Abstract
Background
Disordered amino acid metabolism is observed in cerebral malaria (CM). This study sought to determine whether abnormal amino acid concentrations were associated with level of consciousness in children recovering from coma. Twenty-one amino acids and coma scores were quantified longitudinally and the data were analysed for associations.
Methods
In a prospective observational study, 42 children with CM were enrolled. Amino acid levels were measured at entry and at frequent intervals thereafter and consciousness was assessed by Blantyre Coma Scores (BCS). Thirty-six healthy children served as controls for in-country normal amino acid ranges. Logistic regression was employed using a generalized linear mixed-effects model to assess associations between out-of-range amino acid levels and BCS.
Results
At entry 16/21 amino acid levels were out-of-range. Longitudinal analysis revealed 10/21 out-of-range amino acids were significantly associated with BCS. Elevated phenylalanine levels showed the highest association with low BCS. This finding held when out-of-normal-range data were analysed at each sampling time.
Conclusion
Longitudinal data is provided for associations between abnormal amino acid levels and recovery from CM. Of 10 amino acids significantly associated with BCS, elevated phenylalanine may be a surrogate for impaired clearance of ether lipid mediators of inflammation and may contribute to CM pathogenesis.
Similar content being viewed by others
Background
Of the complications associated with Plasmodium falciparum infection, unarousable coma, the hallmark of cerebral malaria (CM), may lead to long-term disability and death [1, 2]. Recent research aided by autopsy and imaging indicates that coma may be the result of generalized brain swelling due to accumulation of sequestered infected erythrocytes in the microvasculature [3,4,5,6]. Sequestration is the physical adherence of infected cells to activated endothelial cells within brain capillaries and venules. Microscopic, sequestered foci with patchy distribution restrict blood flow with downstream consequences including anoxia and acidosis. Leakage of intravascular fluid and, in some cases, erythrocytes into adjacent brain parenchyma through endothelial junctions adds to pathogenic events [7, 8].
In addition to the anatomical pathology in the brain, there are systemic abnormalities involving carbohydrate [9, 10] lipid [11] and amino acid [12, 13] metabolism. None are specific for CM—indeed, they are also seen in septic syndrome and other severe inflammatory diseases [14,15,16]. Metabolic causes of coma in CM have been sought, but no convincing pathogenic mechanism has been found. Nevertheless, these changes may contribute to coma in ways not yet recognized.
Research on the role of nitric oxide in malaria pathogenesis noted abnormalities in the plasma levels of several amino acids in children presenting with CM [17, 18]. All abnormal amino acid levels were below the normal ranges, except for one, phenylalanine, which was consistently above 80 micromolar . Healthy controls (HC) rarely exceeded this cutoff concentration. With resolution of coma in treated survivors, phenylalanine levels normalized. This study questioned whether longitudinal measurements of abnormal amino acid levels might be associated with the level of consciousness as children recovered. Such analysis may uncover an amino acid or group of amino acids involved in P. falciparum pathogenesis.
To address this question, a prospective observational study was conducted in Ghana, in which children entering hospital in coma due to malaria were observed frequently (every 12–24 h) for 60 h and their level of consciousness was quantified by Blantyre coma score(s) (BCS) [19]. At the same time intervals, blood samples were obtained and processed for 21 plasma amino acid levels. The a priori hypothesis was that the levels of one or more amino acid(s) would be most closely associated with the BCS as participants regained consciousness. A healthy control group was enrolled to determine whether amino acid levels in Ghanaian children matched reference laboratory normal ranges for healthy children in the US. The control group also provided disease versus normal comparisons at the time of presentation to hospital.
Methods
Study design and site
The study was a prospective, observational, longitudinal investigation at Komfo Anokye Teaching Hospital in Kumasi, Ashanti Region, Ghana (6.697479° N 1.631690° W) from 2004 to 2006. Enrolled were children who presented to the hospital with CM, as well as healthy control children who were asymptomatic hospital visitors or attendees at well-clinic check-ups. Children with uncomplicated malaria were not included, as the study was designed to address amino acid levels associated with recovery from coma. Study participants largely resided in Kumasi and its surrounding districts. The study was conducted by senior Ghanaian physicians in the Department of Pediatrics assisted by infectious diseases faculty, fellows, and medical students from the University of Utah. Plasma samples for amino acid analysis were obtained at enrollment (i.e., at admission) and at least every 24 h during hospitalization.
