Abstract
Objective
The purpose of this study was to evaluate the prognostic impact of body composition parameters based on computed tomography (CT) in patients with non-small cell lung cancer (NSCLC) who received ICI treatment.
Methods
This retrospective study analyzed the data from advanced NSCLC patients treated with ICI therapy between 2013 and 2019. We included patients with NSCLC who underwent baseline CT scans. The exclusion criteria included patients who received three or more lines of chemotherapy, those with insufficient clinical information, or those without treatment response evaluation.
Results
A total of 136 patients were enrolled. Among the volumetric body composition parameters, patients in the highest quartiles (Q2–4) of the visceral fat index (VFI) exhibited a higher response rate to ICI therapy than those in the lowest quartile (Q1) of VFI (Q1 vs. Q2–4: 18.2% vs. 43.1%, p = 0.012). Patients with a VFI in Q2–4 had significantly prolonged progression-free survival (PFS) and overall survival (OS) (PFS, Q1 vs. Q2–4: 3.0 months vs. 6.4 months, p = 0.043; OS, Q1 vs. Q2–4: 5.6 months vs. 16.3 months, p = 0.004). Kaplan–Meier analysis based on the VFI and visceral fat Hounsfield unit (HU) revealed that patients with VFI in Q1 and HU in Q2–4 had the worst prognosis.
Conclusions
Visceral fat volume is significantly associated with treatment outcomes in ICI-treated patients with NSCLC. Moreover, fat quality may impact the treatment outcomes. This finding underscores the potential significance of both fat compartments and fat quality as prognostic indicators.
Critical relevance statement
Visceral fat volume is significantly associated with treatment outcomes in ICI-treated patients with non-small cell lung cancer. Moreover, fat quality may impact the treatment outcomes. This finding underscores the potential significance of both fat compartments and fat quality as prognostic indicators.
Graphical Abstract

Key points
• We found that visceral fat volume positively correlated with treatment response and survival in patients with non-small cell lung cancer receiving immune checkpoint inhibitors.
• Additionally, a trend toward a negative correlation between visceral fat attenuation and survival was observed.
• The findings highlight the prognostic utility of fat compartments and fat quality.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of cancer-related mortality, with an estimated 2.2 million new cases recorded in 2020 [1]. Immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) can improve survival outcomes in patients with advanced non-small cell lung cancer (NSCLC) and have become the standard treatment [2, 3]; however, their efficacy varies widely among patients, with some exhibiting primary or acquired resistance to ICIs and having poor prognoses [4,5,6]. The ability to identify patients likely to benefit from ICI therapy can lead to more personalized treatment plans, reduced side effects from unnecessary treatments, and cost savings. Moreover, for patients predicted to have low responsiveness to ICIs, alternative treatment options such as targeted therapies, chemotherapy, or participation in clinical trials for novel agents could be considered, emphasizing the importance of monitoring treatment response and adjusting treatment plans as needed [7, 8]. Therefore, numerous studies have sought to identify predictive biomarkers that can determine which patients are likely to benefit from ICI therapy. [9,10,11]. Established predictive biomarkers for ICIs, including PD-L1 expression, tumor mutation burden, microsatellite instability, and tumor-infiltrating lymphocytes, are mainly related to cancer itself or the associated tumor microenvironment [9, 12]. Nonetheless, studies have examined the predictive utility of host-related factors, such as sex, age, and obesity, to establish the efficacy of ICIs [13,14,15].
Obesity-related inflammation can dysregulate the immune response, substantially impacting the efficacy and toxicity of immunotherapy across several cancer types [16,17,18,19,20]. A previous preclinical study has revealed that obesity contributes to PD-1-mediated T cell dysfunction; however, it may increase the responsiveness of tumor cells to ICIs [21]. Moreover, growing evidence has suggested an association between obesity and improved outcomes in patients with NSCLC undergoing ICI therapy [22, 23]. However, studies that employed body mass index (BMI) as a surrogate for obesity have reported inconsistent results regarding the relationship between BMI and the efficacy of ICIs [6, 23,24,25].
