Abstract
Objective
We assessed the predictive capacity of computed tomography (CT)-enhanced radiomics models in determining microvascular invasion (MVI) for isolated hepatocellular carcinoma (HCC) ≤ 5 cm within peritumoral margins of 5 and 10 mm.
Methods
Radiomics software was used for feature extraction. We used the least absolute shrinkage and selection operator (LASSO) algorithm to establish an effective model to predict patients’ preoperative MVI status.
Results
The area under the curve (AUC) values in the validation sets for the 5- and 10-mm radiomics models concerning arterial tumors were 0.759 and 0.637, respectively. In the portal vein phase, they were 0.626 and 0.693, respectively. Additionally, the combined radiomics model for arterial tumors and the peritumoral 5-mm margin had an AUC value of 0.820. The decision curve showed that the combined tumor and peritumoral radiomics model exhibited a somewhat superior benefit compared to the traditional model, while the fusion model demonstrated an even greater advantage, indicating its significant potential in clinical application.
Conclusion
The 5-mm peritumoral arterial model had superior accuracy and sensitivity in predicting MVI. Moreover, the combined tumor and peritumoral radiomics model outperformed both the individual tumor and peritumoral radiomics models. The most effective combination was the arterial phase tumor and peritumor 5-mm margin combination. Using a fusion model that integrates tumor and peritumoral radiomics and clinical data can aid in the preoperative diagnosis of the MVI of isolated HCC ≤ 5 cm, indicating considerable practical value.
Critical relevance statement
The radiomics model including a 5-mm peritumoral expansion is a promising noninvasive biomarker for preoperatively predicting microvascular invasion in patients diagnosed with a solitary HCC ≤ 5 cm.
Key points
• Radiomics features extracted at a 5-mm distance from the tumor could better predict hepatocellular carcinoma microvascular invasion.
• Peritumoral radiomics can be used to capture tumor heterogeneity and predict microvascular invasion.
• This radiomics model stands as a promising noninvasive biomarker for preoperatively predicting MVI in individuals.
Graphical Abstract

Similar content being viewed by others
Introduction
According to survey data released by the International Center for Research on Cancer, primary liver cancers rank as the sixth most prevalent malignant tumors globally, with the third highest mortality rate [1]. The rapid development of medical technology, particularly the advancement of examination technology, has improved the diagnosis and prognosis for individuals afflicted with hepatocellular carcinoma (HCC), resulting in improving trends in morbidity and mortality [2, 3]; however, the prognosis and long-term survival of HCC patients are still not optimistic [4, 5].
Microvascular invasion (MVI) typically occurs in the small branches of the portal veins of precancerous tissues, with occurrences in the hepatic vein branches being uncommon. The Milan criteria are internationally recognized benchmarks used to assess a patient’s suitability for liver transplantation. Among those with early-stage HCC who meet these benchmarks, the 5-year survival rate post-liver transplantation is approximately 75%, making this an efficient use of organ resources [6]. Research shows that MVI is an independent prognostic factor that impacts the outcomes of patients with HCC [7,8,9,10]. In addition, comparative analysis with pathological results has shown that mononodular tumors with outward protrusion and polynodular fusion are more prone to MVI [11]. However, the confirmation of imaging features depends on experienced experts, who may view certain features differently; therefore, the clinical use of subjective image interpretations is limited.
Radiomics involves extracting subtle features from macroscopic images, revealing microscopic heterogeneity within tumors [12]. Li et al. extracted and quantified features from magnetic resonance imaging (MRI) images of breast cancer and found that large tumors were more aggressive and had greater internal heterogeneity [13]. Some scholars conducting feature extraction on MRI images of patients with HCC found that radiomics features could effectively aid in classifying and predicting both the pathological grade of HCC and the presence of MVI [14, 15]. Kim et al. found that their model, including both the tumor and the 3- and 5-mm peritumoral margins, had higher predictive efficiency compared to the simple tumor feature model [16].
There is a significant difference in biological behavior when the tumor is larger than 5 cm. The AJCC-UICC TNM stage 8 designates this threshold as the boundary for T2 classification. Despite many reports on radiomics to predict MVI in HCC using computed tomography (CT) and MRI images, there is a noticeable lack of attention given to this specific subgroup of tumors. Therefore, isolated HCC ≤ 5 cm were the subject of this study, and tumor and peritumoral histological characteristics were extracted to explore and establish a preoperative model capable of accurately predicting the MVI status of HCC before surgery, offering valuable guidance for precision clinical treatment decisions.
