Abstract
Background
It is not clear how physical activity affects body composition in adolescents. Physical activity levels are often reduced during this period, and the relative proportion of body fat mass and lean mass undergo natural changes in growing adolescents. We aimed to examine whether self-reported physical activity in leisure time at baseline or change in activity during follow-up affect changes in four measures of body composition; body mass index (kg/m2), waist circumference, fat mass index (fat mass in kg/m2) and lean mass index (lean mass in kg/m2).
Methods
We used data from the Tromsø Study Fit Futures, which invited all first year students in upper secondary high school in two municipalities in northern Norway in 2010–2011. They were reexamined in 2012–2013. Longitudinal data was available for 292 boys and 354 girls. We used multiple linear regression analyses to assess whether self-reported level of physical activity in leisure time at baseline predicted changes in body composition, and analysis of covariance to assess the effects of change in level of activity during follow-up on change in body composition. All analyses were performed sex-specific, and a p-value of < 0.05 was considered statistically significant.
Results
There were no associations between self-reported leisure time physical activity in the first year of upper secondary high school and changes in any of the considered measure of body composition after 2 years of follow up, with the exception of waist circumference in boys (p = 0.05). In boys, change in fat mass index differed significantly between groups of activity change (p < 0.01), with boys adopting activity or remaining physically active having less increase in fat mass index than the consistently inactive. In girls, change in lean mass index differed significantly between groups of activity change (p = 0.04), with girls adopting physical activity having the highest increase.
Conclusions
Self-reported leisure time physical activity does not predict changes in body composition in adolescents after 2 years of follow up. Change in the level of physical activity is associated with change in fat mass index in boys and lean mass index in girls.
Similar content being viewed by others
Background
Overweight or obesity in adolescence is a major risk factor for the same conditions as an adult [1], and therefore a risk factor for cardiovascular disease, type II diabetes, several types of cancer and musculoskeletal disorders in adulthood [2]. More than 20% of adolescents in Norway were in 2010 classified as overweight or obese [3]. Among adolescents in the Western world there is evidence for a plateauing of the obesity epidemic at a high level [4]. In Norwegian men and women, the prevalence of both overweight and obesity is increasing [5,6,7]. Studies have shown that adolescent lifestyle tend to persist into adulthood [8, 9], emphasizing the importance of preventing overweight and obesity in this period of life. A systematic review on the relationship between body composition and physical activity in adolescents showed conflicting results, with reverse causality suggested as a possible explanation – meaning that overweight and obesity could be both a cause and an effect of low physical activity [10]. The relationship is further complicated by associations with sedentary behavior, nutrition, socio-economic status and genetics [11,12,13,14].
There are several ways to quantify physical activity in adolescents. The use of direct observation of individuals or doubly labelled water has been suggested as gold standards, but questionnaire data are more feasible, domain specific and common in observational studies [10]. However, it must be acknowledged that self-reported physical activity tends to exaggerate the true amount of physical activity when compared to data from, for instance, accelerometers [15].
Arguably, the most common measure of body composition is body mass index (BMI, body weight in kg/m2), but its ability to identify changes in adiposity is limited as it does not distinguish between changes in fat mass and changes in lean mass [16]. This is a challenge when studying body composition in growing adolescents because boys naturally tend to gain more muscle mass than girls, while girls naturally gain more fat mass [17]. In addition to BMI, we therefore included waist circumference, fat mass index (FMI, fat mass in kg/m2) and lean mass index (LMI, lean mass in kg/m2) as measures of body composition in the present study. Waist circumference is an anthropometric measure which is specific to abdominal fatness [18]. FMI and LMI has been advocated as good measures of changes in adiposity in longitudinal studies because they measure fat mass and lean mass in relation to height [17, 19]. There are few studies investigating the longitudinal association between self-reported physical activity and tissue specific measures of body composition in adolescents [10], with a majority of those available using BMI as the primary outcome. To our knowledge, no studies modelling the association between physical activity and changes in FMI or LMI have been performed in Norway. Some international evidence points to a positive association between physical activity over the course of adolescence and LMI at age 18, but a less clear relationship with FMI [20]. Furthermore, higher self-reported physical activity has been associated with a positive change in lean mass [21], but not in fat mass [22].