Enrollment criteria
Children were 6 months to 6 years of age (median age, 2.8). WHO case definition for CM was used as inclusion criteria [20]: (1) any level of P. falciparum parasitaemia on peripheral blood film; (2) unarousable coma as assessed by BCS ≤ 2 not attributable to hypoglycaemia (i.e., blood glucose level < 40 mg/dl); (3) coma persisting more than 60 min after any convulsion; and (4) no other identifiable cause of coma. Exclusion criteria were any of the following: (1) microscopic or culture evidence of bacterial or viral co-infection; (2) oral or intravenous quinine or oral artemisinin-based combination therapy initiated > 18 h prior to enrollment; (3) haemoglobin < 5 mg/dl when blood transfusion was unavailable at the study site.
Similar aged healthy children were prospectively enrolled as HC. Weight was not included as criterion for enrollment and therefore there is significant missing data for this variable (Table 1). Eligible HC had no symptoms or signs of active illness, no febrile illness within the past 2 weeks, no history or evidence of an active inflammatory condition, and negative blood film for malaria parasites. Availability of HCs for enrollment at the study site was limited and resulted in fewer than anticipated children in this group.
Clinical evaluation and management
Demographic information, clinical history, and physical examination were documented using standardized case report forms. History of last food or liquid intake for CM participants was recorded to assess potential confounding influence of protein intake on plasma amino acid concentrations. The severity of CM resulting in anorexia ruled out this possible confounder for the majority of participants. Capillary blood samples were obtained for malaria thick and thin blood films and were prepared by Giemsa staining. Venous samples for routine laboratory analysis included complete blood count, electrolytes, creatinine, and lactate. Because laboratory service was not always available, there are significant missing data (Table 2) for these analytes. Urine was obtained for dipstick analysis and culture. Blood and urine laboratory results were immediately available to clinicians. Blood cultures were obtained on all participants with CM. Lumbar puncture was done to investigate possible bacterial meningitis unless it was clear to the presiding clinicians that this diagnosis was unlikely. Thirty-four CM cases received lumbar puncture. Cerebrospinal fluid analysis included: (a) determination of glucose and protein concentrations; (b) cell count with differential performed by personnel trained in microscopy; (c) Gram stain and bacterial and fungal cultures.
Children with CM received anti-malarial therapy and supportive care as per standard Ghanaian Ministry of Health protocols for the years during which the study was conducted (intravenous quinine or intravenous artesunate as recommended by WHO protocols). Treatment was initiated as soon as the diagnosis of malaria was suspected.
BCS was assessed at presentation and at least every 24 h until hospital discharge or death. For some participants (year 1 of the study) BCS was measured at admission and at 12-h intervals for up to 60 h. For years 2 and 3, midnight blood draws became impractical due to transportation and safety concerns, and samples were taken every 24 h. For each child at each interval, three clinicians independently assessed BCS. The assessments were done by a Ghanaian faculty physician, a US infectious diseases faculty or fellow, and a Ghanaian or US medical student. After assessment, the three clinicians met to obtain a consensus for BCS. For some patients this required returning to the bedside to review the findings. In all cases, consensus was reached by the three examiners and one BCS was recorded. At the time of BCS assessments the clinician examiners were unaware of any data pertaining to plasma amino acid concentrations.
Plasma amino acid analysis
Blood samples were collected into heparin tubes, mixed, and immediately centrifuged to sediment blood cells. Supernatant plasma was placed into polypropylene freezer tubes and stored at − 80 °C until shipment. Samples were transported in a liquid nitrogen dry shipper to the US for amino acid analysis. All amino acid analyses for the 3-year study were performed within 1 month after collection for each year. Amino acid analyses were performed at the Biochemical Genetics Section, ARUP Laboratories, University of Utah School of Medicine in collaboration with Dr. Marzia Pasquali. The amino acid analyzer employed ion exchange chromatography for separation and quantification. With two exceptions, all plasma amino acids were quantified. Exceptions were tryptophan, which emerged from the column lastly at a long retention time unsuitable for analysis, and hydroxyproline, which gave frequent values of zero or below the level of sensitivity of the assay. Computer output results from the amino acid analyzer were electronically transferred to Excel spread sheets for subsequent data analysis.