Methods
This retrospective study received approval from the ethics committees of the local institutions. Enrolled patients and their families were not required to provide informed consent.
Patient selection
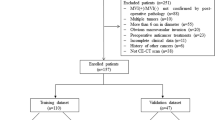
We retrospectively analyzed 206 patients diagnosed with isolated HCC (≤ 5 cm) at our hospital from 2017 to 2021. Among them were 177 men and 29 women. The inclusion criteria comprised (1) the presence of a solitary HCC with a maximum diameter of ≤ 5 cm, (2) lack of extrahepatic metastasis or major vascular invasion per preoperative imaging, (3) no previous HCC-related treatments, (4) comprehensive histopathologic descriptions of HCC, and (5) preoperative enhanced CT with adequate image quality performed within 1 week preceding the surgery.
The exclusion criteria comprised (1) incomplete pathological information, (2) tumors exhibiting diffuse growth and indeterminable edges, (3) concurrent existence of additional malignant tumors, and (4) inadequate clinical baseline data.
The external validation group included 60 patients from another hospital who met the inclusion criteria.
We used SPSS software to randomly split the study population into the training and validation groups. The enrolled patients’ clinical baseline data were obtained by accessing electronic medical records.
CT imaging protocol
The CT scanning equipment used in this study included the Discovery 750 HD CT (GE Medical, USA), the SOMATOM Force CT (Siemens Medical, Germany), the Brilliance ICT (PHILIPS, The Netherlands), and the Aquilian One 640 (Toshiba, Japan).
Patient scanning position
A 120-kV tube voltage with automatic tube current technology was used. The contrast agent (370 mg/mL) was injected via the right anterior elbow vein using a high-pressure syringe (rate = 3 mL/s; dose = 1.5 mL/kg). A rinse of physiological saline followed (20 mL). Using the small-dose trigger technique, imaging was initiated when the descending aorta reached 100 HU post-contrast agent injection. The arterial phase images were captured 10 s afterward, while the portal vein phase images were captured 30 s afterward.
Radiomics feature analysis
Image segmentation and preprocessing
The images obtained by different machines were reconstructed with the standard algorithm. The slice thickness and the layer spacing were 1 mm each. The window width was adjusted to 220 HU, while the window position was calibrated to 40 HU. In the absence of pathological results, the features of lesions on the images were assessed by two liver disease imaging specialists (one with 10 years of experience and the other with 15). When the two radiologists disagreed on certain features, a third physician with 20 years of experience double-checked the features. As shown in Fig. 1, the imaging features included the following: (1) tumor size; (2) tumor margin (smooth or rough); (3) capsule (no capsule, complete capsule, or incomplete capsule); (4) peritumoral enhancement; (5) intratumoral artery; (6) peritumoral low-density rim; and (7) liver-tumor interface differences.
Traditional diagnostic imaging features of MVI
RVI (radiogenomic venous invasion) was characterized by the existence of intratumoral arteries without the presence of a low-density rim and without notable differences in the liver-tumor interface [17]. TTPVI (two-trait predictor of venous invasion) was described as the occurrence of intratumoral arteries and the lack of a low-density rim around the tumor [18].
Delineated volume area of interest (VOI)
The reconstructed images (in DICOM format) were fed into the radiomics software platform for VOI determination. The tumors were outlined using a semi-automatic general segmentation method (Fig. 2). Each of the two radiologists confirmed the region of interest within the specified range, making manual adjustments as needed to acquire the tumor VOI (Vtumor). After confirming the Vtumor, an automatic expansion algorithm expanded the area in all three dimensions. The peritumoral contour was automatically obtained. Care was taken to avoid blood vessels, gas, bones, and bile ducts, resulting in nonuniform Vtumor and Vperitumoral. The acquired VOIs from the arterial and portal vein phases were matched. To assess the consistency of VOI delineation, the process was repeated for 50 randomly selected patients at 1-month intervals. Intra-observer and inter-observer correlation coefficients (ICC) were strong (ICC > 0.80).
VOI was delineated and feature extraction: a and b represent those two patients and outline the volume process of interest. The yellow line represents the tumor boundary, the red line represents the physical expansion of 5 mm to the peritumor, the blue line represents the expansion of 10 mm, and the green line represents the expansion of 15 mm. In this study, 5-mm and 10-mm peritumor areas are discussed. b shows that peritumoral expansion terminates when it reaches the edge of the liver
Feature extraction and model building
Once the VOIs were delineated, the features were computed using radiomics software. All features from the original radiomics software were used in the calculations. Consequently, each patient possessed six groups of features related to the arterial and portal vein phases, along with the peritumoral area. Each group included 1691 parameters, encompassing morphological, first-order, texture, filter transform, and wavelet transform features. Therefore, prioritizing dimensionality reduction screening was the primary approach when constructing a model.
