luzonensis Genetics. 2012;191:163.
Takehana Y, Matsuda M, Myosho T, Suster ML, Kawakami K, Shin-I T, et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat Commun. 2014;5:4157.
Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, et al. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc Natl Acad Sci USA. 2012;109:2955–9.
Li MH, Sun YL, Zhao JE, Shi HJ, Zeng S, Ye K, et al. A Tandem Duplicate of Anti-Mullerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia. Oreochromis niloticus Plos Genet. 2015;11: e1005678.
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, et al. A Trans-Species Missense SNP in Amhr2 Is Associated with Sex Determination in the Tiger Pufferfish, Takifugu rubripes (Fugu). Plos Genet. 2012;8: e1002798.
Reichwald K, Petzold A, Koch P, Downie BR, Hartmann N, Pietsch S, et al. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell. 2015;163:1527–38.
Chen SL, Zhang GJ, Shao CW, Huang QF, Liu G, Zhang P, et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet. 2014;46:253.
Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA. 2002;99:11778–83.
Yano A, Guyomard R, Nicol B, Jouanno E, Quillet E, Klopp C, et al. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr Biol. 2012;22:1423–8.
Zheng SQ, Wang XS, Zhang S, Long J, Tao WJ, Li MH, et al. Screening and characterization of sex-linked DNA markers and marker-assisted selection in the Southern catfish (Silurus meridionalis). Aquaculture. 2020;517:8.
Liew WC, Orban L. Zebrafish sex: a complicated affair. Brief Funct Genomics. 2014;13:172–87.
Pradhan A, Olsson PE. Regulation of zebrafish gonadal sex differentiation. Aims Mol Sci. 2016;3:567–84.
von Hofsten J, Olsson PE. Zebrafish sex determination and differentiation: involvement of FTZ-F1 genes. Reprod Biol Endocrinol. 2005;3:63.
Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364.
Hill RL, Janz DM. Developmental estrogenic exposure in zebrafish (Danio rerio): I. Effects on sex ratio and breeding success. Aquat Toxicol. 2003;63:417–29.
Lee SLJ, Horsfield JA, Black MA, Rutherford K, Gemmell NJ. Identification of sex differences in zebrafish (Danio rerio) brains during early sexual differentiation and masculinization using 17 alpha-methyltestoterone. Biol Reprod. 2018;99:446–60.
Pradhan A, Khalaf H, Ochsner SA, Sreenivasan R, Koskinen J, Karlsson M, et al. Activation of NF-kappaB protein prevents the transition from juvenile ovary to testis and promotes ovarian development in zebrafish. J Biol Chem. 2012;287:37926–38.
Pradhan A, Olsson PE. Juvenile ovary to testis transition in zebrafish involves inhibition of ptges. Biol Reprod. 2014;91:33.
Schulz RW, Bogerd J, Male R, Ball J, Fenske M, Olsen LC, et al. Estrogen-induced alterations in amh and dmrt1 expression signal for disruption in male sexual development in the zebrafish. Environ Sci Technol. 2007;41:6305–10.
Pradhan A, Olsson PE. Germ cell depletion in zebrafish leads to incomplete masculinization of the brain. Gen Comp Endocrinol. 2018;265:15.
Siegfried KR, Nusslein-Volhard C. Germ line control of female sex determination in zebrafish. Deve Biol. 2008;324:277–87.
Kurokawa H, Saito D, Nakamura S, Katoh-Fukui Y, Ohta K, Baba T, et al. Germ cells are essential for sexual dimorphism in the medaka gonad. Proc Natl Acad Sci U S A. 2007;104:16958–63.
Slanchev K, Stebler J, de la Cueva-Mendez G, Raz E. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA. 2005;102:4074–9.
Rodriguez-Mari A, Canestro C, Bremiller RA, Nguyen-Johnson A, Asakawa K, Kawakami K, et al. Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. Plos Genet. 2010;6: e1001034.
Webster KA, Schach U, Ordaz A, Steinfeld JS, Draper BW, Siegfried KR. Dmrt1 is necessary for male sexual development in zebrafish. Dev Biol. 2017;422:33–46.
Lau ES, Zhang Z, Qin M, Ge W. Knockout of Zebrafish Ovarian Aromatase Gene (cyp19a1a) by TALEN and CRISPR/Cas9 leads to all-male offspring due to failed ovarian differentiation. Sci Rep. 2016;6:37357.
