Abstract
Background
Oral HIV pre-exposure prophylaxis (PrEP) for HIV prevention is highly effective, but uptake remains low in Africa, especially among young women who are a priority population for HIV prevention services. HIV self-testing (HIVST) has been proven to increase HIV testing in diverse populations but has been underutilized to support linkage to HIV prevention services. Most young women who initiate PrEP in Africa do so through informal peer referral. We wanted to test a model of formalized peer referral enhanced with HIVST delivery among young Kenyan women.
Methods
The Peer PrEP Trial is a two-arm hybrid effectiveness-implementation cluster-randomized controlled trial being conducted in central Kenya. Eligible participants (i.e., peer providers, n = 80) are women (≥ 16–24 years) refilling or initiating PrEP at public healthcare clinics who can identify at least four peers who could benefit from PrEP and not enrolled in another HIV study. Peer providers will be 1:1 randomized to (1) formal peer PrEP referral + HIVST delivery, where they will be encouraged to refer four peers (i.e., peer clients, ≥ 16–24 years) using educational materials and HIVST kits (two per peer client), or (2) informal peer PrEP referral, where they are encouraged to refer four peer clients using informal word-of-mouth referral. In both arms, peer providers will deliver a standard PrEP referral card with information on nearby public clinics delivering PrEP services. Peer providers will complete surveys at baseline and 3 months; peer clients will complete surveys at 3 months. Our primary outcome is PrEP initiation among peer clients, as reported by peer providers at 3 months. Secondary outcomes include PrEP continuation (any refilling), HIV testing (past 3 months), sexual behaviors (past month), and PrEP adherence (past month) among peer clients, as reported by both peer providers and clients at 3 months. Implementation outcomes will include participants’ perceived acceptability, appropriateness, and feasibility of the intervention as well assessments of the intervention’s fidelity and cost.
Discussion
Evidence from this trial will help us understand how HIVST could support health systems by facilitating linkage to PrEP services among young women who could benefit in Kenya and similar settings.
Trial registration
ClinicalTrials.gov NCT04982250. Registered on July 29, 2021.
Similar content being viewed by others
Introduction
Pre-exposure prophylaxis (PrEP) for HIV prevention is highly effective [1,2,3,4,5] but uptake and continuation remain low among young African women, a priority population for the delivery of HIV prevention services [6]. In Kenya, a country with the fourth largest HIV epidemic worldwide [7, 8], the government began delivering oral PrEP on a national scale in 2017 [9, 10]. One of the priority groups for HIV incidence reduction for the Kenya Ministry of Health is adolescent girls and young women (AGYW, ≥ 16–24 years), who account for 33% of the total new HIV infections in Kenya yet comprise only 10% of the population [11,12,13]. Barriers to PrEP initiation for this population are multi-faceted and include community (e.g., stigma associated with use) and intra-personal (e.g., lack of PrEP knowledge or self-efficacy) factors [6, 14, 15]. Thus, innovative models for PrEP delivery that can help overcome these barriers are needed.
The World Health Organization has recommended HIV self-testing (HIVST) as a strategy to increase HIV testing [16], but the tool has been underutilized to support health systems. Many studies have demonstrated that HIVST increases recent and frequent HIV testing among diverse populations (e.g., female sex workers [FSWs], HIV serodiscordant couples, men, and women) and settings, including Kenya [17,18,19,20,21]. However, few interventions have explored how HIVST could facilitate linkage to HIV services, and those that have primarily focused on linkage to treatment services [22,23,24,25]. Most people who test for HIV test, however, will test negative; thus, HIVST, when packaged with other appropriate implementation strategies, has great potential to support linkage to HIV prevention services, including PrEP.
The opinion of peers often influences the behaviors and preferences of young women, including behaviors related to the uptake of health services, such as contraception [26,27,28,29]. Peer referrals and peer-delivered interventions have been demonstrated to be feasible and effective among populations with tight social connectivity (e.g., men who have sex with men, FSWs) to increase identification of new individuals living with HIV and facilitate linkage to treatment interventions [30,31,32]. In Kenya, most AGYW initiate PrEP following informal (i.e., word-of-mouth) referral from peers [33]. A model of formalized peer PrEP referral enhanced with HIVST delivery could help increase PrEP initiation among this population at an increased risk of HIV acquisition.
Formative qualitative research conducted by this team found that young Kenyan women would be willing to engage in a peer PrEP referral model supported with HIVST delivery [33]. Specifically, young women engaged in PrEP services anticipated they would be willing to educate their peers about PrEP use and safety, deliver and assist peers with HIVST, and support peers with linkage to PrEP services and PrEP adherence, if needed. With input from Kenyan stakeholders (including young female PrEP users and non-users) during a one-day stakeholder meeting, we designed such a model for formal testing [34]. A hybrid effectiveness-implementation randomized-controlled trial aims to measure the effect of this intervention on PrEP initiation among AGYW while assessing implementation outcomes to expedite the translation of research into practice [35].
Methods
This paper has been developed in accordance to the Standard Protocol Items: Recommendations for Interventional Trials (i.e., SPIRIT) reporting guidelines [36] (Table 1).
