Abstract
Background
Knee osteoarthritis (KOA) is one of the most common musculoskeletal disorders. Acupotomy may be effective for KOA, but the evidence is limited. This trial aims to determine the effectiveness and safety of acupotomy for KOA.
Methods/design
This is a parallel-group, assessor-blinded randomized controlled trial. Two hundred patients with KOA will be recruited and randomly assigned to two groups (group A or group D) in a 1:1 ratio. Patients in group A will receive acupotomy and topical diclofenac diethylamine for 4 weeks, while patients in group D will receive topical diclofenac diethylamine alone for 4 weeks. The primary outcome will be the response rate—the proportion of patients who achieve the minimal clinically important improvement in pain and function at week 4 compared with baseline. Secondary outcomes will include pain, function, quality of life, the use of rescue medicine (loxoprofen sodium), and adverse events at weeks 4, 8, and 24 after randomization. Besides, joint fluid and serum will be collected to assess the level of inflammatory cytokines, like TNF-α, IL-1β, and MMP-3.
Discussion
This study will contribute to a better understanding of the effectiveness and safety of acupotomy in combination with topical nonsteroidal anti-inflammatory drugs. If the hypothesis is confirmed, acupotomy may be recommended as adjunctive therapy for patients with KOA. Results of the study will be of great importance for the guidelines of clinical therapy.
Trial registration
Chinese Clinical Trial Registry ChiCTR2100043005 Registered on 4 February 2021.
Similar content being viewed by others
Background
Symptomatic osteoarthritis is common in elderly people, and the knee is the most common site [1, 2]. Almost 50% of people aged 50 and over report knee pain over the 12-month period, and more than 25% have severe and even intolerable pain [3]. A survey in China showed that the prevalence of symptomatic knee osteoarthritis (KOA) was 8.1% and the prevalence increased with age [4]. As the populations of many countries are aging rapidly, the incidence of KOA is rapidly rising [5]. The high prevalence and its impact on quality of life make KOA become a vital public health problem.
The aim of treating KOA is to alleviate pain and improve function [6]. The topical non-steroidal anti-inflammatory drugs (NSAIDs) are used as first-line medicine for symptomatic KOA according to the Chinese clinical guideline for osteoarthritis (2018 Edition) [7] and American osteoarthritis guideline [8]. Diclofenac diethylamine emulgel, a type of topical NSAID, is commonly used for local pain. However, some knee pain cannot be alleviated by using topical NSAIDs alone [9]. Exercise therapy, as another recommended method, remains a problem in wide implementation and long-term adherence [1]. Besides, additional treatment includes total knee replacement, but this approach is the final choice for severe KOA and is not very acceptable to some patients.
Acupotomy, also named needle-scalpel, is the combination of the needle of acupuncture and the scalpel of western medicine [10]. Acupotomy combines traditional Chinese medicine meridian theory and modern surgical principles [11]. Acupotomy could alleviate the pain and reinstate the function of the joint by reducing the tension of local soft tissue and releasing the surrounding tissue adhesions [12,13,14]. Meanwhile, several studies indicated that acupotomy can reduce the expression of local inflammatory factors [15, 16]. In China, acupotomy combined with topical NSAIDs for treating KOA is widely accepted by both acupotomy doctors (a type of TCM doctor who majors in acupotomy) and patients [17]. Although acupotomy is used for KOA in clinical practice to improve pain, joint function, and life quality [18,19,20,21], its effect has not been verified through strict clinical controlled trials (RCTs). This study was designed as an RCT which will add acupotomy in addition to topical NSAIDs, to determine the additive effect of acupotomy.
Methods
Objectives
The aim of this study is to assess the effectiveness and safety of acupotomy combined with diclofenac diethylamine emulgel compared with diclofenac diethylamine emulgel in patients with KOA.
Study design
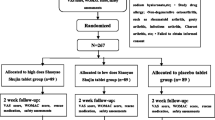
This is a single-center, two-armed, parallel-group RCT that will be conducted at Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University. The protocol has been registered on the Chinese Clinical Trial Registry (No. ChiCTR2100043005) and will be conducted following the Declaration of Helsinki. The protocol will be reported following the SPIRIT guidelines (Additional file 1), and the study will be reported following the CONSORT Statement. Figure 1 shows the flow diagram of the trial.