As an independent factor, BMI may be insufficient to accurately represent the complexity and heterogeneity of body composition owing to its low sensitivity, as indicated by discrepancies between BMI and central obesity [26, 27]. Accordingly, studies have explored the relationships of other adipose tissue indicators with clinical outcomes in patients with cancer [23, 28]. For example, computed tomography (CT) allows volumetric measurement of body composition, which enables analysis using fat volume and quality instead of BMI-based obesity. The recent advancements in artificial and machine learning have enhanced the speed and accuracy of extracting body composition indicators from CT scans, and measurements of these parameters before treatment have been demonstrated to play a crucial role in predicting health, offering the potential to improve patient outcomes when applied clinically [29, 30].
Herein, we aimed to evaluate the relationship between body composition parameters, as determined through pre-treatment CT, and clinical outcomes in patients with NSCLC receiving ICI therapy.
Methods
Study design and patients
This study is a single-center, retrospective investigation conducted in accordance with the Declaration of Helsinki and approved by the institutional review board (IRB No. 2001-069-1094). Informed consent was waived as the study relied on anonymous clinical data and images. We reviewed the electronic medical records of patients with advanced NSCLC who received ICI therapy between 2013 and 2019. The inclusion criteria were as follows: (a) patients with pathologically confirmed NSCLC aged 18 years or older, (b) patients who underwent baseline chest and abdominopelvic CT scans, and (c) patients who received ICI therapy. We excluded the following cases: (a) cases where ICI was administered after three or more lines of chemotherapy, (b) patients who had received prior ICI therapy, (c) patients with other malignancies requiring treatment, (d) patients without a baseline CT scan, (e) patients with insufficient clinical information, and (f) patients who did not undergo treatment response evaluation during ICI therapy.
Clinicopathological parameters and treatment outcomes
We collected data on the patient’s age at the time of ICI treatment initiation, sex, BMI (kg/m2), European Cooperative Oncology Group performance status (ECOG PS), smoking status, histology, driver oncogene mutations, PD-L1 immunohistochemistry findings, previous systemic therapies, treatment outcomes, and survival status. Furthermore, the treatment response and progression-free survival (PFS) were evaluated using Response Evaluation Criteria in Solid Tumors, version 1.1.
CT protocol
All contrast-enhanced CT scans were obtained during the portal venous phase using one of the following multi-detector CT scanners from four manufacturers: Philips Medical Systems (Ingenuity CT [n = 31], Brilliance 64 [n = 16], iCT 256 [n = 5]), GE Healthcare (Discovery CT750 HD [n = 28], Revolution [n = 21]; Siemens Healthineers (SOMATOM Definition [n = 17], SOMATOM Force [n = 4]), and Canon Medical Systems (Aquilion One [n = 14]). The scans were acquired using voltage settings of 100 to 140 kVp (100 kVp [n = 53], 120 kVp [n = 74], and 140 kVp [n = 9]) with automatic exposure control. All CT images were reconstructed with a soft tissue kernel and had a slice thickness of 3 mm or less.
CT analysis
Abdominal CT images were imported into a commercially available deep learning-based body composition analysis software (DeepCatch, v1.0.0.0, MEDICALIP Co. Ltd., Seoul, Korea). The software comprised a three-dimensional (3D) U-Net that segmented CT images into seven classes: skin, muscle, bone, abdominal visceral fat, subcutaneous fat, internal organs/vessels, and central nervous system. In the external validation, the 3D U-Net achieved an average Dice score of 92.3–99.3% for muscle, visceral fat, and subcutaneous fat [31]. A representative CT image is shown in Additional file 1: Fig. S1. An experienced radiologist (SHY) with 17 years of experience in body CT interpretation reviewed the segmentation results obtained with the software and revised the results as appropriate. Subsequently, the software yielded CT-derived parameters, including total fat volume (cm3), visceral fat volume (cm3), subcutaneous fat volume (cm3), skeletal muscle volume (cm3), visceral fat attenuation (Hounsfield units [HUs]), and subcutaneous fat attenuation (HU) at the waist, which was defined according to the