Abstract
Background
Pyrethroid resistance is one of the major threats for effectiveness of insecticide-treated bed nets (ITNs) in malaria vector control. Genotyping of mutations in the voltage gated sodium channel (VGSC) gene is widely used to easily assess the evolution and spread of pyrethroid target-site resistance among malaria vectors. L1014F and L1014S substitutions are the most common and best characterized VGSC mutations in major African malaria vector species of the Anopheles gambiae complex. Recently, an additional substitution involved in pyrethroid resistance, i.e. V402L, has been detected in Anopheles coluzzii from West Africa lacking any other resistance alleles at locus 1014. The evolution of target-site resistance mutations L1014F/S and V402L was monitored in An. coluzzii and Anopheles arabiensis specimens from a Burkina Faso village over a 10-year range after the massive ITN scale-up started in 2010.
Methods
Anopheles coluzzii (N = 300) and An. arabiensis (N = 362) specimens collected both indoors and outdoors by different methods (pyrethrum spray catch, sticky resting box and human landing collections) in 2011, 2015 and 2020 at Goden village were genotyped by TaqMan assays and sequencing for the three target site resistance mutations; allele frequencies were statistically investigated over the years.
Results
A divergent trend in resistant allele frequencies was observed in the two species: 1014F decreased in An. coluzzii (from 0.76 to 0.52) but increased in An. arabiensis (from 0.18 to 0.70); 1014S occurred only in An. arabiensis and slightly decreased over time (from 0.33 to 0.23); 402L increased in An. coluzzii (from 0.15 to 0.48) and was found for the first time in one An. arabiensis specimen. In 2020 the co-occurrence of different resistance alleles reached 43% in An. coluzzii (alleles 410L and 1014F) and 32% in An. arabiensis (alleles 1014F and 1014S).
Conclusions
Overall, an increasing level of target-site resistance was observed among the populations with only 1% of the two malaria vector species being wild type at both loci, 1014 and 402, in 2020. This, together with the co-occurrence of different mutations in the same specimens, calls for future investigations on the possible synergism between resistance alleles and their phenotype to implement local tailored intervention strategies.
Similar content being viewed by others
Background
Malaria vector control is still deeply dependent on the use of pyrethroids, the primary class of World Health Organization (WHO)-recommended insecticides for treating bed nets (ITNs). Pyrethroid-based control tools have been the milestone of malaria prevention in Africa for almost two decades and have led to an incontestable success in the fight against the disease: ITNs alone drove over 68% of 663 million prevented case in 2000–2015 [1]. Unfortunately, the longstanding usage of pyrethroids as pest control in agriculture and their massive scale up in the field of public health [2,3,4,5] have contributed to the current scenario of widespread resistance among mosquito vector populations across all Africa [6, 7]. This is even worsened by cross-resistance, for which an insecticide can elicit the resistance to another chemical compound of a different class but sharing the same mode of action. This has probably been the case of the past extensive use in agriculture of DDT which share with pyrethroids the same target site, i.e. the voltage-gated sodium channel (VGSC) [8, 9]. Pyrethroid resistance is seriously threatening the success of malaria vector control tools, contributing to the current stalling progress towards malaria elimination [10].
To face the global issue of insecticide resistance, WHO has recently approved two new classes of ITN containing pyrethroids mixed with Chlorfenapyr (pyrrole insecticide disrupting oxidative pathways) or Pyriproxyfen (an insect growth regulator) [11], which act on different target sites than sodium channel. This new generation of ITNs is expected to be a game changer in restoring ITN effectiveness [11,12,13,14].
Pyrethroid insecticide resistance is mainly imputable to: 1) non-synonymous mutation in the vgsc gene encoding for the paratype voltage-gated sodium channel and 2) metabolic mechanisms which increase the activity of enzymes detoxifying the insecticide (i.e. cytochrome P450 monooxygenases, esterases, and glutathione S-transferases). Other mechanisms as cuticular resistance and binding/sequestration can act in addition to determine mosquito physiological resistance to pyrethroids [9, 15,16,17].
Molecular testing of mutations in the vgsc is widely used to easily assess presence and frequencies of target site mutations of insecticide resistance and represents also an early informative approach to follow the evolution/spread of resistance among field populations [7]. This is a fundamental aspect in the context of pyrethroid resistance management, especially considering the complicated genetics of target site resistance [18] and its combined effect with metabolic pathways [7, 19].