Study setting, recruitment, and ethics
The recruitment will be conducted at Beijing Hospital of Traditional Chinese Medicine. The study has been approved by the medical ethical review committee of Beijing Hospital of Traditional Chinese Medicine (No. 2020BL02-057-02). KOA patients diagnosed according to the American College of Rheumatology (ACR) criteria [7] will be recruited from outpatients in the pain department and the massage (Tuina) department. Meanwhile, posters will be posted on the corresponding author’s We-Media (Tik Tok) and the outpatient hall. Researchers will screen and qualify the subjects for the study according to the inclusion criteria and exclusion criteria. Before signing the written informed consent, the researcher needs to explain the study in detail with potential patients, for example, the study purpose, procedures, as well as the potential risks and benefits associated with participation in the study. The written informed consent will be provided by all eligible participants. The confidentiality of patient records will be protected. Data will be processed in a “data anonymous” manner, omitting information that can identify individual subjects. If the results of the trial are published, the identity information of the subjects shall remain confidential.
Inclusion criteria
-
1.
Age 40–80 years, male or female
-
2.
Diagnosis of knee osteoarthritis according to the ACR criteria
-
3.
Symptoms have been present for more than 6 months
-
4.
Radiologic confirmation of knee osteoarthritis (Kellgren-Lawrence grade [22] II to IV in the recent 6 months)
-
5.
An average knee pain severity of 4 or more out of 10 on an 11-point numerical rating scale (NRS) [23] over the past week
-
6.
Signed written informed consent
Exclusion criteria
-
1.
History of knee surgery or waiting for surgery (knee replacement or knee arthroscopy)
-
2.
Knee pain caused by other diseases (such as autoimmune diseases, infection, malignant tumors, trauma, fracture, severe effusion of joint cavity, joint bodies, etc).
-
3.
History of arthroscopy within 1 year or intraarticular injection within 6 months
-
4.
History of receiving acupotomy or acupuncture treatment within 3 months
-
5.
Severe acute/chronic organic or mental diseases
-
6.
Pregnant and lactating women
-
7.
Coagulation disorders (such as hemophilia, etc.)
-
8.
Participation in another clinical study in the past 3 months
-
9.
Fear to acupotomy
Drop-out criteria
The drop-out criteria for this study include unwillingness/inability to return for treatment/evaluation or presence of serious adverse events.
Randomization and allocation concealment
Eligible patients will be randomly assigned to the acupotomy group (A group), or the diclofenac diethylamine emulgel group (D group) by using block randomization in a 1:1 ratio. The randomization sequence will be generated by an independent statistician with SPSS 20.0 software and will be put into the sealed and opaque brown envelopes with a serial number affixed to ensure the concealment of the random allocation scheme. Researcher who recruits participants will open envelopes orderly to mitigate the risk.
Blinding
The nature of acupotomy means that patients and acupotomy doctors cannot be blinded during the treatment. Outcome assessors and statisticians will be blinded to group assignments. In this study, acupotomy doctor, outcome assessors, and statisticians will be independent.
Interventions
Patients in both groups will be prescribed diclofenac diethylamine emulgel (Voltalin, 1 g emulgel contains the main ingredient dichlorophenic acid diethylamine 10 mg, Beijing Novartis Pharma Ltd) of 3–4 times per day. A proper amount of diclofenac diethylamine emulgel will be applied according to the size of the knee pain area, then a gentle rub will be needed to make it penetrate the skin. The course of treatment will be 4 weeks. And each patient will be given a diary to keep track of how many times they used Voltalin each day. During the study period, loxoprofen sodium tablets (Loxonin, Dachi Sankyo Pharma Ltd) will be provided as rescue medication in case of intolerable knee pain, with the usage documented by the outcome assessors. Besides, all other interventions, such as acupuncture, moxibustion, physical therapy, surgical procedures, and intra-articular injections for KOA, will be forbidden. Other medications that do not influence pain will be allowed.