Statistical methods
Data was compared for CM cases and healthy controls at entry (Tables 1, 2, 3). Data was analysed using GraphPad Prism version 10.1.1. Data sets for each variable were tested for normality. Parametric data (Tables 1, 2) for both CM and healthy control groups were compared for significant difference using two-tailed Student’s t-test. For non-parametric data (Table 3) the two-tailed Mann–Whitney test was used. A significant difference was defined as P ≤ 0.05.
Association between BCS values and amino acid levels was investigated using statistical modeling. The aim was to assess if an out-of-range level of each amino acid was linked to a low BCS. Since BCS is based on an ordinal discrete scale from 0 to 5, an ordinal logistic regression, which is the most appropriate modeling technique for ordinal variables, was employed [21, 22]. The model was formulated using a generalized linear mixed-effects model (GLM-EM) [23]. It incorporated children as a random effect to account for variations in the sampling period. The ordinal logistic regression model describes the association between the dependent variable and independent variables by estimating odds ratios (OR). Odds ratio was used to identify the association between out-of-range amino acid levels and BCS. An OR significantly below 1.0 indicated that having an amino acid level outside the normal range was associated with low BCS. An OR above 1.0 indicated that having an amino acid level within the normal range was associated with a high BCS. The model equation was the following:
where BCS was children’s BCS expressed as an ordinal variable from 0 to 5, Year was the sampling year, and Child IDrandom was children’s ID included as a random effect.
A logistic regression GLM-EM was also built to investigate the effect of time on the levels of amino acids [24]. This model aimed to identify the time threshold after which the levels of amino acids returned to the normal range. An OR significantly above 1.0 indicated a high probability that the amino acid level was normal at a given time, while an OR significantly below 1.0 indicated a high probability that the amino acid level was abnormal at a given time. The equation for the GLM-EM was the following:
where Amino acid level (0,1) was a dichotomous variable reporting if the amino acid level was normal (value = 1) or out of normal range (value = 0), Sampling time was the time intervals of sampling, Year was the sampling year, and Child IDrandom was children’s ID included as a random effect.
All statistical modelling was performed using BayesX software through R language interface [25, 26]. All P values were adjusted using the Bonferroni correction [27]. A significant association was defined as P ≤ 0.05.
Ethics
The study was approved by the ethics committee at Komfo Anokye Teaching Hospital and the Institutional Review Board at the University of Utah, USA. Written informed consent was obtained from either parent or guardian of all participants. Consent forms were presented in Twi or in English, depending on the consenting parent or guardian preference. US Department of Health and Human Services guidelines for human subjects research, the University of Utah guidelines, and the guidelines for the Komfo Anokye Teaching Hospital were followed.
Results
Clinical and laboratory findings
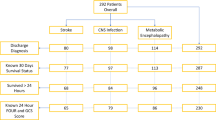
Forty-two children were enrolled with CM due to P. falciparum; of CM cases, 6 were enrolled in 2004, 19 in 2005, and 17 in 2006; note: fewer children were enrolled in year 1 due to lack of dedicated study personnel and difficulties for setting up the Study Protocol requirements. Thirty-six healthy controls were enrolled during these same years. The enrollment periods for each year were the same, i.e., during the long rainy season. Two CM participants were excluded from the analyses due to missing data; six children with CM died (14.3%) during hospitalization, all within the first 48 h of admission.
Clinical characteristics for the two groups are listed in Table 1. The groups were closely matched for age, gender, and weight. Physical findings revealed the degree of illness in the CM group, including significant differences in pulse, respiratory rate, and temperature. Of those CM cases for whom clinical data was available, 68% experienced a witnessed seizure and 59% received diagnostic lumbar puncture. Historical data on most recent food intake indicated that plasma amino acid levels in CM participants were unlikely to be confounded by recent protein ingestion. The length of pre-admission symptoms (mean, 4 days) was consistent with the acute course of CM leading to coma prompting presentation at hospital.
Laboratory findings in CM cases were consistent with CM, including anemia, thrombocytopenia, and metabolic acidosis (Table 2). Elevated creatinine was likely due to acute kidney injury [28]. Hypoglycaemia in CM participants was obscured by immediate intravenous glucose-containing fluid begun on all children presenting in coma.
Plasma amino acid levels at entry
Plasma samples collected from 40 CM enrollees at various time-points during hospitalization were available for amino acid analysis with one exception: a single sample at time zero. Of the 36 healthy control enrollees there were 6 samples unavailable for analysis because of insufficient venipuncture blood for plasma separation or because samples went missing.