L1014F and L1014S mutations (or L995F and L995S using An. gambiae codon numbering) are the most widely spread and best characterized VGSC mutations in major African malaria vector species of the An. gambiae complex [18]. They cause a substitution of leucine with phenylalanine (TTA→TTT, for L1014F) or with serine (TTA→TCA for L1014S) in the sixth transmembrane segment of domain II of the VGSC, leading to altered channel gating and eventually a reduced sensitivity to pyrethroids (knock-down resistance, kdr) [20]. Both mutations were observed in An. gambiae sensu lato (s.l.) field populations largely before the scaling up of pyrethroids in public health (started in 2000s), with first reports dating around the end of 80’s [21,22,23], and were shown to have emerged multiple times across Africa [24,25,26,27]. L1014F and L1014S were originally described in West and East Africa respectively [21, 22, 28,29,30], but now coexist across sub-Saharan Africa at variable frequencies from site to site among major vector species of the An. gambiae complex, i.e. An. coluzzii, An. gambiae and An. arabiensis [24, 31,32,33,34,35,36,37,38,39,40,41]. Although there is no clear evidence that the presence of 1014 mutations is sufficient to result in vector control failure [7, 9, 19], these alleles are commonly used as markers of target-site resistance to pyrethroids.
Two additional tightly linked non-synonymous mutations in vgsc gene, V402L-I1527T, were recently observed in An. coluzzii populations of Ghana, Burkina Faso and Ivory Coast, in specimens lacking mutations at 1014 locus [18], observing a linkage disequilibrium between 1014F and 402L. Allele 402L is reported in two allelic variants (TTA and CTA) causing a substitution from valine to leucine in segment 6 of domain I, while I1527T causes a change from isoleucine to threonine in segment 6 of domain III. For V402L, it has been demonstrated that this mutation confers resistance to pyrethroids in laboratory colonies without apparent fitness cost under experimental laboratory conditions [42], while L1014F has been shown to have pleiotropic effects resulting in reduced fecundity and longevity, alongside an effect on larval development [19, 43, 44]. This would allow V402L to compete with the L1014F mutation and increase in frequency in case of either reduced insecticide selective pressure, or if its combined effect with other resistance mechanisms (e.g. other mutation as I1527T or metabolic resistance) conferred elevated level of pyrethroid resistance. In fact, first evidence of a drop in frequency of 1014F associated with the rise of the alternative V402L/I1527T haplotype was reported in An. coluzzii wild populations of Southwest Burkina Faso where, from 2016 to 2019, 402L-1527 T frequency increased from 18 to 37% while the frequency of the 1014F allele decreased from 82 to 63% [42].
In this study, the temporal frequencies of 1014F, 1014S and 402L mutations in malaria vectors of a village in central Burkina Faso were analysed, from 2011 to 2020, i.e. since one year after the implementation of the ITN national mass distribution campaign. Differently from the previously quoted study in the South-western region of the country [42], a limited insecticidal pressure derived from insecticide use against agricultural pest is expected in the present study site. In addition, this study site has been characterized entomologically for over ten years, supporting the results here retrieved with other parameters related with vector response to ITN pressure [45,46,47,48].
Methods
Study area
The survey was carried out in Goden, a rural village (12°25ʹ N, 1° 21ʹ W) of < 1,000 people (Bogodogo, Health District survey 2021, unpublished data) located in the central region of Burkina Faso, in a Sudanese savannah area, at 35 km East far from the capital city Ouagadougou. The land use and livelihood profile of the region is mainly based on market gardening and, to a lesser extent, on rice cultivation around dams and on livestock rearing of small ruminants and poultry [49]. The study area is far out (about 480 km) from the major cotton growing belt located in the Southwest of Burkina Faso and scarcely affected by pesticide usage for agricultural purposes [50].
The region is characterized by holoendemic malaria mainly caused by Plasmodium falciparum [51]. ITNs are the vector control tools employed in the region where, as in the rest of the country, five national mass distribution campaigns were implemented in 2010, 2013, 2016, 2019 and 2022 [52, 53].