We examined whether self-reported physical activity during leisure time was associated with change in measures of body composition after 2 years in upper secondary school in a cohort of adolescents in northern Norway; from a first measurement in 2010–2011 to a second measurement in 2012–2013. We further investigated whether changes in body composition differ between adolescents who are persistently inactive, persistently active, adopt activity or quit activity over the same period.
Methods
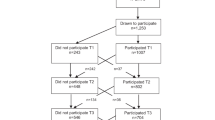
The Tromsø Study Fit Futures is a population-based cohort study, conducted in 2010–2011 (Fit Futures 1) and repeated in 2012–2013 (Fit Futures 2). The study invited all students in their first (Fit Futures 1) and third (Fit Futures 2) year of upper secondary school in the neighboring municipalities of Tromsø and Balsfjord in northern Norway. Fit Futures 1 invited 1117 students, with 1038 (93%) attending. Fit Futures 2 invited 1130 students and 870 (77%) attended. The participants in both studies answered a questionnaire and underwent a clinical examination at the clinical research unit at the University Hospital in Northern Norway, as detailed previously [23]. The present study includes only those participating in both Fit Futures 1 and Fit Futures 2. We excluded participants aged 18 years or older at baseline (Fit Futures 1), those without valid measurements of BMI, waist circumference, FMI and LMI at baseline and follow-up, and participants without information on physical activity at baseline. Altogether 292 boys and 354 girls were eligible for analyses.
Body weight was measured to the nearest 100 g with light clothing and height was measured to the nearest 0.1 cm on a Jenix DS 102 automatic electronic scale/stadiometer (Dong Sahn Jenix, Seoul, Korea). Waist circumference was measured to the nearest cm after expiration and at the height of the umbilicus. Total body fat mass and total body lean mass was measured using GE Lunar Prodigy dual-energy x-ray absorptiometry scanner (Lunar Corporation, Madison, Wisconsin, USA). Lean mass is comprised of all bodily tissue except fat and bone. Based on these measurements, Fat Mass Index (FMI, fat in kg/height in meters2) and Lean Mass Index (LMI, lean mass in kg/height in meters2) was calculated.
The prevalence of overweight or obesity in Fit Futures 1 was determined by applying the International Obesity Task Force body mass index reference values for adolescent populations, using age in half years [24, 25]. The participants were classified as underweight, normal weight, overweight or obese. These reference values correspond to an adult (aged 18 and above) BMI of < 18.5 kg/m2, 18.5 ≤ BMI < 25 kg/m2, 25.0 ≤ BMI < 30 kg/m2, and BMI ≥ 30.0 kg/m2, respectively.
The outcomes in this study were change in BMI, waist circumference, FMI and LMI between Fit Futures 1 and Fit Futures 2. The other variables included in the analyses were derived from the questionnaires. Our primary exposure was self-reported physical activity in leisure time, measured using the question “Are you physically active outside school hours? Yes/no”. Those answering “No” were labelled as physically inactive. Those answering “Yes” were asked “How many hours per week are you physically active outside of school hours?”. This question was used in the Health Behavior in School Children study and was validated for an adolescent population [26]. There are six response categories, from none to more than 7 h per week. One person in Fit Futures 1 reported “none” on this question, and was therefore also labelled as physically inactive. “About half an hour” and “About 1 to 1.5 hours” were combined, while the other responses were maintained unaltered. Together they formed the categorical physical activity variable used in the analyses.