Study design and setting
The “Peer PrEP” trial is a 2-arm hybrid effectiveness-implementation cluster-randomized controlled trial (ClinicalTrials.gov: NCT04982250). The trial will occur in Central Kenya (Kiambu, Nairobi, and Murang’a counties), which has largely peri-urban and rural populations and a population-level HIV prevalence of ~ 4% [37]. The Partners in Health and Research Development (PHRD) team, located in Kiambu County, will conduct the trial. This team — which includes a site Principal Investigator (PI), study coordinator, nurse counselor, research assistants, recruitment officers, data coordinators, and laboratory technicians — has extensive experience conducting PrEP clinical and implementation research [1, 38,39,40], a strong history of community engagement with diverse populations, and experience working collaboratively with healthcare providers in the region [38, 41, 42].
Study participants
The trial will enroll 2 types of participants: (1) peer providers, who are AGYW who have initiated PrEP and will refer peers to PrEP services, and (2) peer clients, who are AGYW who have not initiated PrEP and will be referred to PrEP services (Table 2). Eligible participants will be female, ≥ 16 to 24 years old (including emancipated minors ≥ 16 to 17 years — i.e., AGYW who are married, mothers, pregnant, or household heads [43,44,45]), and able and willing to provide consent and complete research activities. Eligible peer providers will be those who have initiated PrEP, can identify up to 4 peers who could benefit from PrEP, and are willing to be randomized to the intervention. Eligible peer clients will be those referred to PrEP by a peer provider. Peer providers will be ineligible if they are currently enrolled in another HIV-related study or are illiterate.
Peer providers will be recruited on a rolling basis by PHRD staff from public healthcare clinics delivering PrEP services in the region. Peer clients will be recruited by peer providers, who will be instructed to only recruit peers who meet the eligibility criteria. At the point of recruitment, peer providers will encourage peer clients to call study staff to share their contact information and schedule a study visit 3 months following recruitment. Peer providers and clients will each be compensated 50 Kenyan Shillings (KES; ~ $0.50 US Dollars (USD)) each time a client calls the study line; only peer clients who complete this activity will be contacted for follow-up.
All enrolled participants will complete informed written or verbal (select peer clients only) consent by experienced PHRD research assistants and receive 300 KES (~ $3 USD) for their engagement in research activities (i.e., completion of study surveys). For participants who visit the PHRD clinic to complete surveys in person, additional reimbursement for transportation will be provided.
Study procedures
Following enrollment, peer providers will be randomized 1:1 to either (1) formal peer PrEP referral + HIVST delivery, where peer providers will receive a brief training and be encouraged to refer four peers to PrEP using techniques from the training, PrEP and HIVST educational materials, and HIV self-tests, or (2) informal peer PrEP referral, where peer providers will be encouraged to refer 4 peers to PrEP using informal word-of-mouth referral techniques (Fig. 1).
Randomization
The randomization allocation sequence for this study will be generated using computer-generated random numbers and consistent block sizes without any stratification factors [46]. This sequence will be generated by the author C.C., who has no direct involvement with participants. Once a peer provider has enrolled in the study and completed informed consent, a PHRD research assistant will hand them an opaque, sealed envelope containing their study arm assignment. This envelope will be opened at the point of delivery so the research assistant can document the study arm assignment. Due to the inherent characteristics of the intervention, this trial is unblinded.
Intervention: Formal peer PrEP referral + HIVST delivery
Before peer providers identify and refer peer clients to PrEP services, they will complete a one-day, AGYW-friendly training. This training will occur in small groups (i.e., ~ 4–8 peer providers) on a rolling basis dependent on the pace of peer provider enrollment. This training will be led by PHRD research staff experienced in engaging AGYW on topics related to HIV prevention. The training will include information on PrEP use and safety, other available HIV prevention interventions, and guidance on HIVST use and results interpretation. Additionally, the training will include strategies for initiating conversations around PrEP and facilitating linkage to clinic-based HIV prevention or treatment services. At the training, peer providers will have the opportunity to use an HIVST kit with a fake buffer (i.e., water), which they can take and later use as a demonstration kit during peer referral. This training will emphasize the importance of maintaining peers’ confidentiality, not using stigmatizing language, and addressing potential HIV myths and stereotypes.
Following training, peer providers will receive a bag, designed to meet the style preferences of Kenyan AGYW, with the following intervention materials:
Informational brochures (n = 4). The AGYW-friendly informational brochures will reinforce key facts about PrEP and HIVST use and safety. They will also outline steps that peer clients can follow to link to clinic-based confirmatory HIV testing and HIV prevention and treatment services, based on their HIVST result. A study phone number will also be included in these brochures that peer clients can call if they have any questions about PrEP or HIVST. Each bag will include 4 brochures, 1 for each referred peer client.
HIVST kits (n = 8). This study will use the SURE CHECK® HIV 1/2 Assay (ChemBio Diagnostics, Medford, USA), a blood-based HIVST kit with a sensitivity of 99.7% and specificity of 99.9%, or a comparable blood-based HIVST kit approved by the World Health Organization [18]. These kits will come with written and pictorial instructions from the manufacturer (available in English) to help guide kit use and results interpretation. Each bag will include eight HIVST kits, two per each referred peer client. Peer providers will encourage peer clients to use 1 HIVST kit to test themselves and 1 kit to test with a sexual partner (if desired) or test again later.