According to the national survey “Enquête sur les indicateurs du paludisme au Burkina Faso” [54, 55], about 55%, 86% and 79% of households received at least one ITN, respectively, in the first three distribution campaigns in Goden region. Roughly 3,800,000 ITNs were distributed during these campaigns, reaching up to 96% household coverage (data unpublished, courtesy of Dr. Wamdaogo Moussa Guelbeogo).
ITN enriched with PBO (piperonyl butoxide), a non-toxic synergist of pyrethroids, has been introduced in the Central region since 2019 to manage insecticide resistance, while no IRS is currently used [53, 56].
Entomological collections and specimen processing
Mosquitoes analysed in the context of the current study were part of larger entomological collections conducted in years 2011, 2015 and 2020 [45, 47, 48]. Briefly, in 2011 indoor and outdoor resting collections were carried out by pyrethrum spray catches and sticky resting box, while in 2015 and 2020 host seeking mosquitoes were collected inside and outside houses by human landing catches. A subsample of mosquitoes, already identified as An. coluzzii and An. arabiensis in previous studies [45, 47, 48], was randomly chosen for each sampling year and processed for insecticide resistance allele genotyping.
Insecticide resistance analysis
Genotyping of 1014F and 1014S mutations was carried out by two different Taqman Realtime assays, according to the protocol of Bass et al. [57]. For a subgroup of genotyped mosquitoes, results were double-checked by sequencing the amplification products derived from standard PCR assay of Martinez-Torres and colleagues [28]. According to the protocol, primers Agd1 (5'-ATAGATTCCCCGACCATG-3') and Agd2 (5'-AGACAAGGATGATGAACC-3') were used in the reaction mixture. Agd1 was then provided as sequencing primer.
Genotyping of the two allelic variants of V402L substitution was achieved by amplifying the genomic region flanking the mutations adapting the PCR protocol of Fan and colleagues [58] originally designed to detect the mutation in Aedes aegypti. The reaction was performed using primers AaSCF9 (5′-ATCTGCCTTTCGTCTAATGACCC-3′) and AaSCR10 (5′-TTCCTCGGCGGCCTCTTC-3′) and was conducted in a final volume of 25 μl containing: 0.32 μmol of each primer, 0.08 mM of each dNTP, 3 mM MgCl2, 1 U Taq polymerase (Bioline™; Bioline Reagents Ltd, London, U.K.), and 2.5 μL of DNA extracted from half mosquito. Obtained amplicons were sent for sanger sequencing using primer 402-F (5’-GTGTTACGATCAGCTGGACCG-3’) designed by Williams and colleagues (2021) as sequencing primer. This primer binds downstream to an intronic region of the amplicon allowing to avoid problems in sequence interpretation due to the presence of intron length polymorphisms.
All amplification products were purified and sequenced at Eurofins Genomic Center (GmbH, Ebersberg, Germany). Electropherograms were inspected by Chromas Lite (Technelysium Pty. Ltd., Tewantin, Queensland) to detected target site mutations.
Statistics
For each species, Chi-square and/or Fisher’s exact tests were used to investigate differences in resistant allele frequencies over the years and to explore deviations from Hardy–Weinberg equilibrium (HWE) for the year 2020. For An. arabiensis, HWE was performed only for the three allelic locus 1014, while for An. coluzzii combined frequencies for loci 402 and 1014 were considered. Analyses were performed using VassarStats (Statistical Computation Website). R statistical software version 3.5.0 (R development core Team, 2018) with stats package was used to test the Hardy–Weinberg equilibrium under Fisher’s exact test.
Results
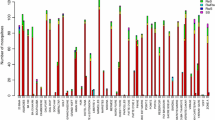
A total of 300 An. coluzzii and 362 An. arabiensis specimens were successfully genotyped for the three target-site mutations for the year 2011, 2015 and 2020. The number of specimens analysed and relative genotypes are summarized in Tables 1 and 2 for the two species in each year. Different trends in the frequency of the three mutations are observed between the two species. In An. coluzzii, 1014F allelic frequency decreases significantly from 0.76 in 2011 to 0.52 in 2020 (χ2 = 29.39, p < 0.0001), while 402L rises from 0.15 in 2011 to 0.48 in 2020 (χ2 = 42.2, p < 0.0001; Fig. 1). L1014S substitution is not observed over the study period in this species. Conversely, in An. arabiensis, 1014F frequency increases significantly from 0.18 in 2011 to 0.70 in 2020 and 1014S allele ranges between 0.23 and 0.33 over the years (χ2 = 183.09, p < 0.0001; Table 2, Fig. 2). Finally, 402L mutation is detected in heterozygosis in a single An. arabiensis specimen collected in 2011 (Table 2). Sequencing of 1014 locus, performed on a subgroup of 130 specimens, confirms the results obtained by the TaqMan assays for L1014F/S substitution detection.