First, stability feature extraction was conducted by evaluating features from 50 randomly chosen cases to determine inter-group and intra-group correlation coefficients. High-stability features (correlation coefficients > 0.80) were retained. Second, we used the Spearman correlation test to remove features with correlations. Third, hypothesis testing was performed. We used T-tests or Mann–Whitney rank sum tests to pick the features correlating with MVI. Fourth, we established the model using LASSO, which imposes constraints to reduce data dimensionality. This algorithm compresses coefficients associated with less influence to zero through a penalty function, ensuring that the sum of absolute values of the coefficients is less than a constant. Post-LASSO, robustness characteristics were obtained, generating an equation inclusive of weighted coefficients. The value determined by this equation was the Radscore.
Statistical analysis
We used the R software package (version 3.6.1, http://www.R-project.org) to conduct the statistical analysis. Clinical baseline data were analyzed, and both univariate and multivariate logistic regression analyses were employed to identify potential risk factors associated with MVI. We used the Delong test to compare different models’ ability to predict MVI in HCC patients. p < 0.05 was deemed statistically significant. We used a decision curve to compare the models’ clinical value. Finally, we constructed a calibration curve to evaluate the model’s calibration capability.
Results
Patient characteristics
We included 206 eligible HCC patients, including 177 men and 29 women, among whom 61 were MVI-positive and 145 were MVI-negative. Out of these, 138 patients were randomly allocated to the training group and 68 to the validation group. The baseline patient data are presented in Table 1. All clinical indicators of the randomly grouped patients were statistically compared, and no statistical differences were observed across any indicators between the training and verification groups.
Univariate and multivariate analyses of clinical and imaging features
The study population was divided based on postoperative pathological MVI status, resulting in 61 MVI-positive patients and 145 MVI-negative patients. The probability of MVI was 29.6% (61/206). We conducted univariate and multivariate logistic regression analyses on all baseline data, including imaging features and pathological findings (Table 2). There was a significant correlation between capsular invasion and MVI. Elevated preoperative alanine aminotransferase (ALT) and aspartate transaminase (AST) levels were also associated with MVI. Factors such as peritumoral enhancement, tumor margin, existence of intratumoral arteries along with a low-density rim surrounding the tumor, integrity of the tumor capsule, and density differences between the tumor and the liver interface were all identified as MVI risk factors. The RVI and TTPVI mentioned in previous studies were also significantly correlated with MVI, indicating that they are risk factors for MVI. Multivariate logistic regression analysis confirmed that capsular invasion was an independent risk factor for MVI. TTPVI, peritumoral enhancement, capsule integrity, and density differences between the tumor and liver interface were also identified as independent MVI risk factors.
Selection and modeling of radiomics features
We excluded features with correlation coefficients > 0.90. Hypothesis testing was performed on the remaining radiomics features to select those with significant statistical differences between the groups characterized by the presence or absence of MVI. Finally, we employed the LASSO algorithm to identify and select the most predictive features for building the model.