World Health Organization definition as “between the 12th rib and the iliac crest” [32]. The total fat volume, visceral fat volume, subcutaneous fat volume, and skeletal muscle area were normalized for height in square meters to calculate the total fat index (TFI), visceral fat index (VFI), subcutaneous fat index (SFI), and skeletal muscle index (SMI), respectively. Given the lack of established cutoff values for fat volume indexes and fat attenuation for evaluating survival outcomes, we arbitrarily split the data into the lowest (Q1) and highest (Q2–4) quartiles. The Q1 cutoff values for TFI, VFI, and SFI were 404.1, 147.6, and 235.0, respectively. The SMI cutoff value was based on the Q1 for each sex (297.0 for males and 257.4 for females)
Statistical analysis
Categorical variables were compared using Pearson’s chi-square test. Univariate and multivariate logistic regression analyses were performed to evaluate the relationships between variables and the objective response to ICI therapy. PFS was defined as the period from initiating ICI treatment to clinical or radiographic progression or death. The overall survival (OS) was defined as the period from initiating ICI treatment to the date of the last follow-up or death. The follow-up period was set at 36 months. The median PFS and OS were calculated using the Kaplan–Meier method. Survival outcomes were compared using the log-rank test. Multivariate analysis of prognostic factors for survival was performed using the Cox proportional hazard model with a forward stepwise method. All statistical tests were two-sided, with statistical significance set at p < 0.05. All statistical analyses were performed using SPSS® Statistics, version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics and treatment response
Out of 228 advanced NSCLC patients treated with ICI, 92 were excluded based on the exclusion criteria, leaving a total of 136 patients enrolled in this study (Additional file 1: Fig. S2). Table 1 summarizes their baseline characteristics. The relationship between clinicopathological variables and the response to ICI treatment is shown in Table 2. Factors related to the treatment response included age, sex, and the number of lines of prior systemic therapy.
Volumetric parameters and differences in treatment outcomes by volumetric parameters
The median SMI, TFI, VFI, and SFI values were 333.6 (range, 45.1–533.0 cm3/m2), 598.0 (range, 30.8–1467.7 cm3/m2), 264.0 (range, 6.4–821.8 cm3/m2), and 314.4 (range, 24.3–764.9 cm3/m2), respectively. Fewer patients had a high VFI in the stable disease/progressive disease group (58/85, 68.2%) than in the partial response group (44/50, 88%) (p = 0.012, Table 2), with the logistic regression analysis showing a similar trend (Additional file 1: Table S1). Significantly higher proportions of patients had initial progressive disease as best response on ICIs in the Q1 group of TFI (Q1 vs. Q2–4: 50.0 % vs. 30.7%, p = 0.042) and Q1 group of VFI (Q1 vs. Q2–4: 60.6% vs. 27.5%, p = 0.001, data not shown) compared with the corresponding Q2–4 groups. There were no differences in SMI and SFI between the groups with different treatment responses.
Differences in survival outcomes by volumetric parameters
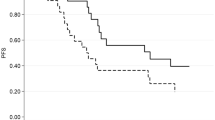
Kaplan–Meier survival analysis was performed using BMI and volumetric parameters. Obesity was defined as a BMI of 25 kg/m2 or higher [33]. Patients with obesity had a significantly longer PFS than those without obesity (BMI < 25 vs. ≥ 25 kg/m2; 3.9 months vs. 12.5 months, p = 0.025 using the log-rank test) (Fig. 1). Moreover, the Q2–4 group of VFI had a significantly longer PFS than the Q1 group (Q1 vs. Q2–4: 3.0 months vs. 6.4 months, p = 0.043 by log-rank test). Similar findings were obtained for the median OS in the obese group (BMI < 25 vs. ≥ 25 kg/m2: 12.6 months vs. 31.5 months, p = 0.024 by log-rank test) and Q2–4 group of VFI (Q1 vs. Q2–4: 5.6 vs. 16.3 months, p = 0.004 by log-rank test) (Fig. 2). However, no significant between-group differences were observed for other parameters, including SMI, TFI, and SFI.
We performed a Cox proportional hazard analysis to assess the impact of the clinicopathological and volumetric parameters on PFS and OS. In the multivariate analysis, VFI was an independent indicator for improved PFS (Q1 vs. Q2–4: hazard ratio [HR] 0.497, p = 0.004 using Cox proportional hazards regression) and OS (Q1 vs. Q2–4: HR 0.466, p = 0.002 using Cox proportional hazards regression) (Table 3).