PrEP referral cards (n = 4). The PrEP referral cards will be modeled after standard Kenya Ministry of Health community PrEP referral cards and include information on the location of nearby public healthcare clinics with free HIV prevention and treatment services. Each bag will include 4 referral cards, 1 for each referred peer client.
The peer providers will be encouraged to refer four peer clients with known behaviors associated with HIV risk (e.g., condomless sex with partners of unknown HIV status, engagement in transactional sex) to PrEP services using the strategies gained and materials obtained from the peer provider training. The peer provider will be encouraged to assist peer clients with HIVST if the client is interested and both parties are comfortable. At the point of HIVST delivery, the peer provider will emphasize that the HIVST kits are for HIV screening and that confirmatory clinic-based HIV testing is necessary for PrEP initiation. Although not a requirement of the intervention, peer providers randomized to this arm will be encouraged to escort peers to clinics to facilitate linkage to HIV prevention or treatment services and support peers’ adherence to these services through informal check-ins, if they feel comfortable. Over the duration of the trial, all interested peer providers will have access to an optional WhatsApp group, monitored by PHRD research staff, where they can ask questions and get support with intervention delivery, if needed.
Control: Informal peer PrEP referral
Peer providers randomized to the control arm will also be encouraged to refer 4 peers with known behaviors associated with risk of HIV acquisition to clinic-based PrEP services. They will not receive any formalized training on PrEP and HIVST or peer referral, nor will they receive any HIVST kits for distribution to peers. The peer providers in this arm will be encouraged to talk to their peers about PrEP in the way they would typically have conversations about sexual behaviors and other health interventions (e.g., contraception). Like in the intervention arm, they will be given PrEP referral cards (the same as those described above) to distribute to their peers (4 cards in total, 1 per referred peer client).
If peer clients referred to PrEP services by peer providers in either the intervention or control arms initiate PrEP at nearby public healthcare clinics, they will receive standard-of-care PrEP services according to Kenya’s national PrEP implementation guidelines without any additional support from the study team [10].
Study visits
Over the duration of the trial, peer providers will complete 2 study visits and peer clients will complete 1 study visit (Fig. 2). All peer providers will complete a baseline (month 0) and follow-up (month 3) visit, while peer clients who contacted study staff at the point of recruitment will only complete a follow-up visit (month 3). This timeline should give interested peer clients referred to PrEP services sufficient time to both initiate PrEP and potentially refill PrEP 1 month following initiation.
Data collection
Information on participants’ demographics (e.g., age, education) and behaviors associated with HIV risk will be collected in surveys conducted at baseline for peer providers and follow-up for peer clients (i.e., their first study visit) (Table 3). At the follow-up visit, self-reported outcomes related to delivery of the intervention and uptake of PrEP services among peer clients will be measured along with several implementation outcomes (described below). All surveys will be conducted by experienced PHRD researcher assistants using CommCare (Dimagi, Cambridge, USA), an electronic data collection tool.
Baseline surveys for peer providers will be conducted at the PHRD research clinic, the public clinics where peer providers are recruited, or other locations convenient to the peer providers. Follow-up surveys will be conducted either in-person at the PHRD research clinic or over the phone to potentially increase retention, especially among peer clients who that might have been recruited outside of Kiambu County. At follow-up, dried blood spot (DBS) samples will be collected to measure PrEP adherence from a random subset of 46 peer clients (23 per arm) who return to the PHRD research clinic for in-person follow-up visits.
Outcomes
This hybrid effectiveness-implementation trial will measure effectiveness and implementation outcomes (Table 4).
Effectiveness outcomes
The primary trial outcome will be PrEP initiation among all referred peer clients, as reported by peer providers at month 3. PrEP initiation is defined as visiting a health clinic and being dispensed PrEP. Peer providers will report this outcome on behalf of peer clients due to anticipated challenges following up with referred peer clients (as experienced in a pilot study that tested this intervention [47]). In studies measuring secondary distribution models, it is common for outcomes among those receiving an intervention to be reported by those delivering the intervention [23, 48,49,50].
Secondary trial outcomes include any recent HIV testing among referred peer clients and any PrEP continuation and adherence among referred peer clients that initiated PrEP, all reported by peer providers except for the adherence outcomes. HIV testing will include any form of peer client HIV testing following referral to PrEP services by peer providers. PrEP continuation will be defined as a peer client returning to a clinic following PrEP initiation and being dispensed PrEP. PrEP adherence will include both self-reported and biological assessments; a 3-item validated scale will be used to assess self-reported adherence [51] and DBS samples will be used to assess biologic adherence (i.e., any detection of tenofovir diphosphate in a 3-mm punch [52,53,54]). Recent PrEP continuation (in the past 3 months) will also be assessed among peer providers.
Implementation outcomes
Peer providers’ and clients’ perceived acceptability, appropriateness, and feasibility of the peer PrEP referral + HIVST delivery model will be assessed, as will the fidelity of intervention delivery. The acceptability assessment will be based on the Theoretical Framework of Acceptability [55], which defines acceptability as a multidimensional construct comprised of seven components (e.g., burden, perceived effectiveness, affective attitude). The appropriateness and feasibility assessments will be based on the Intervention Appropriateness Measure [56] and Feasibility of Intervention Measure [56], respectively. For each outcome, clients will be asked to report how strongly they agree (using a 5-point Likert scale) with statements that assess different component constructs of these outcomes [55]; the percentage of participants that agree or strongly agree with the different statements will be reported. To measure fidelity, participants’ knowledge of PrEP and HIVST use and how many of the intervention core components they received or delivered (e.g., number of HIVST kits, number of PrEP referral cards — with variations by study arm) will be reported.