Sequencing of locus 402 reveals that in An. coluzzii the substitution of valine with leucine is encoded by either TTA nucleotide triplet (in 84% of the cases) or by CTA triplet (N alleles 402L = 191, Additional file S1). In the single An. arabiensis specimen carrying V402L, the mutation is encoded by the CTA triplet.
The 402L mutation appears to be in strong linkage with 1014L wild type allele. The only exceptions (confirmed by sequencing) are observed in: (i) one An. coluzzii specimen collected in 2020 carrying the 402L mutation in homozygosis and 1014F in heterozygosis (Additional file S2); (ii) the An. arabiensis 402L heterozygous specimen which carries both 1014F/S mutations.
For the year 2020 (the most recent sampling) the observed genotype frequency at loci 1014 and 402 doesn’t significantly deflect from the expected values under the Hardy–Weinberg equilibrium for each species.
As visible from Figs. 3 and 4, over the study period it is observed an increasing number of specimens carrying at least one resistance allele: from 95 to 100% for An. coluzzii and from 70 to 98% for An. arabiensis (see also Table 2 and Additional file S2).
Discussion
The availability of historical samples collected in Goden village over a decade following the massive ITN scale-up started in 2010, allowed to highlight an overall increase of resistant alleles in vector populations analysed from 2011 to 2020. For the first time, it is reported the V402L substitution in An. arabiensis and observed a high co-occurrence of different target site mutations which leads to less than 1% of specimens that are wild type at both 1014 and 402 loci in the year 2020. Interestingly, this extremely high target site resistance in the vector population is due to a different genetic response of An. coluzzii and An. arabiensis to the local ITN selective pressure.
In An. coluzzii, the 1014F mutation decreases from 2011 to 2020, but the frequency of 402L allele rises, with both alleles reaching an ~ 50% frequency in 2020. This could be explained by contrasting adaptive selective forces which may have led to a trade-off between maintaining high levels of insecticide resistance and reducing the negative impact of 1014F on population fitness [19, 44, 59]. According to Williams and colleagues [42], no apparent fitness cost has been associated to 402L homozygous laboratory colonies, while its expression in transgenic lines seems to confer a lower level of resistance to pyrethroids in comparison to L1014F. The observed inverse trend for 1014F and 402L mutations is consistent with what already reported in other An. coluzzii populations of West Burkina Faso [42, 60] and confirmed the linkage disequilibrium existing for the two substitutions [18, 42, 61]. Nevertheless, a single specimen carrying a combined 1014F/1014F and 402L/402 V genotypes is found, showing lack of a complete mutual exclusivity between these mutations [18, 42]. The mutation 1014S is never observed in the An. coluzzii samples, consistently with the limited circulation of this allele in other An. coluzzii populations from Burkina Faso [35, 62,63,64,65,66].
Conversely, in An. arabiensis all the three target site mutations are detected, including V402L substitution (observed in a single specimen in 2011). The 1014S was the most frequent target site mutation circulating in An. arabiensis population in 2011 (even if at relatively low level, i.e. 33%), and appears to be overcome by 1014F 10 years later. This can be imputable to the higher resistance conferred by allele 1014F as compared to 1014S [22, 67, 68]. A single specimen of An. arabiensis carried all the three mutations simultaneously (402V/402L and 1014F/1014S genotype), confirming the lack of mutual exclusivity already observed in An. coluzzii.