Finally, to investigate not only the volume of fat but also the impact of fat quality on treatment outcomes, we performed a Kaplan–Meier survival analysis using a visceral fat HU cutoff value of − 91.13 (the first quartile of median HU). As shown in Fig. 3, there was a trend toward a shorter PFS and OS in the Q2–4 group of visceral fat HU than in the Q1 group (median PFS, Q1 vs. Q2–4: 15.3 months vs. 4.2 months, p = 0.213 using the log-rank test; median OS, Q1 vs. Q2–4: 21.7 months vs. 12.8 months, p = 0.142 using the log-rank test, Fig. 3). Subsequently, the study cohort was divided into four groups based on the VFI and visceral fat HU cutoff values; however, only one patient had both VFI and visceral fat HU in Q1 and was excluded from the analysis. Patients in the Q1 group of VFI who were also in the Q2–4 group of visceral fat HU had the shortest PFS and OS (median PFS = 3.0 months, 95% confidence interval [1.4–4.6 months]; median OS = 5.5 months, 95% confidence interval [3.8–7.2 months]; Fig. 3).
Discussion
In the present study, we examined the predictive utility of CT-based body composition parameters for treatment response and prognosis in patients with NSCLC who received ICI therapy. We found that the visceral fat volume positively correlated with the treatment response and survival.
Obesity is a well-established cause of cancer [34]. Specifically, a high BMI is a risk factor for the development of and mortality from several cancer types, including breast, colorectal, and kidney cancer [35]. Conversely, a high BMI has been shown to improve survival in patients with other types of cancers, and this phenomenon has been termed the “obesity paradox” [36]. Obesity can influence treatment response and related toxicities [37, 38]. Recent studies have investigated the role of obesity in patients with cancer undergoing treatment with ICIs [39], with some studies reporting improved treatment responses to ICIs in patients with obesity [16] and others demonstrating that obesity only improves PFS but not OS [40]. Nonetheless, most studies have demonstrated that treatment outcomes for ICI appear to be improved in patients with cancer with a high BMI [41,42,43].
Obesity contributes to chronic inflammatory conditions via multifaceted mechanisms. Specifically, obesity is associated with increased levels of leptin, free fatty acids, and pro-inflammatory cytokines. Additionally, obesity upregulates PD-1 expression in T cells and myeloid-derived suppressive cells. Collectively, the anti-tumor effects of ICIs could be amplified in the obesity-mediated inflammatory environment [39, 44].
It should be noted that BMI is not an optimal surrogate for obesity, given that it fails to accurately reflect body composition [28, 45,46,47]. Accordingly, studies have attempted to establish body composition parameters that better reflect obesity. Recent studies have explored the correlation between treatment outcomes and body composition parameters obtained through CT or positron emission tomography CT in patients with NSCLC treated with ICIs [23, 24]. One study, for example, explored the association between measures of skeletal muscle mass and adiposity (i.e., intramuscular, visceral, and subcutaneous adipose tissue) and changes during treatment, with a focus on disease progression and OS in patients with advanced lung cancer receiving immunotherapy [48]. The evaluation of the response to ICI treatment by measuring CT-based body composition at baseline, as done in the present study, is similar to this previous research. However, a key difference is that the correlation with disease progression was analyzed by calculating the delta value after early CT evaluation within 1–2 months, which allowed for the prediction of response changes [49]. In the present study, we evaluated the body composition using CT-based 3D volumetric analysis, which allows precise qualitative and quantitative analysis of adiposity. Visceral fat volume was associated with improved response and prolonged survival in patients with NSCLC who received ICI therapy. Additionally, visceral fat attenuation showed a tendency of a negative correlation with OS. Furthermore, patients with low VFI and high attenuation had the worst survival.
Visceral and subcutaneous fat display distinct anatomical distributions and gene expression profiles. Studies have reported differences in the expression of inflammation-related genes between abdominal visceral fat and subcutaneous fat [50, 51]. According to a previous study, pro-inflammatory cytokine genes were more abundant in the subcutaneous fat than in the visceral fat [51]. Furthermore, a previous study has reported a positive correlation between cancer mortality and fat attenuation [52]. Fat attenuation may be positively correlated with adipose tissue fibrosis, which is closely related to inflammation and cytokine release. Therefore, the fat compartment and quality may be more important than fat volume in immune regulation and response to ICI in patients with cancer [34].