Wu K, Song W, Zhang Z, Ge W. Disruption of dmrt1 rescues the all-male phenotype of the cyp19a1a mutant in zebrafish - a novel insight into the roles of aromatase/estrogens in gonadal differentiation and early folliculogenesis. Development. 2020;147:182758.
Yu G, Zhang D, Liu W, Wang J, Liu X, Zhou C, et al. Zebrafish androgen receptor is required for spermatogenesis and maintenance of ovarian function. Oncotarget. 2018;9:24320–34.
Zhai G, Shu T, Xia Y, Lu Y, Shang G, Jin X, et al. Characterization of sexual trait development in cyp17a1-deficient zebrafish. Endocrinology. 2018;159:3549–62.
Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–7.
Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–8.
Xu H, Wang F, Liu Y, Yu Y, Gelernter J, Zhang H. Sex-biased methylome and transcriptome in human prefrontal cortex. Hum Mol Genet. 2014;23:1260–70.
Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA. 2014;111:823–8.
Pfeiffer CA. Sexual differences of the hypophyses and their determination by the gonads. Am J Anat. 1936;58:198.
Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–6.
Maclusky NJ, Naftolin F. Sexual-differentiation of the central nervous-system. Science. 1981;211:1294–303.
McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124.
Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–98.
Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Mol Brain Res. 2003;118:82–90.
Lee SI, Lee WK, Shin JH, Han BK, Moon S, Cho S, et al. Sexually dimorphic gene expression in the chick brain before gonadal differentiation. Poultry Sci. 2009;88:1003–15.
Sim H, Argentaro A, Harley VR. Boys, girls and shuttling of SRY and SOX9. Trends Endocrinol Metab. 2008;19:213–22.
Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, et al. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006;16:415–20.
Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11.
Gahr M, Metzdorf R. The sexually dimorphic expression of androgen receptors in the song nucleus hyperstriatalis ventrale pars caudale of the zebra finch develops independently of gonadal steroids. J Neurosci. 1999;19:2628–36.
Wade J, Arnold AP. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc Natl Acad Sci USA. 1996;93:5264–8.
Arnold AP. The gender of the voice within: the neural origin of sex differences in the brain. Curr Opin Neurobiol. 2003;13:759–64.
Francis RC. Sexual lability in teleosts - developmental factors. Q Rev Biol. 1992;67:1–18.
Jorgensen A, Nielsen JE, Nielsen BF, Morthorst JE, Bjerregaard P, Leffers H. Expression of prostaglandin synthases (pgds and pges) during zebrafish gonadal differentiation. Comp Biochem Physiol A. 2010;157:102–8.
Kallivretaki E, Eggen RIL, Neuhauss SCF, Kah O, Segner H. The zebrafish, brain-specific, aromatase cyp19a2 is neither expressed nor distributed in a sexually dimorphic manner during sexual differentiation. Dev Dynam. 2007;236:3155–66.
Trant JM, Gavasso S, Ackers J, Chung BC, Place AR. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio). J Exp Zool. 2001;290:475–83.
Graves JA. Evolution of the testis-determining gene–the rise and fall of SRY. Novartis Found Symp. 2002;244:86–97.
Camper SA. Sox3 and sexual dysfunction: it’s in the head. Nat Genet. 2004;36:217–9.
McCammon JM, Sive H. Addressing the genetics of human mental health disorders in model organisms. Annu Rev Genom Hum G. 2015;16:173–97.
Saleem S, Kannan RR. Zebrafish: an emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 2018;4:45.
Meshalkina DA, Kysil EV, Warnick JE, Demin KA, Kalueff AV. Adult zebrafish in CNS disease modeling: a tank that’s half-full, not half-empty, and still filling. Lab Anim (NY). 2017;46:378–87.
Orban L, Sreenivasan R, Olsson PE. Long and winding roads: testis differentiation in zebrafish. Mol Cell Endocrinol. 2009;312:35–41.
Siegfried KR. In search of determinants: gene expression during gonadal sex differentiation. J Fish Biol. 2010;76:1879–902.
Penman DJ, Piferrer F. Fish gonadogenesis. Part I: genetic and environmental mechanisms of sex determination. Rev Fish Sci. 2008;16:16–34.
Nagahama Y. Molecular mechanisms of sex determination and gonadal sex differentiation in fish. Fish Physiol Biochem. 2005;31:105–9.