Change in physical activity from baseline to follow up was defined by a dichotomous variable – “Active/inactive” – created based on the physical activity variable as described above. Being active was defined as physical activity ≥2 h per week. Those who were active in both surveys were labelled “consistently active” and those who were inactive in both were labelled “consistently inactive”. The participants who became active between surveys (increased level of activity from < 2 h to ≥2 h per week) were labelled “adopters”. Participants who reduced their level of activity from ≥2 h to < 2 h per week were labelled “quitters”. A similar approach has been used in other studies [27, 28]. In addition to the primary exposures, we included baseline measurements of hours per weekday outside of school hours spent in front of a computer or TV (screen time), age in half years, study specialization (which was either general, sports or vocational subjects) and regularity of eating breakfast in the analyses as possible confounders.
Puberty is associated with body composition in adolescents, but in this particular cohort, data from the Pubertal Development Scale (PDS) was missing in a substantial number (17.8%) of boys. We explored the effect of adjusting for PDS or age at menarche (in girls) in complete case analyses, but as this had no substantial impact on results, we did not include the variables in the final model.
Statistics
Results are presented sex-specific. We used descriptive statistics to determine the prevalence of overweight and obesity, levels of physical activity, mean values of BMI, waist circumference, FMI and LMI at baseline and follow-up as well as changes in BMI, waist circumference, FMI and LMI. Categorical variables were presented as proportions in percentages with number of subjects (n), while continuous variables were presented as means with standard deviation (SD) (Table 1). The associations between baseline physical activity and longitudinal changes in BMI, waist circumference, FMI and LMI were assessed using linear regression, with hours of physical activity coded to reflect the number of hours they represent. The associations with changes in activity status were assessed by analysis of covariance. As current body composition may affect the associations between physical activity and change in body composition, we adjusted all analyses for the baseline values. In the fully adjusted model we also included baseline measurements of sedentary behavior (screen time), study specialization, regularity of eating breakfast and age in half years, in addition to the time between baseline and follow-ups. We have presented adjusted beta coefficients for change in outcome at each level of physical activity at baseline (Table 2) or change in activity status (Table 3 and Table 4 in Appendix). A p-value of less than 0.05 was considered significant.
All statistical analyses were performed using STATA, version 14 (StataCorp, College Station, Texas, USA).
Results
Table 1 shows the descriptive characteristics of the study population. Mean BMI increased by 1.2 units for boys, and 0.8 units for girls between the surveys. On average, boys experienced a larger increase of both body height and body weight than girls. In boys, the combined prevalence of overweight and obesity (BMI ≥ 25) increased from 21.2 to 28.1%, while for girls it increased from 18.9 to 20.9%. Waist circumference increased less in girls (1.1 cm) than in boys (3.2 cm). Both sexes experienced a similar increase in FMI (0.7 kg/m2 in boys and 0.6 kg/m2 in girls). Boys experienced a small increase in LMI (0.4 kg/m2), whereas in girls there was no change. The proportion of adolescents classified as active in leisure time (active ≥ 2 h per week) decreased by 6%-points for boys and 12.2%-points for girls between the surveys.
There was no statistically significant linear effect of physical activity levels reported in 2010–2011 on change in neither BMI, FMI nor LMI during the following 2 years (Table 2). This was true for both sexes and also after adjustments. There were indications of a linear, inverse relationship with waist circumference in boys (p = 0.05), whereas a non-significant positive relationship was seen in girls. The most active boys gained less in BMI, waist circumference and FMI relative to the inactive, albeit not statistically significant. In contrast, the most active girls experienced a statistically significant higher adjusted increase in BMI (0.74 (95% CI: 0.04, 1.44)), waist circumference (2.80 (95% CI: 0.02, 5.57)) and FMI (0.90 (95% CI: 0.27, 1.53)) compared to the inactive girls. Stratified analyses including only girls who were active more than 6 h per week at baseline showed no difference in mean increase of BMI, FMI or waist circumference in consistently active girls compared to girls who reduced their level of physical activity. In boys, LMI increased most in those who at baseline were active between 0.5 and 1.5 h per week, but the increase was not significantly different from that observed among the inactive (0.26 (95% CI: − 0.09, 0.61)). In girls, change in LMI differed little across level of activity.