Statistical analysis
Intention-to-treat, complete-case analyses will be used to compare differences in outcome proportions among peer clients between the randomization arms. Risk differences will be estimated with 95% confidence intervals for binary outcomes using mixed-effect models, with the study arm included as a fixed effect and the peer provider included as a random effect. A Gaussian distribution with an identity link will be used to fit the regression models and standard errors robust to clustering at the peer provider level will be used. Pre-specified sensitivity analyses include (1) estimating effect sizes for study outcomes as reported by peer clients (instead of peer providers), (2) study outcomes assessed among all potential referred peer clients (i.e., 4 per peer provider) and not just those referred, and (3) PrEP continuation and adherence outcomes among all peer clients referred to PrEP (not just those who initiated PrEP). Stata 16 (StataCorp, College Station, USA) will be used to run all analyses [57] and significance will be defined at the p = 0.05 level.
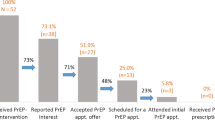
This trial was powered to detect significant differences in the primary outcome, PrEP initiation among peer clients at month 3. In a pilot study testing the peer PrEP referral + HIVST delivery model in Kiambu County, peer providers typically referred 75% of the suggested number of peer clients (n = 4) and PrEP initiation among peer clients referred was 81% (as reported by peer providers) [47]. If 63% of peer clients initiate PrEP with informal peer referral in this trial and there are 40 clusters (i.e., peer providers) per arm, three sampling units (i.e., peer clients) per cluster, and an intra-cluster correlation coefficient of 0.05 (typical for cluster-randomized controlled trials [46]), this trial has 80% power to detect a 17% difference in the proportion of peer clients initiating PrEP between the study arms. This would result in an overall sample of 80 peer providers and 320 peer clients (or 240 peer clients based on our assumption that peer providers will only refer 3 of the suggested 4 peer clients).
Study oversight
Coordinating center
The Fred Hutchinson Cancer Center (Fred Hutch, Seattle, USA) serves as the coordinating center for the trial. The Fred Hutch team includes the study PI, project coordinators, a statistical research associate, and a biostatistics advisor. This team works collaboratively with the PHRD team (Thika, Kenya) to organize weekly team meetings (via Microsoft Teams), perform data quality checks for incoming trial data, monitor study implementation, develop solutions to any identified implementation challenges, ensure the approved institutional review board protocols and survey tools are up to date (requesting any modifications, as needed), and analyze study data (when the timing is appropriate). None of the Fred Hutch team members will have any direct involvement with study participants; all implementation activities (including participant recruitment and enrollment) are the responsibility of the PHRD team.
Data safety and monitoring board (DSMB)
This study will be overseen by an independent DSMB with members experienced in HIV prevention research in the region and implementation science. Every 6 months, the DSMB will review study conduct, progress, and related operational study data in open sessions, and study outcome data by arm in closed sessions. Additionally, the DSMB will review any reports of incident HIV infections, adverse events, and social harms by study arm. Adverse events will include any forms of verbal, physical, emotional, or economic abuse; serious adverse events will include hospitalization (for any condition, including those outside the study) or death. During study visits, participants will be asked to report if they have experienced any of these events and whether they were related to study participation. The DSMB will provide recommendations to the study team following each review, but no formal interim statistical evaluation will be conducted.
Confidentiality
Every effort will be made to protect participants’ privacy and confidentiality. All survey data will be de-identified using a unique study identification number for each enrolled participant, enabling us to link data from individual participants over time. Any records (e.g., locator forms, consent forms) that contain personal identifiers of participants will be stored in a locked file in a room with limited access at the PHRD research site. All local databases will be secured with password-protected access systems.
Dissemination
The study team is committed to public dissemination of the trial findings to participants, local Kenyan policymakers and stakeholders (e.g., members of the Kenya Ministry of Health, young women in the community at HIV risk, community advisory groups, and healthcare providers), and the global scientific community. Findings from this trial will be presented at arranged meetings, at local and international conferences, and in peer-reviewed academic journals.
Discussion
PrEP has great potential to help curb the HIV epidemic; however, for it to achieve this objective, it needs to reach the populations that could benefit most and are not currently engaged in PrEP services [58]. AGYW are a priority population for the delivery of HIV prevention services in Africa, but their uptake of PrEP has been low [6, 59]. The peer PrEP referral + HIVST delivery model combines formalized peer referral and HIVST in a combination HIV prevention package to help increase PrEP uptake among this important population. It is based on evidence that peer-delivered health interventions can increase intervention uptake among populations with close social connectivity [30,31,32, 60,61,62,63], such as AGYW, and that HIVST can increase HIV testing uptake [18, 64, 65], which is along the care pathway to PrEP initiation and could help motivate this behavior among the vast majority of AGYW who will test HIV-negative.