The patients in the acupotomy group will receive acupotomy in the hospital one time per week for 4 weeks. A sterilized, disposable needle-scalpel (Hanzhang No.4, length: 60 mm, diameter: 0.6 mm, Beijing Hanzhang Medical Device Co., Ltd) will be used. To keep the consistency and standardization of the operation, the acupotomy will be performed by an experienced doctor who has a Chinese medicine practitioner license and has been qualified for at least 15 years according to the basic operating procedures of acupotomy in the Principles of Acupotomy. The appropriate body position will be chosen depending on the location of the operation, such as supine, prone or side-lying position. The medial collateral ligaments, lateral collateral ligaments, hamstring muscle, quadriceps femoris, iliotibial band, popliteus muscle, anserine bursa, subpatellar fat pad, and the peripatellar area will be touched and pressed, then the spots with induration and cordlike tissue will be identified as positive points. Generally, 5–7 positive points will be selected in light of the specific pain sites, functional limitations, and imaging examination results of the patients’ joints. The selected points will be marked with gentian violet, and then the surrounding skin will be disinfected with iodophor. After sterile skin preparation, a needle-scalpel will penetrate the skin and gradually go deeper. When feeling a sense of toughness or resistance under the needle-scalpel, the manipulation of acupotomy is performed. After treatment completion, the doctor will check for any abnormality and bleeding, then apply the Band-Aid to the pinhole.
Outcomes
If only one knee meets the ACR criteria and Kellgren-Lawrence grade II to IV, then this knee will be evaluated. When both knees are in accordance with the inclusion criteria (ACR and Kellgren–Lawrence grade II to IV), the more painful knee will be chosen for assessment. Figure 2 shows the treatment and outcome measurement schedules.
Primary outcome measurement
The primary outcome will be the response rate. The response rate is the percentage of patients with improvement in average pain NRS of at least 2 points [24], which is regarded as a minimum clinically important difference, and in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [25] function subscale score of at least 6 points [24] at week 4 compared with baseline. The NRS is a digital scale using “0” to “10” to indicate the severity of the average pain over the last week, with “0” representing painless and “10” representing the most severe pain. The WOMAC function subscale (Likert version 3.1) consists of 17 questions (each scored from 0 to 4) relating to the difficulty experienced due to knee osteoarthritis, with higher scores referring to poorer knee function.
Secondary outcome measurements
The NRS and WOMAC pain subscale will be used to measure the average pain over the last week at weeks 0, 4, 8, and 24. WOMAC pain subscale ranges from 0 to 20. It has five items assessing the severity of pain in five different conditions, with higher scores indicating worse pain.
The WOMAC function subscale will be used to measure the physical function at weeks 0, 4, 8, and 24. The WOMAC function subscale ranges from 0 to 68 and includes 17 items, with lower scores indicating better function.
The WOMAC stiffness subscale will be used to measure the degree of stiffness of the knee at weeks 0, 4, 8, and 24. The WOMAC stiffness subscale ranges from 0 to 8 and includes two items, with higher scores indicating more stiffness.
The 12-item Short-Form Health Survey (SF-12) [26] will be used to assess the quality of life at weeks 0, 4, 8, and 20. SF-12 includes the mental and physical domains. Each domain ranges from 0 to 100, with higher scores indicating a better quality of life.
The use of rescue medicine (loxoprofen sodium tablets) will also be recorded throughout the trial.
Laboratory outcome measurement
Joint fluid and venous serum will be collected at week 0 and week 4, and the levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and matrix metalloproteinase-3 (MMP-3) will be tested.
Adverse events
All adverse events will be recorded throughout the trial by researchers using a record book, which consists of the time, severity, and duration of the adverse event, the disposition taken, and the prognosis of the event. Serious adverse events refer to events that require hospitalization or prolonged hospitalization, disability, affect work ability, or threaten life or death during the clinical trial. If serious adverse events occur, the clinical trial should be withdrawn and appropriate treatment measures should be taken immediately for the subjects. However, according to long-term clinical observation, we have not found the occurrence of serious adverse events caused by acupotomy. Common treatment-related adverse events include bleeding, subcutaneous hematoma, infection, continuous post-needling pain, dizziness, and syncope [27].