Table 3 presents changes in BMI, waist circumference, FMI and LMI according to change in activity status from 2010 to 2011 to 2012–2013. In both sexes, neither quitting activity nor adopting activity, relative to remaining inactive, was significantly associated with change in BMI or waist circumference. The consistently active boys had a significantly lower increase in waist circumference compared to the consistently inactive (− 2.32 (95% CI: − 4.40, − 0.24)). The largest increase in BMI and FMI (and for girls, also waist circumference) was observed among those quitting activity during follow-up, but this was not statistically significantly different from change among those who remained inactive.
In boys, changes in FMI were significantly different between activity groups (p < 0.01), with adopters (− 1.04 (95% CI -1.76, − 0.32)) and the consistently active (− 0.62 (95% CI: − 1.17, − 0.06)) gaining significantly less FMI than the consistently inactive. The difference in change in FMI comparing adopters and quitters was also statistically significant (− 1.06 (95% CI: − 1.83, − 0.28)) (Table 4 in Appendix). In girls there was no statistically significant difference in change of FMI between categories of activity, with the exception of the consistently active which gained less than those quitting activity (− 0.53 (95% CI: − 1.00, − 0.05)) (Table 4 in Appendix).
In boys, there was no statistically significant difference in change in LMI between the groups. In girls, change in LMI differed significantly between groups (p = 0.04). Girls who adopted activity between surveys experienced greater increase in LMI than the consistently inactive, but the difference was not of statistical significance (0.23 (95% CI: − 0.02, 0.49)). Compared to those quitting activity, girls who were consistently active (0.22 (95% CI: 0.03, 0.41)) or adopted physical activity (0.32 (95% CI: 0.07, 0.58)) experienced a statistically significantly higher increase in LMI (Table 4 in Appendix).
Discussion
In this population-based longitudinal study of changes in body composition in adolescents, there was, with the exception of waist circumference in boys, no linear association between self-reported leisure time physical activity and 2-year changes in indices of body composition. Change in physical activity was associated with statistically significant different changes in FMI. Boys who increased their physical activity during follow-up decreased their FMI compared to groups of boys quitting or remaining inactive, while consistently active girls experienced less increase than those reducing activity. Change in physical activity in girls was associated with statistically significant different changes in LMI. Girls who adopted physical activity increased their LMI compared to girls quitting activity.
Body weight, BMI and waist circumference increase during natural growth in children and adolescents, and it is therefore challenging to separate healthy- from unhealthy body development. Although the direction and magnitude of change will vary between individuals, a general increase in all the included measures of body composition is expected during this phase of life given the bodily- and hormonal changes that naturally takes place in adolescents [21]. Physical activity has positive health effects, but the associations with changes in adiposity among adolescents is complicated and conflicting results have been reported [29]. We found weak relationships between the frequency of leisure time physical activity at baseline and change in body composition, suggesting that change in body composition in this age group was mainly independent of level of self-reported physical activity. Girls who were most active at baseline had put on adipose tissue after 2 years (Table 2). A possible explanation could be that the increase occurred in girls who were active at baseline, but reduced their activity during follow up. Stratified analyses in categories of girls who were active more than 6 h per week at baseline did not support this explanation. Our findings are, however, in line with those of Kettaneh et al., who found that girls in the highest category of activity also experienced the largest increase in BMI, waist circumference, sum of skinfolds and percent body fat [17]. LMI remained unaltered between Fit Futures 1 and Fit Futures 2 (Table 1), suggesting that LMI changes little in females during late adolescence.