The number of parameters of the 5-mm peritumoral radiomics models in the arterial phase is 21. Radscore A (Vper5mm) = 0.29714285714285715
-
− 0.074353 X_wavelet-HHL_glcm_Imc2
-
− 0.057030 X_original_shape_Elongation
-
− 0.053594 X_original_glcm_MaximumProbability
-
− 0.049559 X_wavelet-LHH_glcm_ClusterShade
-
− 0.037649 X_square_ngtdm_Busyness
-
− 0.037427 X_exponential_glszm_SmallAreaEmphasis
-
− 0.020075 X_original_glszm_SmallAreaLowGrayLevelEmphasis
-
− 0.019563 X_wavelet-HLH_glcm_InverseVariance
-
− 0.016828 X_logarithm_glcm_Imc1
-
− 0.014204 X_log-sigma-0–5-mm-3D_firstorder_Median
-
− 0.009435 X_original_glcm_Imc1
-
+ 0.000737 X_square_glszm_LowGrayLevelZoneEmphasis
-
+ 0.002325 X_original_glcm_Imc2
-
+ 0.004567 X_wavelet-LHL_firstorder_Kurtosis
-
+ 0.006704 X__wavelet-LLL_glcm_Correlation
-
+ 0.008527 X_exponential_glcm_MCC
-
+ 0.017787 X_log-sigma-4–5-mm-3D_gldm_SmallDependenceEmphasis
-
+ 0.018892 X_wavelet-HLL_glcm_Correlation
-
+ 0.041008 X_original_glcm_Correlation
-
+ 0.041567 X_squareroot_firstorder_Kurtosis
-
+ 0.049917 X_wavelet-LLL_glszm_GrayLevelNonUniformityNormalized
The number of parameters of the 10-mm peritumoral radiomics models in the arterial phase is 12. Radscore A (Vper10mm) = 0.2894528153595409
-
− 0.028023 X_square_ngtdm_Busyness
-
− 0.019626 X_wavelet-HLH_firstorder_Mean
-
− 0.013075 X_original_glcm_MaximumProbability
-
+ 0.001592 X_original_glcm_Correlation
-
+ 0.005310 X_exponential_glcm_Idmn
-
+ 0.011947 X_exponential_glcm_Correlation
-
+ 0.014276 X_original_shape_Maximum3DDiameter
-
+ 0.017465 X_log-sigma-2–5-mm-3D_gldm_SmallDependenceEmphasis
-
+ 0.024618 X_squareroot_glcm_Imc2
-
+ 0.033227 X_original_glcm_Imc2
-
+ 0.035365 X_original_gldm_DependenceEntropy
-
+ 0.046991 X_wavelet-LHL_firstorder_Kurtosis
The number of parameters of the 5-mm peritumoral radiomics models in the portal venous phase is 14. Radscore V (Vper5mm) = 0.2971428571428572
-
− 0.036178 X_wavelet-LLL_ngtdm_Busyness
-
− 0.030546 X_original_shape_Elongation
-
− 0.020929 X_wavelet-HHL_glcm_Imc2
-
− 0.020450 X_squareroot_glcm_Imc1
-
− 0.014857 X_wavelet-LLL_glszm_SmallAreaLowGrayLevelEmphasis
-
− 0.014509 X_wavelet-HLL_firstorder_Median
-
− 0.009716 X_wavelet-LHH_glcm_InverseVariance
-
+ 0.009029 X_original_shape_Maximum3DDiameter
-
+ 0.012752 X_log-sigma-4–5-mm-3D_firstorder_90Percentile
-
+ 0.019192 X_wavelet-HLL_glrlm_RunVariance
-
+ 0.022603 X_squareroot_glcm_Imc2
-
+ 0.027566 X_wavelet-LHH_glcm_Imc1
-
+ 0.042216 X_log-sigma-0–5-mm-3D_ngtdm_Busyness
-
+ 0.046826 X_wavelet-LLH_firstorder_InterquartileRange
The number of parameters of the 10-mm peritumoral radiomics models in the portal venous phase is 25. Radscore V (Vper10mm) = 0.29714285714285743
-
− 0.071165 X_wavelet-HHH_glcm_InverseVariance
-
− 0.064444 X_wavelet-HHH_glszm_SizeZoneNonUniformityNormalized
-
− 0.046372 X_original_glszm_ZoneEntropy
-
− 0.037803 X_wavelet-LLL_ngtdm_Busyness
-
− 0.034973 X_logarithm_gldm_SmallDependenceLowGrayLevelEmphasis
-
− 0.033692 X_wavelet-LHL_gldm_SmallDependenceLowGrayLevelEmphasis
-
− 0.031802 X_original_glcm_JointEnergy
-
− 0.028619 X_log-sigma-1–5-mm-3D_firstorder_Median
-
− 0.025689 X_wavelet-LLH_glrlm_RunEntropy
-
− 0.016416 X_squareroot_glszm_ZoneEntropy
-
− 0.014365 X_wavelet-LLL_glcm_JointEnergy
-
− 0.012163 X_squareroot_glrlm_ShortRunLowGrayLevelEmphasis
-
− 0.007045 X_original_ngtdm_Busyness
-
+ 0.002252 X_original_gldm_DependenceEntropy
-
+ 0.007024 X_log-sigma-1–5-mm-3D_glszm_GrayLevelNonUniformity
-
+ 0.011931 X_logarithm_firstorder_RobustMeanAbsoluteDeviation
-
+ 0.013431 X_logarithm_glszm_GrayLevelNonUniformityNormalized
-
+ 0.023832 X_log-sigma-0–5-mm-3D_gldm_SmallDependenceLowGrayLevelEmphasis
-
+ 0.028906 X_original_shape_Maximum3DDiameter
-
+ 0.030032 X_logarithm_glcm_MCC
-
+ 0.033813 X_log-sigma-4–5-mm-3D_firstorder_90Percentile
-
+ 0.035153 X_wavelet-LLH_firstorder_RobustMeanAbsoluteDeviation
-
+ 0.047734 X_original_firstorder_InterquartileRange
-
+ 0.066691 X_wavelet-LLL_glcm_Imc2
-
+ 0.113210 X_wavelet-HHL_glrlm_LongRunLowGrayLevelEmphasis