For healthy controls, normal distribution was usually the case. However, all amino acid data for the CM group showed non-parametric distributions. For 5 of the 21 amino acids (Asp, Cys, Leu, Tyr, Val), there were no significant differences between CM versus healthy controls at entry (Table 3). In all cases but one, the levels of the remaining amino acids (Ala, Arg, Cit, Glu, Gln, Gly, His, Ilu, Lys, Met, Orn, Pro, Ser, Tau, Thr) were significantly lower in the CM group compared to healthy controls. One amino acid (Phe) was significantly elevated in CM participants compared to healthy controls. Also shown in Table 3 are the normal ranges (mean ± 2SD) for each amino acid, which were established at the ARUP diagnostic laboratory based on a large age-dependent database of healthy US children. For Ghanaian healthy control children, amino acid levels were largely within the US normal ranges.
Longitudinal association between BCS and amino acid levels outside the normal range
Results obtained from the ordinal GLM-EM showed that BCS was significantly associated with amino acid levels outside the normal range for 10 of the 21 amino acids (Table 4). Nine of the 10 amino acids were associated with significant probability for having a low BCS; the lower the odds ratio the greater the probability of having a low BCS. A phenylalanine level outside the normal range was associated with the highest probability of having a low BCS (lowest odds ratio). Conversely, valine out-of-range levels were significantly associated with a high probability of having a high BCS (highest odds ratio). Odds ratios calculated using statistical modeling (GLM-EM) do not specify whether an amino acid association significance is above or below its normal range. In this regard it is notable that only one of the ten amino acids (phenylalanine) with significant association for a low BCS is above its normal range. All other significant amino acid associations are below their normal ranges.
For completeness box plots showing raw data for out-of-range versus normal range individual samples for each amino acid at a given BCS are shown in Supplementary data (Supplementary Fig. 1, panels A–D).
Logistic regression assessment of time to reach normal range levels for the 10 amino acids significantly associated with BCS
For clarity, the time course was plotted for plasma levels (mean ± SEM) of each of the ten amino acids (Table 4) significantly associated with a low BCS (Fig. 1). In each panel the right axis reproduces the mean ± SEM BCS. A dashed horizontal line marks the normal range lower limit for nine of the ten amino acids. For these nine all abnormal levels were below the normal range. The one exception is phenylalanine where the dashed line shows the upper limit of normal, commensurate with hyperphenylalaninaemia for this amino acid. Some amino acids (Arg, Cit, Gln) exhibit a time lag before returning to within their normal ranges while BCS rose to a near conscious level (BCS ~ 3–4) by 24 h. Others (Gly, Ilu, Lys, Ser, Thr) showed borderline low levels, but rose to within their normal ranges as time progressed. Exceptionally, high phenylalanine levels promptly fell in concert with rising BCS.
Out-of-range amino acid levels significantly associated with Blantyre Coma Score at sampling time intervals. Plasma levels of each amino acid (circles, mean ± SEM) significantly associated with BCS (Table 4) are shown at sampling times. Blantyre Coma Score (triangles, mean ± SEM) at the same sampling times are reproduced for each amino acid panel
A logistic regression model was used to determine the time after which there was a high probability that amino acid levels reached the normal range (Fig. 2). Bar graphs for each of the ten amino acids show odds ratios (± 95% CI) at each sampling time. Odds ratios below 1.0 with Asterix denote significant association with levels outside normal ranges. Odds ratios above 1.0 with Asterix denote significant association with levels within normal ranges. Bars without Asterix are without significance. Almost all amino acids normalized by 48–60 h post admission and initiation of treatment. However, the analyses showed marked variability across amino acids: for example, phenylalanine and isoleucine reached their normal range at 36 h, while for other amino acids it took 48 and 60 h to reach the normal range. An outlier was valine, with significant normal range values at zero and 12 h and abnormal range values at 36, 48 and 60 h. The changes were associated with increasing BCS as time elapsed. For reference, Fig. 2 (lower right-most panel) shows BCS data at each sampling time.