Lean mass is comprised of muscles and all bodily tissue except fat mass and skeletal mass. Since muscles are particularly important for oxidization of fat, they are also determinants of energy balance [30], and although physical activity increases muscle mass it is not the sole component of energy expenditure. Total energy expenditure is comprised of resting metabolic rate, the thermic effect of food, bodily movement and, for children and adolescents; energy required for growth [31]. This means that although physical activity declines, the effect on total energy expenditure is modest [17]. Adiposity is the result of a whole range of lifestyle-, sociocultural- and genetic factors. It is therefore difficult to pinpoint the impact of one behavior, and it is possible that factors other than physical activity – and changes in these, exert more influence on change in body composition [32].
Physical activity levels change rapidly in adolescents [33], thus challenging our ability to measure and capture the effect of physical activity on body composition in adolescents. Thus, a baseline measurement may be only modestly associated with prior- or future physical activity [31]. For instance, O’Loughlin et al. reported effects of physical activity on changes in adiposity after 1 year, but not 2 years in girls, and only after 2 years in boys. The authors hypothesized that change in levels of physical activity over follow-up may have contributed to the differences [34].
Boys adopting activity experienced a slight decrease in FMI between surveys. This finding differs from the observed increase in all other measures of body composition in both sexes, and in all other sub-groups of activity change. With the exception of waist circumference and FMI in boys, change in all measures of body composition among the consistently active did not differ statistically significantly from changes in the consistently inactive. Physical activity has a limited potential to affect the difference between these groups [32]. In the consistently inactive, there is less room for unhealthy weight gain as a result of inactivity. Conversely, among the consistently active there is less potential for preventing unhealthy weight gain through increased activity. These groups may be more susceptible to unhealthy weight gain through factors other than, or in addition to, physical activity. This can be considered as floor- and ceiling effects of physical activity, and means that the potential for activity related changes in adiposity is greatest among those who change their level of activity. The prevalence of physically active adolescents declined in our study, and for both sexes there was a rather consistent, albeit not statistically significant, pattern of the highest increase in BMI, waist circumference (not in boys) and FMI in those quitting activity. These findings indicate that those who reduce their level of activity over the course of adolescence are susceptible to unhealthy weight gain. This is of concern, since total activity decreases by 7% annually in adolescents [33]. Boys who adopted physical activity reduced their FMI between surveys and had the highest increase in LMI, indicating that the inactive may profit from increasing level of physical activity. In girls, we observed a statistically significant difference in change of FMI between those who were consistently active and those quitting activity, suggesting that there are negative consequences of reducing level of physical activity. However, girls naturally increase fat mass over the course of adolescence, whereas the same is true for lean mass in boys [17]. It is therefore possible that an increase in FMI in girls occurs regardless of activity level, whereas for boys, this may be prevented through activity. This can also explain why there was no significant associations between change in activity and change in BMI, as BMI does not distinguish between the overweight inactive (with high FMI) and the overweight active (with high LMI) [35].
Individuals may have, and report, high levels of physical activity because they try to lose weight, or they may have low (or high) body weight because of high activity. The problem of reverse causality applies also to longitudinal studies, as overweight adolescents may avoid engaging in physical activity on account of feeling inferior relative to their active peers [31, 36]. Self-reported physical activity is prone to information bias [26] and individuals tend to overestimate the true amount of their physical activity. This can potentially dilute an association with measures of body composition [15]. Furthermore, self-reported physical activity in leisure time does not capture the total level of activity, which can include active transportation to school and friends, physical education and other types of leisure time activity. Objective measures of physical activity can produce more accurate estimates, but are not necessarily associated with changes in adiposity [37]. Finally, studies have suggested that the intensity of activity is more important than the total amount of activity for adiposity [38, 39]. In our study, complete data on perceived physical activity intensity were not available, but in complete case analyses the inclusion of self-reported intensity did not affect results.