The ROC illustrating model effectiveness is shown in Figs. 3 and 4. In the arterial phase, the 5-mm peritumor validation set AUC value was 0.759 and that of the training set was 0.887. Comparatively, for the 10-mm peritumoral validation set, it was 0.657, and for the training set, it was 0.811. In the portal venous phase, the AUC value of the 5-mm verification set was 0.626, and that of the training set was 0.851. The verification and training sets had AUC values of 0.693 and 0.943, respectively. The model validation results indicated higher diagnostic efficiency of the 5-mm peritumoral area in the arterial phase compared to the 10-mm peritumoral area, while the diagnostic efficiency of the 5- and 10-mm peritumoral areas in the portal vein phase showed similar diagnostic efficiency in the validation set. Therefore, the 5-mm peritumoral area is more efficient than the 10-mm area for predicting MVI.
In clinical practice, the diagnostic process often combines multi-stage imaging features. Therefore, this study combined the radiomics features of these four independent volumes of interest in pairs to generate combined models. The models including peritumoral radiomics features showed superior predictive efficacy compared to the models including tumors alone, and the arterial phase provided more diagnostic information to predict the MVI of HCC compared to the portal vein phase. The AUC values of the combined model’s training set were stable (> 0.85). The highest AUC value of the combined model’s test set was 0.82, which was defined as the optimal combined model. The model was established by combining the tumor and the 5-mm peritumoral imaging features in the arterial phase. Finally, 28 parameters were selected, 13 from tumor and 15 from 5-mm peritumoral imaging.
Radscore A(Vt) + A(Vper) = 0.29714285714285704
-
− 0.061300 X(A)_original_glcm_MaximumProbability
-
− 0.056516 X(A)_original_shape_Elongation
-
− 0.045483 X(A)_wavelet-LHH_glcm_ClusterShade
-
− 0.043287 X(A)_square_ngtdm_Busyness
-
− 0.035068 X(A)_exponential_glszm_SmallAreaEmphasis
-
− 0.034264 X(Aper)_wavelet-HHL_glcm_MCC
-
− 0.028729 X(Aper)_wavelet-HLL_glrlm_ShortRunEmphasis
-
− 0.024973 X(Aper)_wavelet-HHH_glcm_Contrast
-
− 0.022994 X(Aper)_wavelet-LLL_firstorder_Range
-
− 0.016860 X(A)_wavelet-HHL_glcm_Imc2
-
− 0.014491 X(Aper)_wavelet-HHH_gldm_LowGrayLevelEmphasis
-
− 0.011767 X(A)_original_glszm_SmallAreaLowGrayLevelEmphasis
-
− 0.006108 X(Aper)_wavelet-LLL_firstorder_10Percentile
-
− 0.005779 X(Aper)_logarithm_glcm_Imc1
-
− 0.003976 X(A)_log-sigma-0–5-mm-3D_firstorder_Median
-
− 0.002726 X(Aper)_wavelet-HHL_glcm_Imc2
-
− 0.002492 X(Aper)_original_glszm_LowGrayLevelZoneEmphasis
-
+ 0.005516 X(Aper)_log-sigma-2–5-mm-3D_gldm_SmallDependenceEmphasis
-
+ 0.014060 X(A)_original_glcm_Correlation
-
+ 0.021479 X(Aper)_logarithm_firstorder_Kurtosis
-
+ 0.025073 X(A)_wavelet-LLL_glcm_Correlation
-
+ 0.025199 X(Aper)_log-sigma-4–5-mm-3D_firstorder_90Percentile
-
+ 0.030258 X(Aper)_logarithm_gldm_SmallDependenceHighGrayLevelEmphasis
-
+ 0.030717 X(A)_wavelet-LLL_glszm_GrayLevelNonUniformityNormalized
-
+ 0.031095 X(A)_wavelet-HLL_glcm_Correlation
-
+ 0.044388 X(A)_squareroot_firstorder_Kurtosis
-
+ 0.045011 X(Aper)_wavelet-LHL_glszm_SizeZoneNonUniformityNormalized
-
+ 0.051918 X(Aper)_square_firstorder_Skewness
Comparison between the optimal radiomics and clinical models
After the multivariate logistic analysis, preoperative indicators were selected from the clinical baseline data to establish a preoperative diagnostic model of MVI based on traditional clinical data. The indicators included peritumoral enhancement, capsule status, density differences between the tumor and liver interface, and TTPVI. The fusion model was then established by combining optimal radiomics and clinical data. The comparative efficiencies of the three models are shown in Fig. 5. In terms of diagnostic efficiency, the optimal radiomics model had greater diagnostic efficiency than the traditional clinical model, and the fusion model had the highest diagnostic efficiency.