Data monitoring and auditing
The case report form (CRF) will be completed in paper form by a data entry operator (DEO) and then entered into an Excel spreadsheet by two electronic data interchange (EDI) administrators independently to ensure the accuracy of the data. The researchers will keep all trial data, including signed informed consent, CRF, and operation procedure records. The results of this study may be published in a peer-review medical journal, but we will not disclose the privacy of patients. The research supervision department, the ethics committee of the hospital, and its relevant personnel will audit the trial per year.
Quality
Before the inclusion of patients, all the researchers will be trained on the implementation program, including scale evaluation, data measurement, and CRF filling. A special quality examiner will be set up to check the quality of the research regularly. The CRF should be strictly managed, and the researchers should truthfully, meticulously record as required to ensure that the contents of the CRF are reliable and completed. Regular follow-up will be conducted to ensure the compliance of patients during the study, and the drop-out rate should be controlled within 20%. A study calendar will be used to determine the exact time point for follow-up. Moreover, all treatments, acupotomy, diclofenac diethylamine emulgel, and loxoprofen sodium, will be provided for patients for free.
Protocol amendments
If the protocol amendments occur during the implementation of the study, researchers will communicate the important protocol changes and report changes to the ethics committee. And CRFs will be updated timely to record all the first-hand clinical trial data of the patients.
Sample size
The primary outcome in this study is the response rate, and the allocation ratio between the two groups is 1:1. A bilateral difference test will be used to calculate the sample size. According to a previous review, the response rate of the diclofenac diethylamine emulgel for KOA is assumed to be 60% [28]. The response rate in the acupotomy group is assumed to be 80% based on preliminary clinical experience. The sample size of each group is 80 calculated by PASS 11.0.7 (NCSS, LLC, USA) software with a two-sided significance level of 5% and power of 80%. Considering a 20% drop-out rate, the final sample size of each group will be 100, with a total of 200.
Statistical analysis
SPSS 19.0 software will be used. For all statistical tests, P < 0.05 will be considered to be statistically significant. Continuous data will be presented as mean ± standard deviation (M±SD), while dichotomous data will be presented as a proportion. The independent sample t test or χ2 test will be used to compare demographic data and other baseline indicators to measure the equilibria between the two groups.
Covariance analysis will be used for continuous data and logistic regression analysis will be used for dichotomous data, which will be adjusted according to baseline and dosages of loxoprofen sodium. The results will be presented as mean difference or odds ratio, providing a 95% confidence interval (CI). Intention to treat analysis (ITT) will be carried out for all the randomized patients. The last observation carried forward (LOCF) method and the maximum likelihood regression will be used for the missing data of primary outcome.
The per-protocol (PP) set will include only those who complete at least three sessions and all planned follow-ups and have no major protocol violations (taking other drugs during the trial, etc.). The ITT set will be used for primary analysis, and the PP set will be used as in the sensitivity analysis.
Discussion
KOA is a common public health problem and a leading cause of disability. This study will focus on patients with mild to severe KOA and will explore whether acupotomy is a viable and effective treatment.
The mechanism of acupotomy in KOA is still unclear. Several studies showed that acupotomy may work through regulating signaling pathways such as FAK-PI3K, Wnt/β-catenin, and PI3K/Akt [29,30,31], as well as improving gene and protein expression of cartilage integrin β1 [32]. Evidence also showed acupotomy can lower the level of MMP-3 in rabbits probably by adjusting the mechanics-related signal pathway of the articular chondrocytes [32] and inhibit the expression of inflammatory cytokines, such as TNF-α, IL-1β [33].
This trial will be performed by researchers, who are experienced at acupotomy for KOA, following the study protocol. To keep the consistency and standardization of acupotomy, all operations will be performed by the same doctor. Rigorous supervision and monitoring will be applied to maintain the methodological rigor and implementary normativity.
This trial has some limitations. First, due to the nature of acupotomy, patients and acupotomy doctor cannot be blinded during the treatment. However, outcome assessors and statisticians will be blinded to group assignments to ensure the reliability of results. Second, it is a single-center study that is easy to control the quality and has high internal authenticity; however, the extrapolation of the conclusion is limiting. Besides, since all the acupotomy operations in this study will be performed by one experienced doctor, it is not certain whether the effects can be the same as this study if performed by young doctors.