This study had several strengths, including the longitudinal design, the high participation rate and the inclusion of four objective measures of body composition. A limitation is the use of self-reported physical activity and the lack of full adjustment for dietary habits, since a validated food-frequency questionnaires or similar was not included in the study. Another limitation is the lack of adjustment for pubertal development due to missing data. However, in boys, the vast majority (≈73%) of complete cases reported pubertal maturation to be “underway”, meaning that the effect of adjusting for PDS would likely be small. Inclusion of PDS in complete case analyses did not indicate confounding by pubertal development. Another limitation is lack of adjustment for socioeconomic status. In the Fit Futures survey, a substantial number of participants reported not knowing parental level of education, thus limiting the possibilities for adjusting for this variable. However, the inclusion of study specialization in the analyses likely adjusts for some of the variance in socioeconomic status in adolescents [40, 41]. Lastly, in our study the length of follow-up was approximately 2 years, but in a population undergoing natural changes in body composition, it may take more time before physical inactivity manifests in body composition. The 3rd survey of the Fit Futures Study is in planning and will enable further research on how physical activity in late adolescence affects changes in body composition in early adulthood.
Conclusion
In this longitudinal study of changes in objectively measured body composition, we found that consistently inactive boys increased significantly more in fat mass index compared to those adopting physical activity or remaining consistently active, and that girls adopting physical activity increased their lean mass index significantly more than those who reduced physical activity. Adolescence is a time of transformation and it is challenging to pinpoint the effect of one behavior on change in body composition. Physical activity should nevertheless be encouraged because of the health benefits other than the prevention of adiposity.
Availability of data and materials
The data that support the findings of this study are available from UiT – The Arctic University of Norway, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of UiT – The Arctic University of Norway.
Abbreviations
- BMI:
-
Body Mass Index
- FMI:
-
Fat Mass Index
- LMI:
-
Lean Mass Index
- PDS:
-
Pubertal Development Scale
References
Engeland A, Bjørge T, Tverdal A, Søgaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology. 2004;15:79–85.
World Health Organization. Obesity and Overweight Fact Sheet. 2018. http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed Dec 2018.
Evensen E, Emaus N, Kokkvoll A, Wilsgaard T, Furberg AS, Skeie G. The relation between birthweight, childhood body mass index, and overweight and obesity in late adolescence: a longitudinal cohort study from Norway, the Tromsø study. BMJ Open. 2017. https://doi.org/10.1136/bmjopen-2016-015576.
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017. https://doi.org/10.1016/S0140-6736(17)32129-3.
Jacobsen BK, Aars NA. Changes in body mass index and the prevalence of obesity during 1994-2008: repeated cross-sectional surveys and longitudinal analyses. BMJ Open. 2015. https://doi.org/10.1136/bmjopen-2015-007859.
Midthjell K, Lee CM, Langhammer A, Krokstad S, Holmen TL, Hveem K, et al. Trends in overweight and obesity over 22 years in a large adult population: the HUNT study. Clin Obes. 2013;3:12–20.
Norwegian Institute of Public Health. Public Health Report: Health Status in Norway 2018. Oslo: Norwegian Institute of Public Health; 2018.
Boreham C, Robson PJ, Gallagher AM, Cran GW, Savage JM, Murray LJ. Tracking of physical activity, fitness, body composition and diet from adolescence to young adulthood: the young hearts project. Int J Behav Nutr Phys Act. 2004;1:14.
Deforche B, Van Dyck D, Deliens T, De Bourdeaudhuij I. Changes in weight, physical activity, sedentary behaviour and dietary intake during the transition to higher education: a prospective study. Int J Behav Nutr Phys Act. 2015;12:16.
Reichert FF, Baptista Menezes AM, Wells JC, Carvalho Dumith S, Hallal PC. Physical activity as a predictor of adolescent body fatness: a systematic review. Sports Med. 2009;39:279–94.