Volff JN, Nanda I, Schmid M, Schartl M. Governing sex determination in fish: regulatory putsches and ephemeral dictators. Sex Dev. 2007;1:85–99.
Desjardins J, Fernald R. Fish sex: why so diverse? J Curr opin neurobiol. 2009;19:648.
Endo A, Ingalls TH. Chromosomes of the zebra fish: A model for cytogenetic, embryologic, and ecologic study. J Heredity. 1968;59:382–4.
Schreeb K, Groth G, Sachsse W, Freundt K. The karyotype of the zebrafish (Brachydanio rerio). J Exp Anim Sci. 1993;36:27–31.
Gornung E, Gabrielli I, Cataudella S, Sola L. CMA3-banding pattern and fluorescence in situ hybridiz ation with 18S rRNA genes in zebrafish chromosomes. Chromosome Res. 1997;5:40–6.
Phillips RB, Reed KM. Localization of repetitive DNAs to zebrafish (Danio rerio) chromosomes by fluorescence in situ hybridization (FISH). Chromosome Res. 2000;8:27–35.
Sharma KK, Sharma OP, Tripathi NK. Female heterogamety in Danio rerio (Cypriniformes: Cyprinidae). Proc Natl Acad Sci India Sect B. 1998;68:123–6.
Fontana F, Chiarelli B, Rossi A. Il Cariotipo di Alcune Specie di Cyprinidae, Centrarchidae, Characidae Studiate Mediante Colture in vitro. Caryologia. 1970;23:549–64.
Anderson JL, Rodriguez Mari A, Braasch I, Amores A, Hohenlohe P, Batzel P, et al. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS ONE. 2012;7: e40701.
Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, Orban L. Polygenic sex determination system in zebrafish. PLoS ONE. 2012;7: e34397.
Takatsu K, Miyaoku K, Roy SR, Murono Y, Sago T, Itagaki H, et al. Induction of female-to-male sex change in adult zebrafish by aromatase inhibitor treatment. Sci Rep. 2013;3:3400.
Yin Y, Tang H, Liu Y, Chen Y, Li G, Liu X, et al. Targeted disruption of aromatase reveals dual functions of cyp19a1a during sex differentiation in zebrafish. Endocrinology. 2017;158:3030–41.
Yang YJ, Wang Y, Li Z, Zhou L, Gui JF. Sequential, divergent, and cooperative requirements of Foxl2a and Foxl2b in ovary development and maintenance of zebrafish. Genetics. 2017;205:1551–72.
Pradhan A, Olsson PE. Zebrafish sexual behavior: role of sex steroid hormones and prostaglandins. Behav Brain Funct. 2015;11:23.
Shu TT, Zhai G, Pradhan A, Olsson PE, Yin Z. Zebrafish cyp17a1 knockout reveals that androgen-mediated signaling is important for male brain sex differentiation. Gen Comp Endocr. 2020;295: 113490.
Wang X, Zhang J-B, He K-J, Wang F, Liu C-F. Advances of Zebrafish in neurodegenerative disease: from models to drug discovery. J Front Pharmacol. 2021;12:1802.
Kishimoto N, Shimizu K, Sawamoto K. Neuronal regeneration in a zebrafish model of adult brain injury. Dis Model Mech. 2012;5:200–9.
Marz M, Schmidt R, Rastegar S, Strahle U. Regenerative response following stab injury in the adult zebrafish telencephalon. Dev Dyn. 2011;240:2221–31.
Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–41.
Cosacak MI, Papadimitriou C, Kizil C. Regeneration, Plasticity, and Induced Molecular Programs in Adult Zebrafish Brain. Biomed Res Int. 2015;2015:10.
Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18:225–42.
Lenz KM, McCarthy MM. A Starring Role for Microglia in Brain Sex Differences. Neuroscientist. 2015;21:306–21.
Schwarz JM, McCarthy MM. Cellular mechanisms of estradiol-mediated masculinization of the brain. J Steroid Biochem Mol Biol. 2008;109:300–6.
Nelson LH, Warden S, Lenz KM. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav Immun. 2017;64:11–22.
Baksi S, Pradhan A. Thyroid hormone: sex-dependent role in nervous system regulation and disease. Biol Sex Differ. 2021;12:25.
Ampatzis K, Makantasi P, Dermon CR. Cell Proliferation Pattern in Adult Zebrafish Forebrain Is Sexually Dimorphic. Neuroscience. 2012;226:367–81.