This study will contribute to a better understanding of the effectiveness and safety of acupotomy in combination with topical NSAIDs. Results of the study will be of great importance for the guidelines of clinical therapy, and more patients suffering from KOA may benefit from acupotomy.
Trial status
This recruitment has begun on March 25, 2021. Trial recruitment is expected by the end of June 2022.
Availability of data and materials
Queries about the full protocol for the study can be consulted with the corresponding author. The results of the trial may be published in peer-reviewed journals, no matter positive or negative results. Personal information of patients will not be disclosed due to privacy protection.
Abbreviations
- KOA:
-
Knee osteoarthritis
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- NRS:
-
Numerical rating scale
- SF-12:
-
12-item Short-Form Health Survey
- WOMAC:
-
Western Ontario and McMaster Universities Osteoarthritis Index
- ITT:
-
Intention-to-treat
- PP:
-
Per-protocol
- LOCF:
-
Last observation carry forward
- CI:
-
Confidence interval
- CRF:
-
Case report form
- DEO:
-
Data entry operator
- EDI:
-
Electronic data interchange
References
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59. https://doi.org/10.1016/S0140-6736(19)30417-9.
Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: part I. Caspian J Intern Med. 2011;2(2):205–12.
Jinks C, Jordan K, Ong BN, Croft P. A brief screening tool for knee pain in primary care (KNEST). 2. Results from a survey in the general population aged 50 and over. Rheumatology. 2004;43:55–61.
Tang X, Wang S, Zhan S, Niu J, Tao K, Zhang Y, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China Health and Retirement Longitudinal Study. Arthritis Rheum. 2016;68(3):648–53. https://doi.org/10.1002/art.39465.
Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73(9):1659–64. https://doi.org/10.1136/annrheumdis-2013-203355.
Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12(2):92–101. https://doi.org/10.1038/nrrheum.2015.135.
Chinese Orthopaedic Association. Clinical Guidelines for Osteoarthritis (2018 Edition). Chin J Orthop. 2018;38:705–15.
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020;72(2):149–62. https://doi.org/10.1002/acr.24131.
Ferreira RM, Duarte JA, Gonçalves RS. Non-pharmacological and non-surgical interventions to manage patients with knee osteoarthritis: an umbrella review. Acta Reumatol Port. 2018;43:182–200.
Sun J, Zhao Y, Zhu R, Chen Q, Song M, Xue Z, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283–17. https://doi.org/10.1155/2020/2168283.
Kwon CY, Yoon SH, Lee B. Clinical effectiveness and safety of acupotomy: An overview of systematic reviews. Complement Ther Clin Pract. 2019;36:142–52. https://doi.org/10.1016/j.ctcp.2019.07.002.
An XY, Wang T, Zhang W, Yu HL, Zhao RC, Guo Y, et al. Chondroprotective effects of combination therapy of acupotomy and human adipose mesenchymal stem cells in knee osteoarthritis rabbits via the GSK3β-Cyclin D1-CDK4/CDK6 signaling pathway. Aging Dis. 2020;1:1116–32.
Huang XS, Geng K, Luo SY, Liu CB, Yang KN, Zhai A, et al. Mechanism of action of acupotomy in inhibiting chondrocyte apoptosis in rabbits with KOA through the PI3K/Akt signaling pathway. Evid Based Complement Alternat Med. 2020;10:4241917.
Wang T, Guo Y, Shi XW, Gao Y, Zhang JY, Wang CJ, et al. Acupotomy contributes to suppressing subchondral bone resorption in KOA rabbits by regulating the OPG/RANKL signaling pathway. Evid Based Complement Alternat Med. 2021;8168657:1–17. https://doi.org/10.1155/2021/8168657.
Xiu ZB, Zhang CX, Liu H, Zhang LZ, Liu J, Lu AH. Clinical effect observation and mechanism discussion on needle knife release in treatment of knee osteoarthritis. J Liaoning Univ Tradit Chin Med. 2018;20:15–8.