Goisis A, Sacker A, Kelly Y. Why are poorer children at higher risk of obesity and overweight? A UK cohort study. Eur J Pub Health. 2015. https://doi.org/10.1093/eurpub/ckv219.
Croezen S, Visscher TL, Ter Bogt NC, Veling ML, Haveman-Nies A. Skipping breakfast, alcohol consumption and physical inactivity as risk factors for overweight and obesity in adolescents: results of the E-MOVO project. Eur J Clin Nutr. 2009;63:405–12.
Silventoinen K, Jelenkovic A, Sund R, Hur YM, Yokoyama Y, Honda C, et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the COllaborative project of development of anthropometrical measures in twins (CODATwins) study. Am J Clin Nutr. 2016;104:371–9.
Janssen I, Katzmarzyk PT, Boyce WF, Vereecken C, Mulvihill C, Roberts C, et al. Comparison of overweight and obesity prevalence in school-aged youth from 34 countries and their relationships with physical activity and dietary patterns. Obes Rev. 2005;6:123–32.
Belcher BR, Moser RP, Dodd KW, Atienza AA, Ballard-Barbash R, Berrigan D. Self-reported versus accelerometer-measured physical activity and biomarkers among NHANES youth. J Phys Act Health. 2015;12:708–16.
Reichert FF, Wells JC, Ekelund U, Menezes AM, Victora CG, Hallal PC. Prospective associations between physical activity level and body composition in adolescence: 1993 Pelotas (Brazil) birth cohort. J Phys Act Health. 2015;12:834–9.
Kettaneh A, Oppert JM, Heude B, Deschamps V, Borys JM, Lommez A, et al. Changes in physical activity explain paradoxical relationship between baseline physical activity and adiposity changes in adolescent girls: the FLVS II study. Int J Obes. 2005;29:586–93.
Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3-19 y. Am J Clin Nutr. 2000;72:490–5.
Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. 2014;99:1020–4.
Ramires VV, Dumith SC, Wehrmeister FC, Hallal PC, Menezes AM, Goncalves H. Physical activity throughout adolescence and body composition at 18 years: 1993 Pelotas (Brazil) birth cohort study. Int J Behav Nutr Phys Act. 2016;13:105.
Baxter-Jones AD, Eisenmann JC, Mirwald RL, Faulkner RA, Bailey DA. The influence of physical activity on lean mass accrual during adolescence: a longitudinal analysis. J Appl Physiol. 2008;105:734–41.
Hallal PC, Reichert FF, Ekelund U, Dumith SC, Menezes AM, Victora CG, et al. Bidirectional cross-sectional and prospective associations between physical activity and body composition in adolescence: birth cohort study. J Sports Sci. 2012;30:183–90.
Winther A, Ahmed LA, Furberg AS, Grimnes G, Jorde R, Nilsen OA, et al. Leisure time computer use and adolescent bone health--findings from the Tromsø study, fit futures: a cross-sectional study. BMJ Open. 2015. https://doi.org/10.1136/bmjopen-2014-006665.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3.
Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335:194.
Booth ML, Okely AD, Chey T, Bauman A. The reliability and validity of the physical activity questions in the WHO health behaviour in schoolchildren (HBSC) survey: a population study. Br J Sports Med. 2001;35:263–7.
Rangul V, Holmen TL, Bauman A, Bratberg GH, Kurtze N, Midthjell K. Factors predicting changes in physical activity through adolescence: the young-HUNT study. J Adolesc Health. 2011;48:616–24.
Hermansen R, Broderstad AR, Jacobsen BK, Mahonen M, Wilsgaard T, Morseth B. The impact of changes in leisure time physical activity on changes in cardiovascular risk factors: results from the Finnmark 3 study and SAMINOR 1, 1987-2003. Int J Circumpolar Health. 2018;77:1459145.
Ekelund U, Hildebrand M, Collings PJ. Physical activity, sedentary time and adiposity during the first two decades of life. Proc Nutr Soc. 2014;73:319–29.
Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–82.
Must A, Tybor DJ. Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. Int J Obes. 2005;29(Suppl 2):S84–96.
Collings PJ, Wijndaele K, Corder K, Westgate K, Ridgway CL, Sharp SJ, et al. Objectively measured physical activity and longitudinal changes in adolescent body fatness: an observational cohort study. Pediatr Obes. 2016;11:107–14.
Dumith SC, Gigante DP, Domingues MR, Kohl HW 3rd. Physical activity change during adolescence: a systematic review and a pooled analysis. Int J Epidemiol. 2011;40:685–98.
O'Loughlin J, Gray-Donald K, Paradis G, Meshefedjian G. One- and two-year predictors of excess weight gain among elementary schoolchildren in multiethnic, low-income, inner-city neighborhoods. Am J Epidemiol. 2000;152:739–46.
Basterfield L, Reilly JK, Pearce MS, Parkinson KN, Adamson AJ, Reilly JJ, et al. Longitudinal associations between sports participation, body composition and physical activity from childhood to adolescence. J Sci Med Sport. 2015;18:178–82.
Laxer RE, Cooke M, Dubin JA, Brownson RC, Chaurasia A, Leatherdale ST. Behavioural patterns only predict concurrent BMI status and not BMI trajectories in a sample of youth in Ontario. PLoS One. 2018;13:e0190405.
Wilks DC, Besson H, Lindroos AK, Ekelund U. Objectively measured physical activity and obesity prevention in children, adolescents and adults: a systematic review of prospective studies. Obes Rev. 2011;12:e119–29.
van Sluijs EM, Sharp SJ, Ambrosini GL, Cassidy A, Griffin SJ, Ekelund U. The independent prospective associations of activity intensity and dietary energy density with adiposity in young adolescents. Br J Nutr. 2016;115:921–9.
Deere K, Sayers A, Davey Smith G, Rittweger J, Tobias JH. High impact activity is related to lean but not fat mass: findings from a population-based study in adolescents. Int J Epidemiol. 2012;41:1124–31.
Friestad C, Klepp KI. Socioeconomic status and health behaviour patterns through adolescence: results from a prospective cohort study in Norway. Eur J Pub Health. 2006;16:41–7.
Hagquist CE. Health inequalities among adolescents: the impact of academic orientation and parents' education. Eur J Pub Health. 2007;17:21–6.
Acknowledgements
The authors thank the participants in the study, as well as the staff at the Clinical Research Unit at the University Hospital of North Norway for data collection and clinical measurements. We also thank the Fit Futures Steering Committee in both studies.
Funding
This particular manuscript has not received any specific funding, while the Fit Futures surveys were funded by, inter alia, the Northern Norway Regional Health Authority and UiT – The Arctic University of Norway, Tromsø, and the National Public Health Institute, Oslo.
Author information
Authors and Affiliations
Contributions
NAA wrote the draft of the manuscript, which was revised and edited by BKJ, BM, NE and SG several times during the process. BKJ contributed to the statistical analyses, and BM specifically contributed to the discussion of physical activity. NE was the principal investigator in Fit Futures 2 and contributed significantly to the acquisition of data. SG formulated the research question and conceived the study. All authors have substantially contributed to the study, and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by The Regional Committee of Medical and Health Research Ethics in northern Norway (REK North), as part of the Tromsø Study Fit Futures cohort. Written consent was obtained from all participants aged ≥16 years. Those under 16 years of age brought signed, written consent from their parent or legal guardian.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Aars, N.A., Jacobsen, B.K., Morseth, B. et al. Longitudinal changes in body composition and waist circumference by self-reported levels of physical activity in leisure among adolescents: the Tromsø study, Fit Futures. BMC Sports Sci Med Rehabil 11, 37 (2019). https://doi.org/10.1186/s13102-019-0150-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-019-0150-8