Effect of sampling time on having an amino acid level outside of, or within, the normal range. Bar graphs for each amino acid show odds ratios (± 95% CI) at each sampling time. Odds ratios below 1.0 with Asterix denote significant association with levels outside normal ranges. Odds ratios above 1.0 with Asterix denote significant association with levels within normal ranges. Bars without Asterix are without significance. Box plot in the lower right hand panel shows median BCS with variance measures at sampling intervals. Boxes represent interquartile ranges (IQR). Dark lines within boxes are medians. Whiskers indicate minimum and maximum values within 1.5 times the IQR
Discussion
Plasma amino acid abnormalities in CM at hospital entry
Abnormal amino acid levels based on age-dependent normal ranges were defined for 21 amino acids as established for healthy US children in the reference laboratory. To determine whether these normal ranges applied to healthy Ghanaian children, an age-matched healthy control group was enrolled and their amino acid plasma levels were measured. The healthy control levels were within normal ranges save for slight median decreases of cystine and glutamine (Table 3).
Amino acid levels were compared in CM versus healthy control cohorts at hospital entry. Sixteen of the 21 amino acids were significantly different. The results mirror those reported by others for children [13] and adults [12] with severe malaria, including CM. Fifteen of the 16 were below the normal range and one (phenylalanine) was elevated. The hypercatabolic state induced by the inflammatory response in severe malaria, in which amino acids are oxidatively degraded to yield chemical energy likely contributes to the low levels observed [29, 30] Additionally, gluconeogenesis in liver consumes glucogenic amino acids. Acute kidney injury associated with amino acid reabsorption abnormalities poses yet another source of loss [13]. Significantly low levels of glutamine, glutamate, proline, ornithine, citrulline and arginine comprise the pathway of de novo arginine synthesis [18] a finding consistent with low nitric oxide production [31]. Longitudinal data found low arginine levels associated with nitric oxide-dependent endothelial dysfunction [32].
Longitudinal associations between out-of-range amino acid levels and BCS
These results extended amino acid abnormalities by longitudinal measurements with analysis for association with BCS. By employing the GLM-EM model the data showed that ten of the sixteen out-of-range amino acids at entry were significantly associated with BCS (P ≤ 0.05, Table 4). Six of the ten (arginine, glycine, isoleucine, phenylalanine, threonine, and valine) showed high significance for association with BCS (P ≤ 0.01). Of this set, phenylalanine and valine stood out. Phenylalanine was the only amino acid with levels above the normal range in CM participants compared to healthy controls. Of all significant BCS-associated amino acids, phenylalanine showed the highest association (lowest odds ratio, 0.25). Valine out-of-range values were associated with high BCS.
For the ten amino acids significantly associated with BCS, the out-of-range values at each sampling time were analysed by calculating odds ratios with the GLM-EM model. With time, 9 of 10 amino acids normalized by 60 h post admission and initiation of treatment (odds ratios > 1.0). At 24 to 36 h post admission and initiation of treatment, levels transitioned to normal ranges for isoleucine, lysine, phenylalanine, and threonine. At these same time-points median BCS rose from about 1 to 4–5. Thus, regaining consciousness occurred at the time when these four amino acids normalized. The outlier was valine with significant normal range values at entry and at 12 h with transition to abnormally low levels at 36, 48, and 60 h post admission and initiation of treatment.
Significance of association of abnormal valine levels with high BCS
Of the ten amino acids associated with BCS, a unique association between BCS and valine was found. Out-of-range valine levels bore significant probability to have high, rather than low, BCS. This finding could relate to the complex catabolism of the branched-chain amino acids (Leu, Ilu, Val). In experimental animal models and possibly in humans, high leucine levels antagonize valine degradation at the decarboxylation (keto acid dehydrogenase) step by competitive inhibition [33, 34]. This might delay valine catabolic kinetics resulting in out-of-range levels at later times during recovery, when BCS had risen to 4 or 5.