Decision curve drawing
The light gray diagonal line in Fig. 6 indicates the relationship between all models and the incidence of MVI across all patients. When the threshold probability exceeds 0.1, the benefits of the fusion model are greater than those of the single model, and the benefits derived from the tumor and peritumoral radiomics model surpass those of the traditional clinical model indicating that, in clinical practice, the benefits of the tumor and peritumoral radiomics model are slightly greater than those of traditional models, while the benefits of the fusion model are greater than any single model. The fusion model has good application value in clinical practice. In the external validation cohort, the optimal combination model exhibited a diagnostic AUC value of 0.643, while the AUC value of the clinical and radiomics fusion model reached 0.753.
Decision curves of different models. The green line represents the clinical model, the blue line represents the radiomics model (arterial tumor + arterial peritumor 5 mm), and the red line represents the fusion model. From the analysis of the decision curve, it can be concluded that when the threshold probability range is greater than 0.1, the fusion model can obtain a large net benefit
Discussion
Partial hepatectomy or liver transplantation is often more beneficial for patients with stage T2 tumors (early-stage HCC) than for patients in later disease stages. However, within the subset of early-stage HCC tumors measuring ≤ 5 cm, postoperative prognoses can vary significantly, with worse prognoses for patients with MVI. Therefore, this study focused on accurately predicting preoperative MVI statuses in patients with early-stage HCC in order to formulate individualized treatment plans for this specific patient group. Discussions on this tumor subgroup, particularly concerning CT-based radiomics, are rarely reported.
Regarding the biological behavior of HCC, many scholars have established MVI models to study the radiomics features of tumors through preoperative imaging [19]. Radiomics aims to uncover the biological nature of tumors using high-throughput data. Chen et al. conducted an MRI radiomics study of hepatobiliary-specific contrast agents, showing that the inclusion of radiomics features within a 1-cm peritumoral area could effectively predict intratumor immune score grading [20]. Similarly, an MRI-enhanced radiomics study of breast cancer showed that the combined radiomics features of the tumor and peritumoral area could effectively predict the efficacy of neoadjuvant chemotherapy [21]. A growing body of research indicates that small changes in the structure of normal tissues surrounding tumors can provide information regarding biological tumor behavior. A study of non-small cell lung adenocarcinoma showed that the 3-mm peritumoral region was different from the 3- to 9-mm peritumoral region and that the inclusion of the 3-mm peritumoral region significantly enhanced the radiomics model’s efficacy in predicting distant metastasis [22]. The abovementioned studies indicate that the peritumoral area can be used to obtain biological information about tumors; however, a unified definition of the peritumoral scope remains absent.
The results of this study showed that for HCC less than 5 cm, both the 5- and 10-mm peritumoral histological models held predictive value for MVI. ROC analysis of the model validation set revealed that the 5-mm peritumoral arterial histological model outperformed the 10-mm peritumoral model in diagnostic efficiency. No significant difference in diagnostic efficacy between the 5-mm peritumoral histological model and the 10-mm peritumoral model was noted at the portal vein stage. The diagnostic efficiency of the 5-mm peritumoral model in the arterial stage was the highest. Therefore, in the following discussion, a 5-mm peritumoral range is regarded as the focus of this study. In a separate study of lung adenocarcinoma, researchers found that including peritumoral data enhanced the diagnostic efficiency in distinguishing between benign and malignant nodules, but as the spread distance increased, the efficacy of the peritumoral diagnostic model gradually decreased [23], a trend that aligns with our study’s findings.