Pellegrini E, Mouriec K, Anglade I, Menuet A, Le Page Y, Gueguen MM, et al. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J Comp Neurol. 2007;501:150–67.
Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade I, Pakdel F, et al. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J Comp Neurol. 2005;485:304–20.
Rakic P. Neuronal migration and contact guidance in the primate telencephalon. Postgrad Med J. 1978;54(Suppl 1):25–40.
Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–63.
Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20.
Pradhan A, Kharlyngdoh JB, Asnake S, Olsson PE. The brominated flame retardant TBECH activates the zebrafish (Danio rerio) androgen receptor, alters gene transcription and causes developmental disturbances. Aquat Toxicol. 2013;142–143:63–72.
Hossain MS, Larsson A, Scherbak N, Olsson PE, Orban L. Zebrafish androgen receptor: isolation, molecular, and biochemical characterization. Biol Reprod. 2008;78:361–9.
Olsson PE, Berg AH, von Hofsten J, Grahn B, Hellqvist A, Larsson A, et al. Molecular cloning and characterization of a nuclear androgen receptor activated by 11-ketotestosterone. Reprod Biol Endocrinol. 2005;3:37.
Sorensen PW, Hara TJ, Stacey NE, Goetz FW. F-prostaglandins function as potent olfactory stimulants that comprise the postovulatory female sex-pheromone in goldfish. Biol Reprod. 1988;39:1039–50.
Sorensen PW, Pinillos M, Scott AP. Sexually mature male goldfish release large quantities of androstenedione into the water where it functions as a pheromone. Gen Comp Endocr. 2005;140:164–75.
Colombo L, Marconato A, Belvedere PC, Friso C. Endocrinology of teleost reproduction: a testicular steroid pheromone in the black goby. Gobius jozo L Boll Zool. 1980;47:355–64.
Nagarajan G, Aruna A, Chang CF. Neurosteroidogenic enzymes and their regulation in the early brain of the protogynous grouper Epinephelus coioides during gonadal sex differentiation. Gen Comp Endocrinol. 2013;181:271–87.
Vizziano-Cantonnet D, Anglade I, Pellegrini E, Gueguen MM, Fostier A, Guiguen Y, et al. Sexual dimorphism in the brain aromatase expression and activity, and in the central expression of other steroidogenic enzymes during the period of sex differentiation in monosex rainbow trout populations. Gen Comp Endocrinol. 2011;170:346–55.
Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32.
Lanthier A, Patwardhan VV. Sex steroids and 5-en-3 beta-hydroxysteroids in specific regions of the human brain and cranial nerves. J Steroid Biochem. 1986;25:445–9.
Mathur C, Prasad VVK, Raju VS, Welch M, Lieberman S. Steroids and their conjugates in the mammalian brain. Proc Natl Acad Sci USA. 1993;90:85–8.
Ruiz-Palmero I, Ortiz-Rodriguez A, Melcangi RC, Caruso D, Garcia-Segura LM, Rune GM, et al. Oestradiol synthesized by female neurons generates sex differences in neuritogenesis. Sci Rep. 2016;6:31891.
Sakamoto H, Ukena K, Tsutsui K. Activity and localization of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase in the zebrafish central nervous system. J Comp Neurol. 2001;439:291–305.
Takács S, Gries R, Gries G. Sex hormones function as sex attractant pheromones in house mice and brown rats. ChemBioChem. 2017;18:1391–5.
Witt DM, Young LJ, Crews D. Progesterone and sexual behavior in males. Psychoneuroendocrinology. 1994;19:553–62.
Andersen ML, Tufik S. Does male sexual behavior require progesterone? Brain Res Rev. 2006;51:136–43.
McCarthy EA, Naik AS, Coyne AF, Cherry JA, Baum MJ. Effect of ovarian hormones and mating experience on the preference of female mice to investigate male urinary pheromones. Chem Senses. 2018;43:97–104.
Zhong C, Xiong K, Wang X. Effects of progesterone on the reproductive physiology in zebrafish. bioRxiv. 2017:147280.
Paulos P, Runnalls TJ, Nallani G, La Point T, Scott AP, Sumpter JP, et al. Reproductive responses in fathead minnow and Japanese medaka following exposure to a synthetic progestin. Norethindrone Aquat Toxicol. 2010;99:256–62.