This trial has strengths and limitations. Strengths include the use of HIVST to support health systems by motivating engagement in HIV prevention services. An additional strength includes formalizing the most common way AGYW are already coming into PrEP services — via informal word-of-mouth referral — to ensure they have accurate information on PrEP use, safety, and locations where they can initiate PrEP services. Potential trial limitations include relying on outcomes among peer clients reported by peer providers, but as discussed this is necessary because of anticipated challenges following up with peer clients. Additional limitations include limiting the number of peer clients peer providers are encouraged to recruit and only delivering two HIVST kits to each client. Findings from this trial could inform future adaptations to this model that could change some of these model details.
If this trial demonstrates the formalized peer PrEP referral + HIVST delivery intervention significantly increases PrEP initiation over informal PrEP referral among Kenyan AGYW, this could inform national and international PrEP implementation guidelines [10, 58, 66] on strategies that can be utilized to reach this important population. Additionally, this model could be adapted to increase initiation of other health services that could benefit this population, including newer longer-acting PrEP forms that will soon be available in Kenya (e.g., bi-monthly injectable cabotegravir) [67], human papillomavirus vaccination [68], and family planning services [69].
Trial status
The first trial participant was enrolled on May 3, 2023 and it is anticipated the last trial participant will be enrolled around March 2024 (which will conclude follow-up with all peer providers and clients). This trial was first registered on ClinicalTrials.gov on July 29, 2021. All relevant ethics committees have approved the trial and any relevant modifications. At the time of this publication, the current protocol was version 1.10 (October 2023).
Availability of data and materials
The trial protocol, data, and statistical code will be available upon request to study investigators by qualified researchers.
Abbreviations
- AGYW:
-
Adolescent girls and young women
- DSMB:
-
Data Safety Monitoring Board
- DBS:
-
Dried blood spot
- FSW:
-
Female sex worker
- HIV:
-
Human immunodeficiency virus
- HIVST:
-
HIV self-testing
- KES:
-
Kenyan Shillings
- PHRD:
-
Partners in Health Research Development
- PI:
-
Principal Investigator
- PrEP:
-
Pre-exposure prophylaxis
- SPIRIT:
-
Standard Protocol Items: Recommendations for Interventional Trials
- USD:
-
United States Dollars
References
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410.
Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99.
Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34.
Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok tenofovir study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–90.
Chou R, Evans C, Hoverman A, Sun C, Dana T, Bougatsos C, et al. Preexposure prophylaxis for the prevention of HIV infection: evidence report and systematic review for the US preventive services task force. J Am Med Assoc. 2019;321:2214–30.
Sila J, Larsen AM, Kinuthia J, Owiti G, Abuna F, Kohler PK, et al. High awareness, yet low uptake, of pre-exposure prophylaxis among adolescent girls and young women within family planning clinics in Kenya. AIDS Patient Care STDS. 2020;34:336–43. https://doi.org/10.1089/apc.2020.0037.
Kimanga DO, Ogola S, Umuro M, Ng’ang’a A, Kimondo L, Murithi P, Muttunga J, Waruiru W, Mohammed I, Sharrif S, De Cock KM, Kim AA, KAIS Study Group. Prevalence and incidence of HIV infection, trends, and risk factors among persons aged 15–64 years in Kenya: results from a nationally representative study. J Acquir Immune Defic Syndr. 2014;661(111):S13-26. https://doi.org/10.1097/QAI.0000000000000124. PMID: 24445338; PMCID: PMC4794992.
Kenya National Bureau of Statistics. The 2009 Kenya population and housing census. 2010. Available from: https://s3-eu-west-1.amazonaws.com/s3.sourceafrica.net/documents/21195/Census-2009.pdf. [Accessed 10 Oct 2023].
Masyuko S, Mukui I, Njathi O, M K, Oluoch J, Wamicwe J. PrEP roll out in a national public sector program: the Kenyan case study. Sex Health. 2018;15:578–86.
National AIDS and STI Control Programme, Ministry of Health. Framework for the implementation of pre-exposure prophylaxis of HIV in Kenya. 2017. Available from: https://www.prepwatch.org/wp-content/uploads/2017/05/Kenya_PrEP_Implementation_Framework.pdf. [Accessed 10 Oct 2023].
Cowan F, Pettifor A. HIV in adolescents in sub-Saharan Africa. Curr Opin HIV AIDS. 2009;4:288–93.
Shisana O, Rehle T, Simbayi L, Zuma K, Jooste S, Zungu N, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Launch Edition. Cape Town: HSRC Press; 2014. Available from: https://hsrc.ac.za/uploads/pageContent/4565/SABSSM%20IV%20LEO%20final.pdf. [Accessed 10 Oct 2023].
National AIDS and STI Control Programme (NASCOP). Preliminary KENPHIA 2018 report. Nairobi: NASCOP; 2020. Available from: https://phia.icap.columbia.edu/wp-content/uploads/2020/04/KENPHIA-2018_Preliminary-Report_final-web.pdf. [Accessed 10 Oct 2023].
Mugo NR, Ngure K, Kiragu M, Irungu E, Kilonzo N. The preexposure prophylaxis revolution; from clinical trials to programmatic implementation. Curr Opin HIV AIDS. 2016;11:80–6.