In contrast to a previous report [53], we observed no correlation between SMI and clinical outcomes. Given that previous studies have used inconsistent criteria for sarcopenia, the optimal cutoff values for sarcopenia according to race, sex, or cancer type are yet to be established. A limitation of the present study is that it was a relatively small-scale retrospective study. In addition, the automatic CT segmentation method in this study does necessitate expert verification to confirm accurate segmentation, even though the deep learning algorithm showed high accuracy compared to the reference during its development [31]. However, this method greatly diminishes both time and computational demands, enabling more efficient analysis of extensive CT datasets. Additionally, we did not compare the CT-based body composition parameters with the results of other modalities, including dual-energy X-ray absorptiometry or bioelectrical impedance analysis; however, CT is considered the reference standard for assessing body composition [54]. Nonetheless, this study presents robust findings indicating the potential prognostic utility of abdominal visceral fat volume and attenuation in patients undergoing immunotherapy. A personalized management strategy for these patients could be developed by incorporating body composition parameter assessments into risk stratification and implementing targeted nutritional interventions.
Conclusions
We observed a significant association between visceral fat volume and treatment outcomes in patients with NSCLC who received ICI therapy, indicating the prognostic utility of fat compartments and fat quality. Future large-scale prospective studies are warranted to confirm our findings.
Availability of data and materials
The datasets generated or analyzed during the study are available from the corresponding authors upon reasonable request.
Abbreviations
- 3D:
-
Three-dimensional
- BMI:
-
Body mass index
- CT:
-
Computed tomography
- ECOG PS:
-
European Cooperative Oncology Group performance status
- HR:
-
Hazard ratio
- HU:
-
Hounsfield unit
- ICI:
-
Immune checkpoint inhibitor
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death ligand-1
- PFS:
-
Progression-free survival
- Q1:
-
Lowest quartile
- Q2–4:
-
Highest quartiles
- SFI:
-
Subcutaneous fat index
- SMI:
-
Skeletal muscle index
- TFI:
-
Total fat index
- VFI:
-
Visceral fat index
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–49
Horn L, Spigel DR, Vokes EE et al (2017) Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 35(35):3924–33
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–39
Chen P-L, Roh W, Reuben A et al (2016) Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 6(8):827–37
O’Donnell JS, Teng MWL, Smyth MJ (2019) Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 16(3):151–67
Cohen R, Duval A, Svrcek M (2019) Primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer-beyond the misdiagnosis-in reply. JAMA Oncol 5(5):741
Horvath L, Thienpont B, Zhao L, Wolf D, Pircher A (2020) Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC) - novel approaches and future outlook. Mol Cancer 19(1):141
Walsh RJ, Soo RA (2020) Resistance to immune checkpoint inhibitors in non-small cell lung cancer: biomarkers and therapeutic strategies. Ther Adv Med Oncol 12:1758835920937902
Bruno TC (2020) New predictors for immunotherapy responses sharpen our view of the tumour microenvironment. Nature 577(7791):474–6
Xiao Q, Nobre A, Piñeiro P et al (2020) Genetic and epigenetic biomarkers of immune checkpoint blockade response. J Clin Med 9(1):286
Lee PC, Chao Y, Chen MH et al (2020) Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel) 12(1):182
Yi M, Jiao D, Xu H et al (2018) Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 17(1):129
Conforti F, Pala L, Bagnardi V et al (2018) Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 19(6):737–46
Mojibi Y, Seif F, Mojibi N, Aghamajidi A, Mohsenzadegan M, Torang HA (2022) Efficacy of immunotherapy in obese patients with cancer. Immunopharmacol Immunotoxicol 44(4):471–83
Erbe R, Wang Z, Wu S et al (2021) Evaluating the impact of age on immune checkpoint therapy biomarkers. Cell Reports 36(8):109599
Cortellini A, Bersanelli M, Buti S et al (2019) A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 7(1):57
McQuade JL, Daniel CR, Hess KR et al (2018) Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 19(3):310–22