Possible significance of hyperphenylalaninaemia as a surrogate for malarial coma
Like other amino acids, the phenylalanine degradation supplies carbon to the TCA cycle for oxidation [35]. Why then did investigators find consistently elevated phenylalanine levels in CM? In a previous study, a possible mechanism to explain hyperphenylalaninaemia was found [36]. The liver enzyme, phenylalanine hydroxylase (PHA), requires the cofactor tetrahydrobiopterin (BH4) for mono-oxygenation of phenylalanine to yield its product, tyrosine [37]. In health biopterin is poised in the reduced state (BH4) such that with each phenylalanine to tyrosine reaction, the oxidized cofactors, biopterin (B) and dihydrobiopterin (BH2), are reduced back to BH4 via two pathways (recycling and salvage) [37]. The reducing equivalents for restoring biopterin to its reduced state are supplied by the nicotinamide adenine dinucleotides NADH and NADPH [37]. In the systemic compartment (i.e., plasma and liver) about 2/3 of biopterin is poised in the reduced state (BH4) [38]. This provides sufficient reducing power for tight regulation of plasma phenylalanine below 80 micromolar [39]. Phenylalanine regulation prevents hyperphenylalaninaemia, which is toxic to the brain (e.g., in the congenital disease, phenylketonuria [39]). While total biopterins (B + BH2 + BH4) were slightly increased in CM, the percentage of reduced biopterin fell by 75% [36]. This suggests that hyperphenylalaninaemia in CM results from a diminution of PAH catalysis in the liver due to insufficient BH4 availability, possibly a result of oxidative stress.
Potential role of BH4 in CM pathogenesis
BH4 is a unique cofactor in that there are only 6 enzymes for which it is required [37, 40]. These are the enzymes for (a) synthesis of catecholamines and serotonin in the brain, (b) systemic and CNS synthesis of nitric oxide, (c) PAH in liver, and (d) a little studied liver enzyme for catabolism of membrane ether lipids, alkylglycerol mono-oxygenase (AGMO) [40,41,42]. Accumulation of membrane-derived ether lipids from impaired AGMO activity resulting from limited BH4 availability might adversely affect the CNS [43]. Blood–brain barrier permeability is markedly increased in experimental animals treated with short chain alkyl (ether) glycerols [44, 45]. Limiting AGMO activity could enhance circulating ether lipid mediators such as platelet activation factor (PAF). This possibility is yet to be investigated [46, 47]. There is evidence from malaria models that PAF could enhance sequestration of infected red cells if regulation control mechanisms fail [48, 49]. The possible role for an ether lipid(s) in malaria pathogenesis should be explored.
Limitations
Because a group of children with severe malaria without coma was not included, one cannot infer that amino acid abnormalities described here are specific for the depth of coma itself, but rather could reflect broader associations with severe disease states. The cohort of 40 CM cases is relatively small. BCS are subjective, therefore open to observer bias. The linear mixed effects model can only assign an association between out-of-range amino acid levels and BCS, and thus cannot establish cause and effect. Diagnosis of CM carries a significant false positive fraction [50]. However, 59% of the CM cohort received lumbar puncture to rule out meningitis, thus diminishing the likelihood of non-malarial causes of coma.
Conclusion
Longitudinal data on normalization of amino acid levels with recovery from malarial coma found a highly significant association for phenylalanine. Unlike other coma-associated amino acids, abnormal phenylalanine levels were above, not below, normal range. Hyperphenylalaninaemia likely results from insufficient reducing power normally provided by tetrahydrobiopterin. A previous study found a marked diminution of tetrahydrobiopterin. Therefore, the single other liver tetrahydrobiopterin-dependent enzyme, responsible for degrading biologically active ether lipids (AGMO), may be impaired. Elevated bioactive ether lipids are known to permeabilize the CNS blood–brain barrier and lead to platelet activation, events shown to be critical in CM pathogenesis. If impaired, restoration of AGMO activity could provide efficacious adjunctive therapy.
Data availability
No datasets were generated or analysed during the current study.
References
Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404.
WHO. World Malaria Report 2021. Geneva: World Health Organization; 2021.
Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372:1126–37.
Potchen MJ, Kampondeni SD, Seydel KB, Haacke EM, Sinyangwe SS, Mwenechanya M, et al. 1.5 Tesla magnetic resonance imaging to investigate potential etiologies of brain swelling in pediatric cerebral malaria. Am J Trop Med Hyg. 2018;98:497–504.
Kampondeni S, Seydel KB, Zhang B, Small DS, Birbeck GL, Hammond CA, et al. Amount of brain edema correlates with neurologic recovery in pediatric cerebral malaria. Pediatr Infect Dis J. 2020;39:277–82.
Poespoprodjo JR, Douglas NM, Ansong D, Kho S, Anstey NM. Malaria. Lancet. 2023;402:2328–45.
Darling TK, Mimche PN, Bray C, Umaru B, Brady LM, Stone C, et al. EphA2 contributes to disruption of the blood-brain barrier in cerebral malaria. PLoS Pathog. 2020;16: e1008261.