Kayesu I, Mayanja Y, Nakirijja C, Machira YW, Price M, Seeley J, et al. Uptake of and adherence to oral pre-exposure prophylaxis among adolescent girls and young women at high risk of HIV-infection in Kampala, Uganda: a qualitative study of experiences, facilitators and barriers. BMC Womens Health. 2022;22:1–14.
World Health Organization. Policy Brief: WHO recommends HIV self- testing – evidence update and considerations for success. 2019. Available from: https://iris.who.int/bitstream/handle/10665/329968/WHO-CDS-HIV-19.36-eng.pdf?sequence=. [Accessed 10 Oct 2023].
Hamilton A, Thompson N, Choko AT, Hlongwa M, Jolly P, Korte JE, et al. HIV self-testing uptake and intervention strategies among men in sub-Saharan Africa: a systematic review. Front Public Health. 2021;9:60.
Witzel TC, Eshun-Wilson I, Jamil MS, Tilouche N, Figueroa C, Johnson CC, et al. Comparing the effects of HIV self-testing to standard HIV testing for key populations: a systematic review and meta-analysis. BMC Med. 2020;18:1–13.
Indravudh PP, Choko AT, Corbett EL. Scaling up HIV self-testing in sub-Saharan Africa: a review of technology, policy and evidence. Curr Opin Infect Dis. 2018;31:14–24.
Lyons CE, Coly K, Bowring AL, Liestman B, Diouf D, Wong VJ, et al. Use and acceptability of HIV self-testing among first-time testers at risk for HIV in Senegal. AIDS Behav. 2019;23:130–41.
Neuman M, Hensen B, Mwinga A, Chintu N, Fielding KL, Handima N, et al. Does community-based distribution of HIV self-tests increase uptake of HIV testing? Results of pair-matched cluster randomised trial in Zambia. BMJ Glob Health. 2021;6:1–11.
Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, et al. Uptake, accuracy, safety, and linkage into care over two years of promoting annual self-testing for HIV in Blantyre, Malawi: a community-based prospective study. PLoS Med. 2015;12:e1001873.
Korte JE, Kisa R, Vrana-Diaz CJ, Malek AM, Buregyeya E, Matovu JKB, et al. HIV oral self-testing for male partners of women attending antenatal care in central Uganda: uptake of testing and linkage to care in a randomized trial. J Acquir Immune Defic Syndr. 2020;84:271–9.
Shapiro AE, van Heerden A, Krows M, Sausi K, Sithole N, Schaafsma TT, et al. An implementation study of oral and blood-based HIV self-testing and linkage to care among men in rural and peri-urban KwaZulu-Natal. South Africa J Int AIDS Soc. 2020;23:e25514.
Chipungu J, Bosomprah S, Zanolini A, Thimurthy H, Chilengi R, Sharma A, et al. Understanding linkage to care with HIV self-test approach in Lusaka, Zambia – a mixed method approach. PLoS One. 2017;12(11):e0187998. https://doi.org/10.1371/journal.pone.0187998.
Calhoun LM, Mirzoyants A, Thuku S, Benova L, Delvaux T, van den Akker T, et al. Perceptions of peer contraceptive use and its influence on contraceptive method use and choice among young women and men in Kenya: a quantitative cross-sectional study. Reprod Health. 2022;19:1–12.
Behrman JR, Kohler H-P, Watkins SC. Social networks and changes in contraceptive use over time: Evidence from a longitudinal study in rural Kenya. Demography. 2002;39:713–38.
Dynes M, Stephenson R, Rubardt M, Bartel D. The influence of perceptions of community norms on current contraceptive use among men and women in Ethiopia and Kenya. Health Place. 2012;18:766–73.
Lowe SMP, Moore S. Social networks and female reproductive choices in the developing world: A systematized review. Reprod Health. 2014;11:85.
Ortblad K, Kibuuka Musoke D, Ngabirano T, Nakitende A, Magoola J, Kayiira P, et al. Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: a cluster-randomized controlled health systems trial. PLoS Med. 2017;14:e1002458.
Shangani S, Escudero D, Kirwa K, Harrison A, Marshall B, Operario D. Effectiveness of peer-led interventions to increase HIV testing among men who have sex with men: a systematic review and meta-analysis. AIDS Care. 2017;29(8):1003–13. https://doi.org/10.1080/09540121.2017.1282105.
Chanda MM, Ortblad KF, Mwale M, Chongo S, Kanchele C, Kamungoma N, et al. HIV self-testing among female sex workers in Zambia: a cluster randomized controlled trial. PLoS Med. 2017;14:e1002442. https://doi.org/10.1371/journal.pmed.1002442.
McGowan M, Casmir E, Wairimu N, Mogere P, Jahn A, Ngure K, et al. Assessing young Kenyan women’s willingness to engage in a peer-delivered HIV self-testing and referral model for PrEP initiation: a qualitative formative research study. Front Public Health. 2022;10:932948. https://doi.org/10.3389/fpubh.2022.932948.
Wairimu N, Mogere P, McGowan M, Casmir E, Mugo N, Wagner AD, et al. Design of a peer PrEP referral + HIV self-testing delivery model to increase PrEP initiation among young Kenyan women: use of the nominal group technique. Nairobi, Kenya: Oral presentation at the University of Nairobi STD/HIV/SRH Collaborative Research Group Meeting; 2023.
Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–26.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, SPIRIT, et al. explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;2013:346.
National AIDS Control Council, Ministry of Health. Kenya HIV estimates report 2018. 2018;1–34. Available from: https://nsdcc.go.ke/wp-content/uploads/2018/11/HIV-estimates-report-Kenya-20182.pdf. [Accessed 10 Oct 2023].
Irungu EM, Mugwanya KK, Mugo NR, Bukusi EA, Donnell D, Odoyo J, et al. Integration of prophylaxis services into public HIV care clinics in Kenya : a pragmatic stepped-wedge randomised trial. Lancet Glob Health. 2021;9:e1730–9.
Haberer JE, Bukusi EA, Mugo NR, Pyra M, Kiptinness C, Oware K, et al. Effect of SMS reminders on PrEP adherence in young Kenyan women (MPYA study): a randomised controlled trial. Lancet HIV. 2021;8:e130–7.
Heffron R, Ngure K, Odoyo J, Bulya N, Tindimwebwa E, Hong T, et al. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa [version 2; peer review: 2 approved]. Gates Open Res. 2018;1:3. https://doi.org/10.12688/gatesopenres.12752.2.
Irungu EM, Ngure K, Mugwanya K, Mugo N, Bukusi E, Wamoni E, et al. Training health care providers to provide PrEP for HIV serodiscordant couples attending public health facilities in Kenya. Glob Public Health. 2019;14:1524–34.
Ortblad KF, Mogere P, Roche S, Kamolloh K, Odoyo J, Irungu E, et al. Design of a care pathway for pharmacy-based PrEP delivery in Kenya: results from a collaborative stakeholder consultation. BMC Health Serv Res. 2020;20:1034.
National AIDS and STI Control Programme, Kenya Medical Research Institute (KEMRI). Guidelines for conducting adolescent HIV sexual and reproductive health research in Kenya. 2015. Available from: https://icop.or.ke/wp-content/uploads/2016/10/Adolescents-Guidance-on-HIV-SRH-Research.pdf. [Accessed 10 Oct 2023].
National AIDS and STI Control Programme, Ministry of Health. National implementation guidelines for HIV and STI programming among young key populations. 2018. Available from: https://www.iavi.org/phocadownload/userupload/National%20Implementation%20Guidelines%20for%20HIV%20and%20STI%20Programming%20among%20YKPs%202018.pdf. [Accessed 10 Oct 2023].
Kenya Medical Research Institute (KEMRI), Scientific and Ethics Review Unit (SERU). KEMRI SERU guidelines for the conduct of research during the COVID-10 pandemic in Kenya. 2020. Available from: https://www.kemri.go.ke/wp-content/uploads/2019/11/KEMRI-SERU_GUIDELINES-FOR-THE-CONDUCT-OF-RESEARCH-DURING-THE-COVID_8-June-2020_Final.pdf. [Accessed 10 Oct 2023].
Friedman LM, Furberg CD, Demets DL, Reboussin DM, Granger CB. Fundamentals of clinical trials. 5th ed. Springer. 2015.
Mcgowan M, Wairimu N, Reedy AM, Mogere P, Malen RC, Njeru I, et al. Peer PrEP referral + HIV self-testing delivery for PrEP initiation among adolescent girls and young women in Kenya: a mixed-methods pilot study. Poster presentation at 12th IAS Conference on HIV Science Brisbane, Australia, July 23–26, 2023 Abstract EPE0797. Available from: https://www.iasociety.org/sites/default/files/IAS2023/abstract-book/IAS_2023__Abstracts.pdf. [Accessed 10 Oct 2023].
Joseph Davey DL, Wall KM, Naidoo N, Naidoo D, Xaba G, Serao C, et al. HIV testing and linkage to ART following secondary distribution of HIV self-test kits to male partners of women living with HIV: a pilot randomized control trial in Mpumalanga. South Africa J Int AIDS Soc. 2022;25:e25937.
Masters SH, Agot K, Obonyo B, Napierala Mavedzenge S, Maman S, Thirumurthy H. Promoting partner testing and couples testing through secondary distribution of HIV self-tests: a randomized clinical trial. PLoS Med. 2016;13:e1002166.
Zewdie K, Kiptinness C, Ngure K, Kipkurui N, Wairimu N, Ambiyo F, et al. Targeted Implementation of HIV Self-Testing Increases Testing Uptake among Partners of Index Persons Known to Have HIV in Kenya. J Acquir Immune Defic Syndr. 2022;90(5):524–9 Available from: https://journals.lww.com/jaids/Fulltext/2022/08150/Targeted_Implementation_of_HIV_Self_Testing.7.aspx. [Accessed 10 Oct 2023].
Wilson IB, Lee Y, Michaud J, Fowler FJ, Rogers WH. Validation of a new three-item self-report measure for medication adherence. AIDS Behav. 2016;20:2700–8.
Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–9.
Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125.
Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, Zheng JH, et al. Emtricitabine-Triphosphate in dried blood spots as a marker of recent dosing. Antimicrob Agents Chemother. 2016;60:6692–7.
Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17:1–13.
Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12:1–12.
StataCorp. Stata Statistical Software: Release 16. College Station: StataCorp LLC; 2019. https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/.