De Giorgi U, Procopio G, Giannarelli D et al (2019) Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res 25(13):3839–46
Martini DJ, Kline MR, Liu Y et al (2020) Adiposity may predict survival in patients with advanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer 126(3):575–82
Cortellini A, Bersanelli M, Santini D et al (2020) Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: a multicentre analysis of immune-related adverse events. Eur J Cancer 128:17–26
Wang Z, Aguilar EG, Luna JI et al (2019) Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 25(1):141–51
Ichihara E, Harada D, Inoue K et al (2020) The impact of body mass index on the efficacy of anti-PD-1/PD-L1 antibodies in patients with non-small cell lung cancer. Lung Cancer 139:140–5
Popinat G, Cousse S, Goldfarb L et al (2019) Sub-cutaneous fat mass measured on multislice computed tomography of pretreatment PET/CT is a prognostic factor of stage IV non-small cell lung cancer treated by nivolumab. Oncoimmunology 8(5):e1580128-e
Magri V, Gottfried T, Di Segni M et al (2019) Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Manag Res 11:8201–7
Vithayathil M, D’Alessio A, Fulgenzi CAM et al (2023) Impact of body mass index in patients receiving atezolizumab plus bevacizumab for hepatocellular carcinoma. Hepatol Int 17(4):904–14
Yu D, Zheng W, Johansson M et al (2018) Overall and central obesity and risk of lung cancer: a pooled analysis. J Natl Cancer Inst 110(8):831–42
Romero-Corral A, Somers VK, Sierra-Johnson J et al (2008) Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 32(6):959–66
Song X, Jousilahti P, Stehouwer CDA et al (2013) Comparison of various surrogate obesity indicators as predictors of cardiovascular mortality in four European populations. Eur J Clin Nutr 67(12):1298–302
Elhakim T, Trinh K, Mansur A, Bridge C, Daye D (2023) Role of machine learning-based CT body composition in risk prediction and prognostication: current state and future directions. Diagnostics (Basel) 13(5):968
Tolonen A, Pakarinen T, Sassi A et al (2021) Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: a review. Eur J Radiol 145:109943
Lee YS, Hong N, Witanto JN et al (2021) Deep neural network for automatic volumetric segmentation of whole-body CT images for body composition assessment. Clin Nutr 40(8):5038–46
Ross R, Neeland IJ, Yamashita S et al (2020) Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nature Reviews Endocrinology 16(3):177–89
Kim BY, Kang SM, Kang JH et al (2021) 2020 Korean society for the study of obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr 30(2):81–92
Khaddour K, Gomez-Perez SL, Jain N, Patel JD, Boumber Y (2020) Obesity, sarcopenia, and outcomes in non-small cell lung cancer patients treated with immune checkpoint inhibitors and tyrosine kinase inhibitors. Front Oncol 10:576314
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M (2019) Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism 92:121–35
Lee DH, Giovannucci EL (2019) The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep 8(3):175–81
Sinicrope FA, Foster NR, Yothers G et al (2013) Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer 119(8):1528–36
Wong AL, Seng KY, Ong EM et al (2014) Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res Treat 144(1):143–52
Woodall MJ, Neumann S, Campbell K, Pattison ST, Young SL (2020) The effects of obesity on anti-cancer immunity and cancer immunotherapy. Cancers (Basel) 12(5):1230
Donnelly D, Bajaj S, Yu J et al (2019) The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J Immunother Cancer 7(1):222
Takemura K, Yonekura S, Downey LE, Evangelopoulos D, Heng DYC (2022) Impact of body mass index on survival outcomes of patients with metastatic renal cell carcinoma in the immuno-oncology era: a systematic review and meta-analysis. Eur Urol Open Sci 39:62–71
Imai H, Naito E, Yamaguchi O et al (2022) Pretreatment body mass index predicts survival among patients administered nivolumab monotherapy for pretreated non-small cell lung cancer. Thorac Cancer 13(10):1479–89
Nie RC, Chen GM, Wang Y et al (2021) Association between body mass index and survival outcomes in patients treated with immune checkpoint inhibitors: meta-analyses of individual patient data. J Immunother 44(9):371–5
Wang Z, Monjazeb AM, Murphy WJ (2019) The complicated effects of obesity on cancer and immunotherapy. Immunotherapy 11(1):11–4