The combination of the tumor and peritumoral area in the arterial stage had the greatest diagnostic efficiency, with a training set AUC value of 0.941 and a validation set AUC value of 0.820. This model was determined to be the optimal combination model. MVI can change the hemodynamics around the tumor. Therefore, peritumoral models of the arterial and portal phases provide valuable information, and peritumoral diagnosis of the arterial phase was of greater significance in this study. A fusion model, including the optimal radiomics combination model and clinical indicators after multi-factor regression analysis, was established. Considering the wide application of preoperative enhanced CT in clinical practice, this study incorporated traditional imaging features into clinical data. Peritumoral enhancement is significantly correlated with MVI, which may be caused by changes in peritumoral blood flow and abnormal perfusion [24, 25]. The presence of intratumoral arteries often indicates rapid growth and strong invasiveness of tumors [26, 27]. Capsule integrity acts as a physical barrier to the tumor and can hinder the occurrence of MVI [28]. In this study, when the density difference between the tumor and liver interface was not significant, the incidence of MVI was higher, which is something that is not discussed in many studies. Here, we included the RVI and TTPVI in the clinical model, which has rarely been done by other researchers. Radiomics is a means of presenting the microscopic environment, which is otherwise invisible to the naked eye, in order to improve diagnostic accuracy. The fusion model established in this study also demonstrated optimal clinical application value in the decision curve analysis.
There are several limitations to this study. First, its retrospective nature comes with an inherent bias. While external validation was part of the experimental design, larger cohort sizes and additional external validations are necessary. Second, only 206 patients were included, most of whom had hepatitis-associated HCC. Future studies should also consider alcoholic liver disease or non-alcoholic steatohepatitis. Third, we did not examine the correlation between genomic and radiomics features, which could further clarify the impact of radiomics. Finally, our research, which was based on enhanced CT images, lacks multimodal image data.
Conclusion
In conclusion, a radiomics model including a 5-mm peritumoral expansion stands as a promising noninvasive biomarker for preoperatively predicting MVI in individuals with a solitary HCC ≤ 5 cm. This model can aid in shaping personalized treatment policies.
Availability of data and materials
Please contact the author for data requests.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate transaminase
- AUC:
-
Area under the curve
- CT:
-
Computed tomography
- HCC:
-
Hepatocellular carcinoma
- ICC:
-
Intra-observer and inter-observer correlation coefficients
- LASSO:
-
Least absolute shrinkage and selection operator
- MRI:
-
Magnetic resonance imaging
- MVI:
-
Microvascular invasion
- ROC:
-
Receiver operating characteristic
- RVI:
-
Radiogenomic venous invasion
- TTPVI:
-
Two-trait predictor of venous invasion
- VOI:
-
Volume area of interest
- Vtumor:
-
Tumor VOI
References
Ferlay J, Colombet M, Soerjomataram I et al (2021) Cancer statistics for the year 2020: an overview. Int J Cancer. https://doi.org/10.1002/ijc.33588
Raoul JL, Edeline J (2020) Apr Systemic treatment of hepatocellular carcinoma: standard of care in China and elsewhere. Lancet Oncol 21:479–481. https://doi.org/10.1016/S1470-2045(20)30082-6
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Jan Cancer statistics, 2021. CA Cancer J Clin 71:7–33. https://doi.org/10.3322/caac.21654
Cheng Z, Yang P, Qu S et al (2015) Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 17:422–427. https://doi.org/10.1111/hpb.12367
Ueno S, Kubo F, Sakoda M et al (2008) Efficacy of anatomic resection vs nonanatomic resection for small nodular hepatocellular carcinoma based on gross classification. J Hepatobiliary Pancreat Surg 15:493–500. https://doi.org/10.1007/s00534-007-1312-8
Guo D, Gu D, Wang H et al (2019) Aug Radiomics analysis enables recurrence prediction for hepatocellular carcinoma after liver transplantation. Eur J Radiol 117:33–40. https://doi.org/10.1016/j.ejrad.2019.05.010
Zhang X, Li C, Wen T et al (2015) Aug Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol 27:933–940. https://doi.org/10.1097/MEG.0000000000000383
Yao Z, Dong Y, Wu G, et al. (2018) Preoperative diagnosis and prediction of hepatocellular carcinoma: radiomics analysis based on multi-modal ultrasound images. BMC Cancer 18:1089. https://doi.org/10.1186/s12885-018-5003-4
Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK (2013) Jan A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 20:325–339. https://doi.org/10.1245/s10434-012-2513-1
Lei Z, Li J, Wu D et al (2016) Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 151:356–363. https://doi.org/10.1001/jamasurg.2015.4257
Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL (2014) Sep Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol 203:W253–W259. https://doi.org/10.2214/AJR.13.10595
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Mar Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446. https://doi.org/10.1016/j.ejca.2011.11.036
Li H, Zhu Y, Burnside ES et al (2016) Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer 2:16012. https://doi.org/10.1038/npjbcancer.2016.12
Wu M, Tan H, Gao F et al (2019) Jun Predicting the grade of hepatocellular carcinoma based on non-contrast-enhanced MRI radiomics signature. Eur Radiol 29:2802–2811. https://doi.org/10.1007/s00330-018-5787-2
Yang G, Nie P, Zhao L et al (2020) Aug 2D and 3D texture analysis to predict lymphovascular invasion in lung adenocarcinoma. Eur J Radiol 129:109111. https://doi.org/10.1016/j.ejrad.2020.109111
Yang Y, Fan W, Gu T et al (2021) Radiomic features of multi-ROI and multi-phase MRI for the prediction of microvascular invasion in solitary hepatocellular carcinoma. Front Oncol 11:756216. https://doi.org/10.3389/fonc.2021.756216
Banerjee S, Wang DS, Kim HJ, et al. (2015) A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 62:792–800. https://doi.org/10.1002/hep.27877
Renzulli M, Brocchi S, Cucchetti A et al (2016) May Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma? Radiology 279:432–442. https://doi.org/10.1148/radiol.2015150998
Ma X, Wei J, Gu D et al (2019) Jul Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol 29:3595–3605. https://doi.org/10.1007/s00330-018-5985-y
Chen S, Feng S, Wei J et al (2019) Aug Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur Radiol 29:4177–4187. https://doi.org/10.1007/s00330-018-5986-x
Braman NM, Etesami M, Prasanna P et al (2017) Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res 19. https://doi.org/10.1186/s13058-017-0846-1
Dou TH, Coroller TP, van Griethuysen JJM, Mak RH, Aerts HJWL (2018) Peritumoral radiomics features predict distant metastasis in locally advanced NSCLC. PLoS One 13:e0206108. https://doi.org/10.1371/journal.pone.0206108
Akinci D’Antonoli T, Farchione A, Lenkowicz J et al (2020) Apr CT radiomics signature of tumor and peritumoral lung parenchyma to predict nonsmall cell lung cancer postsurgical recurrence risk. Acad Radiol 27:497–507. https://doi.org/10.1016/j.acra.2019.05.019
Wu S, Zhang N, Wu Z, Ren J, Can EL (2022) Feb Can Peritumoral Radiomics Improve the Prediction of Malignancy of Solid Pulmonary Nodule Smaller Than 2 cm? Acad Radiol 29(Suppl 2):S47–S52. https://doi.org/10.1016/j.acra.2020.10.029
Xu QQ, Shan WL, Zhu Y, Huang CC, Bao SY, Guo LL (2021) Jun Prediction efficacy of feature classification of solitary pulmonary nodules based on CT radiomics. Eur J Radiol 139:109667. https://doi.org/10.1016/j.ejrad.2021.109667
Marquardt JU, Galle PR, Teufel A (2012) Jan Molecular diagnosis and therapy of hepatocellular carcinoma (HCC): an emerging field for advanced technologies. J Hepatol 56:267–275. https://doi.org/10.1016/j.jhep.2011.07.007
Qian X, Lu X, Ma X et al (2022) A multi-parametric radiomics nomogram for preoperative prediction of microvascular invasion status in intrahepatic cholangiocarcinoma. Front Oncol 12:838701. https://doi.org/10.3389/fonc.2022.838701
He M, Zhang P, Ma X, He B, Fang C, Jia F (2020) Radiomic feature-based predictive model for microvascular invasion in patients with hepatocellular carcinoma. Front Oncol 10:574228. https://doi.org/10.3389/fonc.2020.574228
Funding
This research was supported by the Medical Science and Technology Project of Henan Province, Joint Construction Project (LHGJ20210344).
Author information
Authors and Affiliations
Contributions
FW and MC: research design and conception; BD: data collection; WPH and LML: imaging data collection; JBG: review of the manuscript and data analysis. All authors have contributed to the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our hospital ethics committee approved this retrospective study; informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, F., Cheng, M., Du, B. et al. Predicting microvascular invasion in small (≤ 5 cm) hepatocellular carcinomas using radiomics-based peritumoral analysis. Insights Imaging 15, 90 (2024). https://doi.org/10.1186/s13244-024-01649-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-024-01649-0