The divergent allelic response to ITN selective pressure observed in the two vector species can result from several factors. An. coluzzii and An. arabiensis showed different backgrounds of insecticide resistance as early as 2011, i.e. one year after beginning of ITN implementation, with 1014F dominating in An. coluzzii (76%) and 1014S in An. arabiensis (33%) at frequencies in the range of those observed in 2012 and 2009 in other villages of the same eco-climatic zone of Goden (i.e. Sudan Sahelian) [35, 50]. In addition, different biting behaviours occurring in the two sibling species possibly affected their exposure to treated nets. According to our previous surveys conducted in Goden village since 2011, an evasive behaviour to ITN presence was observed in both species, affecting biting rhythms and their degrees of endophagy and anthropophily [45, 47, 48].
However, after ITN implementation, An. coluzzii kept maintaining a higher anthropophily when compared to An. arabiensis, as suggested by its higher densities inside dwellings, human blood index, human biting pressure, and sporozoite rate ([45, 47, 48], and Perugini et al. pers. commun.). Thus, it can be hypothesized that An. arabiensis suffered reduced ITN insecticidal pressure than An. coluzzii, and this may have contributed to the limited increase of 1014F observed in this species from 2011 to 2015. Finally, different levels of metabolic resistance and/or insecticide binding mechanisms may exist between the species over the study period [17, 69]. Future transcriptomic investigations will address to the contribution of other resistance mechanisms in malaria vector species, especially considering the introduction of PBO net in Goden village since 2019.
In the most recent year of the survey (2020), 43% of An. coluzzii and 32% of An. arabiensis specimens were found double mutants for 1014F-410L and 1014F-1014S, respectively (Table 2; Additional file S2). Although genotype frequencies are conformed to Hardy–Weinberg expectations, future investigations are needed to evaluate a possible advantage in the co-occurrence of different target site mutations in the same specimen and the impact on vector control. So far, no information is available about synergism between V402L and L1014F/S but there is evidence that the co-expression of 1014F/1014S mutation confers a more resistant phenotype than those expressed by the heterozygosity of one of the two substitutions [68, 70, 71]. Moreover, 1014F/1014S double mutants seem to express a resistance phenotype almost comparable to that of 1014F homozygotes [71].
In 2020, only 2% of An. arabiensis and no An. coluzzii were found homozygous wild type at both 1014 and 402 loci. In fact, almost all An. coluzzii specimens were homozygous for 1014F, or for 402L or double mutant 1014F/402L (Fig. 3; Additional file S2), while 88% of tested An. arabiensis specimens were 1014F or 1014S homozygous, or 1014F/1014S (Fig. 4; Table 2). Given the high target site resistance observed in the malaria vector population and high co-occurrence of different substitutions in the same specimens (Table 2; Additional file S2), it will be important to evaluate the phenotypic effect of these combined mutations and the level of metabolic resistance, in order to predict their impact on PBO net effectiveness.
The present results, together with bioassays, will inform the choice on the most cost-effective strategy to adopt in the area. In fact, although the new generations of ITNs (containing admixture of phyretroids and chlorfenapyr or pyriproxyfen) are expected to be a game changer in insecticide resistance management, their implementation is more expensive and less sustainable than PBO nets [11,12,13,14]. Thus, local-level studies are needed to unveil different mechanisms involved in insecticide resistance to develop tailored control interventions.
Conclusion
This study showed extremely high levels of target site insecticide resistance in malaria vector populations of Goden village in the central region of Burkina Faso. Here the co-occurrence of 1014F, 1014S and 402L may be reducing the effectiveness of ITNs and, potentially, limiting the impact of PBO nets recently introduced in this region. In fact, the entomological indices calculated in our previous studies revealed a consistent high level of malaria transmission risk over 10 years, despite the large bed net coverage. The results call for future studies to evaluate possible synergism among the different target site mutations and other insecticide resistance mechanisms (i.e. metabolic and sequestration/binding). Taking Goden village as a potential “sentinel site”, obtaining this information could eventually lead to reconsider the current strategies adopted in the central region of Burkina Faso and inform on the choice of the most suitable malaria vector control tool at local scale.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional files. The datasets used and analysed during the present study are available from the corresponding author upon reasonable request.
Abbreviations
- ITN:
-
Insecticide-treated bed nets
- VGSC:
-
Voltage-gated sodium channel
- DDT:
-
Dichloro-diphenyl-trichloroethane
- PBO:
-
Piperonyl butoxide
- HWE:
-
Hardy–Weinberg equilibrium
References
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Diabate A, Baldet T, Chandre F, Akogbeto M, Guiguemde TR, Darriet F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67:617–22.