Zeilinger J, Steger-Hartmann T, Maser E, Goller S, Vonk R, Lange R. Effects of synthetic gestagens on fish reproduction. Environ Toxicol Chem. 2009;28:2663–70.
Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–34.
Nishiike Y, Miyazoe D, Togawa R, Yokoyama K, Nakasone K, Miyata M, et al. Estrogen receptor 2b is the major determinant of sex-typical mating behavior and sexual preference in medaka. Curr Biol. 2021;31(1699–710): e6.
Lu H, Cui Y, Jiang L, Ge W. Functional Analysis of Nuclear Estrogen Receptors in Zebrafish Reproduction by Genome Editing Approach. Endocrinology. 2017;158:2292–308.
Levillain O, Ventura G, Dechaud H, Hobeika M, Meseguer A, Moinard C, et al. Sex-differential expression of ornithine aminotransferase in the mouse kidney. Am J Physiol Renal Physiol. 2007;292:F1016–27.
Small CM, Carney GE, Mo Q, Vannucci M, Jones AG. A microarray analysis of sex- and gonad-biased gene expression in the zebrafish: evidence for masculinization of the transcriptome. BMC Genomics. 2009;10:579.
Di Rosa V, Lopez-Olmeda JF, Burguillo A, Frigato E, Bertolucci C, Piferrer F, et al. Daily rhythms of the expression of key genes involved in steroidogenesis and gonadal function in Zebrafish. PLoS ONE. 2016;11: e0157716.
Wong RY, McLeod MM, Godwin J. Limited sex-biased neural gene expression patterns across strains in Zebrafish (Danio rerio). BMC Genomics. 2014;15:905.
Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122.
Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: Clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–18.
Noda M. Thyroid Hormone in the CNS: Contribution of Neuron-Glia Interaction. Vitam Horm. 2018;106:313–31.
Sharma P, Tang S, Mayer GD, Patino R. Effects of thyroid endocrine manipulation on sex-related gene expression and population sex ratios in Zebrafish. Gen Comp Endocrinol. 2016;235:38–47.
Houbrechts AM, Van Houcke J, Darras VM. Disruption of deiodinase type 2 in zebrafish disturbs male and female reproduction. J Endocrinol. 2019;241:111–23.
Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15:2137–48.
Schneider MJ, Fiering SN, Thai B, Wu SY, St Germain E, Parlow AF, et al. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–9.
Sawyer SJ, Gerstner KA, Callard GV. Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: Gene specific tissue distribution, sex differences, developmental programming, and estrogen regulation. Gen Comp Endocrinol. 2006;147:108–17.
Arslan-Ergul A, Adams MM. Gene expression changes in aging zebrafish (Danio rerio) brains are sexually dimorphic. BMC Neurosci. 2014;15:29.
Hartig EI, Zhu S, King BL, Coffman JA. Chronic cortisol exposure in early development leads to neuroendocrine dysregulation in adulthood. BMC Res Notes. 2020;13:366.
Hao R, Bondesson M, Singh AV, Riu A, McCollum CW, Knudsen TB, et al. Identification of estrogen target genes during zebrafish embryonic development through transcriptomic analysis. PLoS ONE. 2013;8: e79020.
Gans I, Hartig EI, Zhu S, Tilden AR, Hutchins LN, Maki NJ, et al. Klf9 is a key feedforward regulator of the transcriptomic response to glucocorticoid receptor activity. Sci Rep. 2020;10:11415.
Zhou L, Charkraborty T, Zhou Q, Mohapatra S, Nagahama Y, Zhang Y. Rspo1-activated signalling molecules are sufficient to induce ovarian differentiation in XY medaka (Oryzias latipes). Sci Rep. 2016;6:19543.
Xiao L, Guo Y, Wang D, Zhao M, Hou X, Li S, et al. Beta-Hydroxysteroid Dehydrogenase Genes in Orange-Spotted Grouper (Epinephelus coioides): genome-wide identification and expression analysis during sex reversal. Front Genet. 2020;11:161.
Liu Y, Morton RE. Apolipoprotein F: a natural inhibitor of cholesteryl ester transfer protein and a key regulator of lipoprotein metabolism. Curr Opin Lipidol. 2020;31:194–9.
Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, et al. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:643.
Armant O, Gombeau K, Murat-El-Houdigui S, Floriani M, Camilleri V, Cavalie I, et al. Zebrafish exposure to environmentally relevant concentration of depleted uranium impairs progeny development at the molecular and histological levels. PLoS ONE. 2017;12:e0177932.