Moxon C, Alhamdi Y, Storm J, Toh J, Ko JY, Murphy G, et al. Parasite histones mediate blood-brain barrier disruption in cerebral malaria. Clin Med. 2020;20(Suppl 2):s96–7.
White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–6.
Planche T, Krishna S. Severe malaria: metabolic complications. Curr Mol Med. 2006;6:141–53.
Herdman MT, Sriboonvorakul N, Leopold SJ, Douthwaite S, Mohanty S, Hassan MM, et al. The role of previously unmeasured organic acids in the pathogenesis of severe malaria. Crit Care. 2015;19:317.
Leopold SJ, Apinan S, Ghose A, Kingston HW, Plewes KA, Hossain A, et al. Amino acid derangements in adults with severe falciparum malaria. Sci Rep. 2019;9:6602.
Conroy AL, Tran TM, Bond C, Opoka RO, Datta D, Liechty EA, et al. Plasma amino acid concentrations in children with severe malaria are associated with mortality and worse long-term kidney and cognitive outcomes. J Infect Dis. 2022;226:2215–25.
Dinarello CA. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984;311:1413–8.
Lundblad RL. Biotechnology of plasma proteins. Ist. Boca Raton: CRC Press; 2012. p. 460.
van Gassel RJJ, Baggerman MR, van de Poll MCG. Metabolic aspects of muscle wasting during critical illness. Curr Opin Clin Nutr Metab Care. 2020;23:96–101.
Lopansri BK, Anstey NM, Stoddard GJ, Mwaikambo ED, Boutlis CS, Tjitra E, et al. Elevated plasma phenylalanine in severe malaria and implications for pathophysiology of neurological complications. Infect Immun. 2006;74:3355–9.
Rubach MP, Zhang H, Florence SM, Mukemba JP, Kalingonji AR, Anstey NM, et al. Kinetic and cross-sectional studies on the genesis of hypoargininemia in severe pediatric Plasmodium falciparum malaria. Infect Immun. 2019;87:e00655-e718.
Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–59.
WHO. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:S1–90.
Armstrong BG, Sloan M. Ordinal regression models for epidemiologic data. Am J Epidemiol. 1989;129:191–204.
Norris CM, Ghali WA, Saunders LD, Brant R, Galbraith D, Faris P, et al. Ordinal regression model and the linear regression model were superior to the logistic regression models. J Clin Epidemiol. 2006;59:448–56.
Hedeker D. A mixed-effects multinomial logistic regression model. Stat Med. 2003;22:1433–46.
Ugwu CLJ, Zewotir TT. Using mixed effects logistic regression models for complex survey data on malaria rapid diagnostic test results. Malar J. 2018;17:453.
Umlauf N, Adler D, Kneib T, Lang S, Zeileis A. Structured additive regression models: an R interface to BayesX. Stat Softw. 2015;63:1–46.
Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021.
Cabin RJ, Mitchell RJ. To Bonferroni or not to Bonferroni: when and how are the questions. Ecol Soc Am. 2000;81:246–8.
Batte A, Berrens Z, Murphy K, Mufumba I, Sarangam ML, Hawkes MT, et al. Malaria-associated acute kidney injury in African children: prevalence, pathophysiology, impact, and management challenges. Int J Nephrol Renovasc Dis. 2021;14:235–53.
Basler T, Meier-Hellmann A, Bredle D, Reinhart K. Amino acid imbalance early in septic encephalopathy. Intensive Care Med. 2002;28:293–8.
Bröer S, Bröer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J. 2017;474:1935–63.
Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, Misukonis MA, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–67.
Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, et al. Recovery of endothelial function in severe falciparum malaria: relationship with improvement in plasma L-arginine and blood lactate concentrations. J Infect Dis. 2008;198:602–8.
Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54.
Staten MA, Bier DM, Matthews DE. Regulation of valine metabolism in man: a stable isotope study. Am J Clin Nutr. 1984;40:1224–34.
Castillo L, Yu YM, Marchini JS, Chapman TE, Sanchez M, Young VR, et al. Phenylalanine and tyrosine kinetics in critically ill children with sepsis. Pediatr Res. 1994;35:580–8.
Rubach MP, Mukemba J, Florence S, Lopansri BK, Hyland K, Volkheimer AD, et al. Impaired systemic tetrahydrobiopterin bioavailability and increased oxidized biopterins in pediatric falciparum malaria: association with disease severity. PLoS Pathog. 2015;11: e1004655.