Yadouleton A, Martin T, Padonou G, Chandre F, Asidi A, Djogbenou L, et al. Cotton pest management practices and the selection of pyrethroid resistance in Anopheles gambiae population in Northern Benin. Parasit Vectors. 2011;4:60.
Hien AS, Soma DD, Hema O, Bayili B, Namountougou M, Gnankiné O, et al. Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles gambiae s.l. populations from cotton growing areas in Burkina Faso West Africa. PLoS ONE. 2017;12: e0173098.
Reid MC, McKenzie FE. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J. 2016;15:107.
Hancock PA, Hendriks CJM, Tangena JA, Gibson H, Hemingway J, Coleman M, et al. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 2020;18: e3000633.
WHO. Global plan for insecticide resistance management in malaria vectors. Geneva: World Health Organization; 2012.
Akogbeto M, Djouaka R, Noukpo H. [Use of agricultural insecticides in Benin](in French). Bull Soc Pathol Exot. 2005;98:400–5.
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8.
WHO. World malaria report. 20 years of global progress and challenges. Geneva: World Health Organization; 2020. p. 2020.
WHO. Guidelines for malaria, 16 October 2023. Geneva: World Health Organization; 2023.
Tungu PK, Michael E, Sudi W, Kisinza WW, Rowland M. Efficacy of interceptor® G2, a long-lasting insecticide mixture net treated with chlorfenapyr and alpha-cypermethrin against Anopheles funestus: experimental hut trials in north-eastern Tanzania. Malar J. 2021;20:180.
Tchouakui M, Thiomela RF, Nchoutpouen E, Menze BD, Ndo C, Achu D, et al. High efficacy of chlorfenapyr-based net Interceptor® G2 against pyrethroid-resistant malaria vectors from Cameroon. Infect Dis Poverty. 2023;12:81.
Ngufor C, Fagbohoun J, Critchley J, N’Guessan R, Todjinou D, Malone D, et al. Which intervention is better for malaria vector control: insecticide mixture long-lasting insecticidal nets or standard pyrethroid nets combined with indoor residual spraying? Malar J. 2017;16:340.
Nkya TE, Akhouayri I, Kisinza W, David J-P. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol. 2013;43:407–16.
Minetti C, Ingham VA, Ranson H. Effects of insecticide resistance and exposure on Plasmodium development in Anopheles mosquitoes. Curr Opin Insect Sci. 2020;39:42–9.
Ingham VA, Anthousi A, Douris V, Harding NJ, Lycett G, Morris M, et al. A sensory appendage protein protects malaria vectors from pyrethroids. Nature. 2020;577:376–80.
Clarkson CS, Miles A, Harding NJ, O’Reilly AO, Weetman D, Kwiatkowski D, et al. The genetic architecture of target-site resistance to pyrethroid insecticides in the African malaria vectors Anopheles gambiae and Anopheles coluzzii. Mol Ecol. 2021;30:5303–17.
Grigoraki L, Cowlishaw R, Nolan T, Donnelly M, Lycett G, Ransoni H. CRISPR/Cas9 modified An. gambiae carrying kdr mutation L1014F functionally validate its contribution in insecticide resistance and combined effect with metabolic enzymes. PLoS Genet. 2021;17: e1009556.
Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1–17.
Elissa N, Mouchet J, Riviere F, Meunier JY, Yao K. Resistance of Anopheles gambiae s.s. to pyrethroids in cote d´ivoire. Ann Soc Belge Med Trop. 1993;73:291–4.
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7.
Tripet F, Wright J, Cornel A, Fofana A, McAbee R, Meneses C, et al. Longitudinal survey of knockdown resistance to pyrethroid (KDR) in Mali, West Africa, and evidence of its emergence in the Bamako form of Anopheles gambiae s.s. Am J Trop Med Hyg. 2007;76:81–7.
Pinto J, Lynd A, Elissa N, Donnelly MJ, Costa C, Gentile G, et al. Co-occurrence of East and West African kdr mutations suggests high levels of resistance to pyrethroid insecticides in Anopheles gambiae from Libreville. Gabon Med Vet Entomol. 2006;20:27–32.