Ribas L, Vanezis K, Imues MA, Piferrer F. Treatment with a DNA methyltransferase inhibitor feminizes zebrafish and induces long-term expression changes in the gonads. Epigenetics Chromatin. 2017;10:59.
Kamstra JH, Sales LB, Alestrom P, Legler J. Differential DNA methylation at conserved non-genic elements and evidence for transgenerational inheritance following developmental exposure to mono(2-ethylhexyl) phthalate and 5-azacytidine in zebrafish. Epigenet Chromatin. 2017;10:20.
Han J, Hu Y, Qi Y, Yuan C, Naeem S, Huang DJS. High temperature induced masculinization of zebrafish by down-regulation of sox9b and esr1 via DNA methylation. J Environ Sci. 2021;107:160–70.
Chatterjee A, Lagisz M, Rodger EJ, Zhen L, Stockwell PA, Duncan EJ, et al. Sex differences in DNA methylation and expression in zebrafish brain: a test of an extended “male sex drive” hypothesis. Gene. 2016;590:307–16.
Klein HU, De Jager PL. Uncovering the role of the methylome in dementia and neurodegeneration. Trends Mol Med. 2016;22:687–700.
Gebriel M, Prabhudesai S, Uleberg KE, Larssen E, Piston D, Bjornstad AH, et al. Zebrafish brain proteomics reveals central proteins involved in neurodegeneration. J Neurosci Res. 2014;92:104–15.
Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–6.
Uversky VN, Eliezer D. Biophysics of Parkinson’s disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci. 2009;10:483–99.
Grimm S, Hoehn A, Davies KJ, Grune T. Protein oxidative modifications in the ageing brain: consequence for the onset of neurodegenerative disease. Free Radical Res. 2011;45:73–88.
Williams A. Defining neurodegenerative diseases. BMJ. 2002;324:1465–6.
Das T, Kamle A, Kumar A, Chakravarty S. Hypoxia induced sex-difference in zebrafish brain proteome profile reveals the crucial role of H3K9me3 in recovery from acute hypoxia. bioRxiv. 2020:2020.06.15.150052.
Ivanisevic J, Epstein AA, Kurczy ME, Benton PH, Uritboonthai W, Fox HS, et al. Brain Region Mapping Using Global Metabolomics. Chem Biol. 2014;21:1575–84.
Chew H, Solomon VA, Fonteh AN. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front Physiol. 2020;11:598.
Choi WT, Tosun M, Jeong HH, Karakas C, Semerci F, Liu Z, et al. Metabolomics of mammalian brain reveals regional differences. BMC Syst Biol. 2018;12:127.
Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obesity. 2000;24:S109–12.
Vodo S, Bechi N, Petroni A, Muscoli C, Aloisi AM. Testosterone-induced effects on lipids and inflammation. Mediators Inflamm. 2013;2013: 183041.
Blanc M, Alfonso S, Begout ML, Barrachina C, Hyotylainen T, Keiter SH, et al. An environmentally relevant mixture of polychlorinated biphenyls (PCBs) and polybrominated diphenylethers (PBDEs) disrupts mitochondrial function, lipid metabolism and neurotransmission in the brain of exposed zebrafish and their unexposed F2 offspring. Sci Total Environ. 2021;754: 142097.
Acknowledgements
We would like to thank Örebro University, Sweden, and Chinese Academy of Sciences for supporting this study.
Funding
Open access funding provided by Örebro University. This study was financed by the National Key Research and Development Program, China (No. 2018YFD0900205 to ZY), Knowledge Foundation, O.E and Edla Johansson’s Scientific Foundation and Örebro University, Sweden (to AP).
Author information
Authors and Affiliations
Contributions
Manuscript writing and editing: AP, ZY, GZ, JJ and CB. Data analysis: AP, JJ and CB. Funding acquisition: AP and ZY. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Zebrafish-related experiments were approved by the Institute of Hydrobiology, Chinese Academy of Sciences (Permit No. IHB 201324).
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
List of differentially expressed genes for Fig. 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhai, G., Jia, J., Bereketoglu, C. et al. Sex-specific differences in zebrafish brains. Biol Sex Differ 13, 31 (2022). https://doi.org/10.1186/s13293-022-00442-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13293-022-00442-2