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9(7):629–35
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31(12):1539–47
Donohoe CL, Doyle SL, Reynolds JV (2011) Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr 3:12
Khan A, Welman CJ, Abed A et al (2023) Association of computed tomography measures of muscle and adipose tissue and progressive changes throughout treatment with clinical endpoints in patients with advanced lung cancer treated with immune checkpoint inhibitors. Cancers (Basel) 15(5):1382
Crombé A, Kind M, Toulmonde M, Italiano A, Cousin S (2020) Impact of CT-based body composition parameters at baseline, their early changes and response in metastatic cancer patients treated with immune checkpoint inhibitors. Eur J Radiol 133:109340
Wolfs MG, Rensen SS, Bruin-Van Dijk EJ et al (2010) Co-expressed immune and metabolic genes in visceral and subcutaneous adipose tissue from severely obese individuals are associated with plasma HDL and glucose levels: a microarray study. BMC Med Genomics 3:34
Spoto B, Di Betta E, Mattace-Raso F et al (2014) Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr Metab Cardiovasc Dis 24(10):1137–43
Rosenquist KJ, Massaro JM, Pedley A et al (2015) Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J Clin Endocrinol Metab 100(1):227–34
Li S, Wang T, Tong G, Li X, You D, Cong M (2021) Prognostic impact of sarcopenia on clinical outcomes in malignancies treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol 11:726257
Bernardi L, Roesel R, Vagelli F, Majno-Hurst P, Cristaudi A (2022) Imaging based body composition profiling and outcomes after oncologic liver surgery. Front Oncol 12:1007771
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Conceptualization: JEP, MK, and SHY: Data curation: JEP and JJ, Formal analysis: JEP and JJ, Investigation: JEP, MK, and SHY, Methodology: JEP, MK, and SHY, Resources: MK, JY, BK, TMK, and DWK, Supervision: MK and SHY, Visualization: JEP, Writing—original draft: JEP, Writing-review and editing: JEP, JJ, MK, JY, BK, TMK, DWK, and SHY
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board (IRB No. 2001-069-1094) and was conducted according to the Declaration of Helsinki. The requirement for obtaining consent was waived by the IRB.
Consent for publication
Not applicable.
Competing interests
MK received consulting fees from Merck Sharp & Dohme Corp., Ipsen, Bristol-Myers Squibb/Ono Pharmaceutical, Eisai, Boryung, and Yuhan outside the submitted work. TMK received honoraria from or played an advisory role with AstraZeneca; Boryung; F. Hoffmann-La Roche Ltd./Genentech, Inc.; IMBDx, Inc.; Janssen; Novartis; Regeneron; Samsung Bioepis; Sanofi; Takeda; and Yuhan and received research funding from AstraZeneca-Korea Health Industry Development Institute outside the submitted work. SHY works as a chief medical officer in MEDILCAL IP and has a stock option for the firm. The other authors declare that they have no competing interests.
Seoul National University Hospital has received research funding from Alpha Biopharma, Amgen, Astrazeneca/Medimmune, Boehringer-Ingelheim, Bridge BioTherapeutics, Chong Keun Dang, Daiichi-Sankyo, GSK, Hanmi, Janssen, Merck, Merus, Mirati Therapeutics, MSD, Novartis, ONO Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, Yuhan, and Inno outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Logistic regression hazard analysis of the prognostic factors for tumor response. CI, confidence interval; HR, hazard ratio; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; OR, odds ratio; PD-L1, programmed death ligand-1. Fig. S1. Representative image of CT scan analysis. The image in the upper left corner shows 3D, axial, sagittal, and coronal views (clockwise). In the 3D view, there are three transverse planes, and the two green planes represent the waist range (lowest rib to iliac crest), while the middle light green plane indicates the L3 level. Fig. S2. CONSORT Diagram.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, J.E., Jo, J., Youk, J. et al. Prognostic utility of body composition parameters based on computed tomography analysis of advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Insights Imaging 14, 182 (2023). https://doi.org/10.1186/s13244-023-01532-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-023-01532-4