Etang J, Vicente JL, Nwane P, Chouaibou M, Morlais I, do Rosario VL, et al. Polymorphism of intron-1 in the voltage-gated sodium channel gene of Anopheles gambiae s.s. populations from Cameroon with emphasis on insecticide knockdown resistance mutations. Mol Ecol. 2009;18:3076–86.
Santolamazza F, Caputo B, Nwakanma DC, Fanello C, Petrarca V, Conway DJ, et al. Remarkable diversity of intron-1 of the para voltage-gated sodium channel gene in an Anopheles gambiae/Anopheles coluzzii hybrid zone. Malar J. 2015;14:9.
Lynd A, Weetman D, Barbosa S, Egyir Yawson A, Mitchell S, Pinto J, et al. Field, genetic, and modeling approaches show strong positive selection acting upon an insecticide resistance mutation in Anopheles gambiae s.s. Mol Biol Evol. 2010;27:1117–25.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84.
Weill M, Chandre F, Brengues C, Manguin S, Akogbeto M, Pasteur N, et al. The kdr mutation occurs in the Mopti form of Anopheles gambiae s.s. through introgression. Insect Mol Biol. 2000;9:451–5.
Stump AD, Atieli FK, Vulule JMBN. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am J Trop Med Hyg. 2004;70:591–6.
Nwane P, Etang J, Chouaїbou M, Toto JC, Mimpfoundi R, Simard F. Kdr-based insecticide resistance in Anopheles gambiae s.s populations in cameroon: spread of the L1014F and L1014S mutations. BMC Res Notes. 2011. https://doi.org/10.1186/1756-0500-4-463.
Moreno M, Vicente JL, Cano J, Berzosa PJ, De Lucio A, Nzambo S, et al. Knockdown resistance mutations (kdr) and insecticide susceptibility to DDT and pyrethroids in Anopheles gambiae from equatorial Guinea. Trop Med Int Health. 2008;13:430–3.
Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar J. 2006;5:16.
Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, Kollo D, et al. Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock-down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Health. 2014;19:331–41.
Dabiré RK, Namountougou M, Diabaté A, Soma DD, Bado J, Toé HK, et al. Distribution and frequency of kdr mutations within Anopheles gambiae s.l. populations and first report of the Ace.1G119S mutation in Anopheles arabiensis from Burkina Faso (West Africa). PLoS ONE. 2014;9: e101484.
Namountougou M, Diabaté A, Etang J, Bass C, Sawadogo SP, Gnankinié O, et al. First report of the L1014S kdr mutation in wild populations of Anopheles gambiae M and S molecular forms in Burkina Faso (West Africa). Acta Trop. 2013;125:123–7.
Djègbè I, Akoton R, Tchigossou G, Ahadji-Dabla KM, Atoyebi SM, Adéoti R, et al. First report of the presence of L1014S Knockdown-resistance mutation in Anopheles gambiae s.s and Anopheles coluzzii from Togo West Africa. Wellcome Open Res. 2018. https://doi.org/10.12688/wellcomeopenres.13888.1.
da Cruz DL, Paiva MHS, Guedes DRD, de Souza Gomes EC, Pires SG, Gomez LF, et al. First report of the L1014F kdr mutation in wild populations of Anopheles arabiensis in Cabo Verde. West Africa Parasit Vectors. 2021;14:582.
Thiaw O, Doucouré S, Sougoufara S, Bouganali C, Konaté L, Diagne N, et al. Investigating insecticide resistance and knock-down resistance (kdr) mutation in Dielmo, Senegal, an area under long lasting insecticidal-treated nets universal coverage for 10 years. Malar J. 2018;17:123.
Etang J, Fondjo E, Chandre F, Morlais I, Brengues C, Nwane P, et al. First report of knockdown mutations in the malaria vector Anopheles gambiae from cameroon. Am J Trop Med Hyg. 2006;74:795–7.
Bandibabone J, McLoughlin C, N’Do S, Bantuzeko C, Byabushi V, Jeanberckmans M, et al. Investigating molecular mechanisms of insecticide resistance in the Eastern Democratic Republic of the Congo. Malar J. 2021;20:464.
Williams J, Cowlishaw R, Sanou A, Ranson H, Grigoraki L. In vivo functional validation of the V402L voltage gated sodium channel mutation in the malaria vector An. gambiae. Pest Manag Sci. 2021;78:1155–63.