Blau N, Thony B, Cotton RGH, Hyland K. Disorders of tetrahydrobiopterin and related biogenic amines. In: CR Scriver AB, WS Sly, D Valle, (eds.) The Metabolic and molecular bases of inherited disease. 8 edn. Vol II. New York: McGraw-Hill; 2001.
Hyland K. Estimation of tetrahydro, dihydro and fully oxidised pterins by high-performance liquid chromatography using sequential electrochemical and fluorometric detection. J Chromatogr. 1985;343:35–41.
Kaufman CS. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: CR Scriver AB, WS Sly, D Valle, (eds). The metabolic and molecular bases of inherited disease. 8th edn VII. New York: McGraw-Hill; 2001, 1667–724.
Werner ER. Three classes of tetrahydrobiopterin-dependent enzymes. Pteridines. 2013;24:7–11.
Watschinger K, Werner ER. Alkylglycerol monooxygenase. IUBMB Life. 2013;65:366–72.
Sailer S, Keller MA, Werner ER, Watschinger K. The emerging physiological role of AGMO 10 years after its gene identification. Life. 2021;11:88.
Dorninger F, Forss-Petter S, Wimmer I, Berger J. Plasmalogens, platelet-activating factor and beyond - ether lipids in signaling and neurodegeneration. Neurobiol Dis. 2020;145: 105061.
Erdlenbruch B, Alipour M, Fricker G, Miller DS, Kugler W, Eibl H, et al. Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br J Pharmacol. 2003;140:1201–10.
Erdlenbruch B, Jendrossek V, Eibl H, Lakomek M. Transient and controllable opening of the blood-brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. Exp Brain Res. 2000;135:417–22.
Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci. 2003;40:643–72.
Gupta S, Seydel K, Miranda-Roman MA, Feintuch CM, Saidi A, Kim RS, et al. Extensive alterations of blood metabolites in pediatric cerebral malaria. PLoS ONE. 2017;12: e0175686.
Lacerda-Queiroz N, Rodrigues DH, Vilela MC, Rachid MA, Soriani FM, Sousa LP, et al. Platelet-activating factor receptor is essential for the development of experimental cerebral malaria. Am J Pathol. 2012;180:246–55.
Lacerda-Queiroz N, Rachid MA, Teixeira MM, Teixeira AL. The role of platelet-activating factor receptor (PAFR) in lung pathology during experimental malaria. Int J Parasitol. 2013;43:11–5.
Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5.
Acknowledgements
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002538. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or their employers. John Hibbs Jr., J. Brice Weinberg, and Guy Zimmerman (deceased) provided useful comments. Tsin Yeo and Nick Anstey reviewed the manuscript and raised important suggestions for statistical analysis. Mary MacFarland aided in the preparation of the manuscript. Thanks to Will J. Fennelly for data entry and to Arthur L. Granger for arranging statistical assistance. Special appreciations go to Justice Sylverken, Clinical Assistant, and the pediatric nursing staff at Komfo Anokye Teaching Hospital for their dedicated patient care and service to the study.
Presentation
This work has not been presented in any form at a scientific meeting.
Funding
NIH/NIAID: K23 AI 116869 BKL (awardee), DLG (sponsor).
Author information
Authors and Affiliations
Contributions
D.L.G wrote the main manuscript text. B.K.L., R.R., and D.B provided edits to the manuscript. D.L.G. and D.B. assembled the Tables and Figures. D.A., T.A., M.S.L., B.A.B., D.C.H., and B.K.L. carried out the study protocol in Kumasi, Ghana. D.B., R.R., and D.L.G. analyzed the data. D.B. and R.R. provided statistical expertise. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12936_2024_5077_MOESM1_ESM.zip
Additional file1Supplementary Fig. 1. Box plots show out-of-range versus normal-range amino acid plasma levels at given BCS. Fig. 1 A–1D show results for all 21 amino acids analysed. Coloured circles represent individual amino acid levels for participants with cerebral malaria. An Asterix denotes significant association between amino acid levels within or outside the normal ranges at given Blantyre Coma Scores as determined by the generalized linear mixed-effects model
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Granger, D.L., Ansong, D., Agbenyega, T. et al. Longitudinal associations of plasma amino acid levels with recovery from malarial coma. Malar J 23, 253 (2024). https://doi.org/10.1186/s12936-024-05077-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-05077-9