Wang Y, Wang N, Qiao YY, Duan YF. Clinical study of acupotomology in the treatment of knee osteoarthritis. New Tradit Chin Med. 2020;52:116–9.
Quan K, He DF, Li MT. Clinical analysis of needle-knife in the treatment of osteoarthritis in knee joint. Chin Pract Med. 2016;11:45–7.
Zhan HJ. Guiding significance of aponeurotic system theory for needle-knife in treating knee osteoarthritis. Chin Med Herald. 2016;13:165–8.
Chen DY, He J, Wang X, Zhang MC, Chen YC, Zhan HS. Clinical study of myofascial release therapy by acupotomy for nonspecific low back pain. Shanghai J Tradit Chin Med. 2012;46:53–4.
Ding Y, Wang Y, Shi X, Luo Y, Gao Y, Pan J. Effect of ultrasound-guided acupotomy vs electro-acupuncture on knee osteoarthritis: a randomized controlled study. J Tradit Chin Med. 2016;36(4):450–5.
Zhang Y, Guo CQ, Zhang XF. Study of the clinical efficacy of acupotomy for treating Knee Osteoarthritis: a randomized, controlled trial. Chin Sci Technol Inform. 2007;18:219–22.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. https://doi.org/10.1136/ard.16.4.494.
Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–26. https://doi.org/10.1016/0304-3959(86)90228-9.
Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64(1):29–33. https://doi.org/10.1136/ard.2004.022905.
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40.
Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
Jeong JK, Kim E, Yoon KS, Jeon JH, Kim YI, Lee H, et al. Acupotomy versus manual acupuncture for the treatment of back and/or leg pain in patients with lumbar disc herniation: a multicenter, randomized, controlled, assessor-blinded clinical trial. J Pain Res. 2020;13:677–87. https://doi.org/10.2147/JPR.S234761.
Derry S, Conaghan P, Da Silva JA, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;4:Cd007400.
Ma S, Xie Z, Guo Y, Yu J, Lu J, Zhang W, et al. Effect of acupotomy on FAK-PI3K signaling pathways in KOA rabbit articular cartilages. Evid Based Complement Alternat Med. 2017;2017:11. https://doi.org/10.1155/2017/4535326.
Lietman C, Wu B, Lechner S, Shinar A, Sehgal M, Rossomacha E, et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight. 2018;3(3):e96308. https://doi.org/10.1172/jci.insight.96308.
Huang X, Geng K, Luo S, Liu C, Yang K, Zhai A, et al. Mechanism of action of acupotomy in inhibiting chondrocyte apoptosis in rabbits with KOA through the PI3K/Akt signaling pathway. Evid Based Complement Alternat Med. 2020;2020:4241917–0. https://doi.org/10.1155/2020/4241917.
Liang C, Guo Y, Tao L, Xiao H, Liu Q, Ma H, et al. Effects of acupotomy intervention on regional pathological changes and expression of carti- lage-mechanics related proteins in rabbits with knee osteoarthritis. Zhen Ci Yan Jiu. 2015;40:119–24.
Lin M, Li X, Liang W, Liu J, Guo J, Zheng J, et al. Needle-knife therapy improves the clinical symptoms of knee osteoarthritis by inhibiting the expression of inflammatory cytokines. Exp Ther Med. 2014;7(4):835–42. https://doi.org/10.3892/etm.2014.1516.
Acknowledgements
The authors would like to thank Beijing Municipal Health Commission and all patients participating in the trial.
Funding
This study was supported by Capital's Funds for Health Improvement and Research (No.2020-2-2231).
Author information
Authors and Affiliations
Contributions
SML and LTL: conception, design of the study, and manuscript writing. CWZ: design of the study and manuscript writing. GR, CP, DWS, and YMZ: conceived the study. KSB: critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Research Ethical Committee of Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University (No. 2020BL02-057-02). Written informed consent will be obtained from all patients before they enroll in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, SM., Li, TL., Guo, R. et al. Effectiveness and safety of acupotomy for knee osteoarthritis: study protocol for a randomized controlled trial. Trials 22, 824 (2021). https://doi.org/10.1186/s13063-021-05786-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-021-05786-5