World Health Organization. WHO Implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection: module 5: monitoring and evaluation. World Health Organization. 2018. Available from: https://apps.who.int/iris/handle/10665/279834. Accessed 10 Oct 2023.
Mayanja Y, Kamacooko O, Lunkuse JF, Muturi-Kioi V, Buzibye A, Omali D, et al. Oral pre-exposure prophylaxis preference, uptake, adherence and continuation among adolescent girls and young women in Kampala, Uganda: a prospective cohort study. J Int AIDS Soc. 2022;25:25909. https://doi.org/10.1002/jia2.25909.
George A, Blankenship KM. Peer outreach work as economic activity: implications for HIV prevention interventions among female sex workers. PLoS ONE. 2015;10:1–14.
Safren SA, O’Cleirigh C, Skeer MR, Driskell J, Goshe BM, Covahey C, et al. Demonstration and evaluation of a peer-delivered, individually-tailored, HIV prevention intervention for HIV-infected MSM in their primary care setting. AIDS Behav. 2011;15:949–58. https://doi.org/10.1007/s10461-010-9807-8.
Bhattacharjee P, Prakash R, Pillai P, Isac S, Haranahalli M, Blanchard A, et al. Understanding the role of peer group membership in reducing HIV-related risk and vulnerability among female sex workers in Karnataka. India AIDS Care. 2013;25(Suppl 1):s46-54. https://doi.org/10.1080/09540121.2012.736607.
Shahmanesh M, Patel V, Mabey D, Cowan F. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: A systematic review. Tropical Med Int Health. 2008;13:659–79.
Kelvin EA, Akasreku B. The Evidence for HIV self-testing to increase HIV testing rates and the implementation challenges that remain. Curr HIV/AIDS Rep. 2020;17:281–9.
Njau B, Damian DJ, Abdullahi L, Boulle A, Mathews C. The effects of HIV self-testing on the uptake of HIV testing, linkage to antiretroviral treatment and social harms among adults in Africa: A systematic review and meta-analysis. PLoS One. 2021;16:e0245498.
National AIDS and STI Control Programme, Ministry of Health. Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya. Ministry Of Health; 2018. Available from: https://cquin.icap.columbia.edu/wp-content/uploads/2017/04/ICAP_CQUIN_Kenya-ARV-Guidelines-2018-Final_20thAug2018.pdf. [Accessed 10 Oct 2023].
Muchangi J. Kenyan-tested bimonthly injection to prevent HIV approved. The Star, Kenya (Online Newspaper). 2021. Available from: https://www.the-star.co.ke/news/2021-12-22-kenyan-tested-bimonthly-injection-to-prevent-hiv-approved/. [Accessed 10 Oct 2023].
Kutz JM, Rausche P, Gheit T, Puradiredja DI, Fusco D. Barriers and facilitators of HPV vaccination in sub-saharan Africa: a systematic review. BMC Public Health. 2023;23:974.
Ochako R, Mbondo M, Aloo S, Kaimenyi S, Thompson R, Temmerman M, et al. Barriers to modern contraceptive methods uptake among young women in Kenya: A qualitative study. BMC Public Health. 2015;15:1–9.
Acknowledgements
We would like to acknowledge the administrative teams at the Partners in Health and Research Development site, University of Washington (Department of Global Health), and Fred Hutchinson Cancer Center (Public Health Sciences Division) who helped support the acquisition of funding for this trial.
Funding
This trial was funded by the National Institute of Mental Health (R00 MH121166). The funder had no role in the design of the study; collection, analysis, and interpretation of data; or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
NW, RCM, AMR, CC, and KFO drafted the manuscript. NW, RCM, AMR, PM, MM, KN, and KFO drafted the study protocol, obtained ethical approval, and developed study forms. KFO obtained the funding for this trial. KN and JMB provided oversight on the trial design and funding application. All authors reviewed and edited the manuscript and approved the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial protocol (version 1.10, October 2023), informed consent forms, and participant education and recruitment materials have all been approved by the Scientific Ethics Review Unit at the Kenya Medical Research Institute (Nairobi, Kenya) and the Institutional Review Board at the Fred Hutchinson Cancer Center (Seattle, United States). All participants will provide written informed consent (available in English and Kiswahili); peer providers prior to enrollment and randomization, and peer clients prior to their month 3 follow-up visit. Peer clients will have the option for either written or verbal consent (using a consent script) based on whether they complete their follow-up visit in person at the PHRD research site or remotely over the phone. All consent forms include an option to opt-out of future sharing of identifiable data; the peer client consent form included an option to opt-out of DBS collection for PrEP adherence testing. At any point during study implementation, participants may discontinue participation. Any potential protocol modifications will be reviewed by our ethics boards before implementation and, if necessary, participants notified of these changes and the Clinicaltrials.gov trial registry will be updated.
Consent for publication
Not applicable.
Competing interests
JMB is a current employee of Gilead Sciences, outside of the present work. KN has received research funding from the Merck Investigator Program. All other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wairimu, N., Malen, R.C., Reedy, A.M. et al. Peer PrEP referral + HIV self-test delivery for PrEP initiation among young Kenyan women: study protocol for a hybrid cluster-randomized controlled trial. Trials 24, 705 (2023). https://doi.org/10.1186/s13063-023-07734-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-023-07734-x