Platt N, Kwiatkowska RM, Irving H, Diabaté A, Dabire R, Wondji CS. Target-site resistance mutations (kdr and RDL), but not metabolic resistance, negatively impact male mating competiveness in the malaria vector Anopheles gambiae. Heredity (Edinb). 2015;115:243–52.
Nkahe DL, Kopya E, Djiappi-Tchamen B, Toussile W, Sonhafouo-Chiana N, Kekeunou S, et al. Fitness cost of insecticide resistance on the life-traits of a Anopheles coluzzii population from the city of Yaoundé Cameroon. Wellcome Open Res. 2020;5:171.
Pombi M, Calzetta M, Guelbeogo WM, Manica M, Perugini E, Pichler V, et al. Unexpectedly high Plasmodium sporozoite rate associated with low human blood index in Anopheles coluzzii from a LLIN-protected village in Burkina Faso. Sci Rep. 2018;8:1–10.
Calzetta M, Perugini E, Seixas G, Sousa CA, Guelbeogo WM, Sagnon N, et al. A novel nested polymerase chain reaction assay targeting Plasmodium mitochondrial DNA in field-collected Anopheles mosquitoes. Med Vet Entomol. 2018;32:372–7.
Perugini E, Guelbeogo WM, Calzetta M, Manzi S, Virgillito C, Caputo B, et al. Behavioural plasticity of Anopheles coluzzii and Anopheles arabiensis undermines LLIN community protective effect in a Sudanese-savannah village in Burkina Faso. Parasit Vectors. 2020;13:277.
Perugini E, Guelbeogo WM, Guglielmo F, Poggi C, Gabrieli E, Ranson H, et al. The interplay between malaria vectors and human activity accounts for high residual malaria transmission in a Burkina Faso village with universal ITN coverage. Parasit Vectors. 2023;16:101.
Famine Early WarningSystems Network, USAID. Livelihood zoning and profiling report: Burkina Faso. 2010. https://fews.net/sites/default/files/documents/reports/bf_profile_en.pdf. Accessed 20 Feb 2024
Dabiré RK, Diabaté A, Namountougou M, Djogbénou L, Wondji C, Chandre F, et al. Trends in insecticide resistance in natural populations of malaria vectors in Burkina Faso West Africa: 10 Years’ Surveys Insecticides Pest Engineering. IntehOpen. 2012;22:479–502.
Ouédraogo A, Tiono AB, Diarra A, Sanon S, Yaro JB, Ouedraogo E, et al. Malaria morbidity in high and seasonal malaria transmission area of Burkina Faso. PLoS ONE. 2013;8: e50036.
Koenker H, Olapeju B, Toso M, Millward J, Ricotta E. Insecticide-Treated Nets (ITN): Access and Use Report. Breakthrough ACTION and PMI VectorWorks projects, Johns Hopkins Center for Communication Programs. 2019. https://itnuse.org/. Accessed 20 Feb 2024
U.S. President’s Malaria Initiative. Burkina Faso Malaria Operational Plan FY 2023. www.pmi.gov. Accessed 20 Feb 2024.
Institut National de la Statistique et de la Démographie (INSD), Programme d’Appui au Développement Sanitaire (PADS), Programme National de Lutte contre le Paludisme (PNLP) et ICF. Enquête sur les Indicateurs du Paludisme au Burkina Faso (EIPBF) 2014. Rockville, Maryland, USA; 2015.
Institut National de la Statistique et de la Démographie (INSD), Programme d’Appui au Développement Sanitaire (PADS), Programme National de Lutte contre le Paludisme (PNLP) et ICF.. Enquête sur les indicateurs du paludisme au Burkina Faso, 2017–2018. Rockville, Maryland, USA; 2018.
Supplemental environmental assessment for nationwide IRS in Burkina Faso ( 2018–2022 ). Abt Associates, Inc., Rockville M 20852, 2022.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;13(6):111.
Fan Y, O’Grady P, Yoshimizu M, Ponlawat A, Kaufman PE, Scott JG. Evidence for both sequential mutations and recombination in the evolution of kdr alleles in Aedes aegypti. PLoS Negl Trop Dis. 2020;14: e0008154.
Diop MM, Moiroux N, Chandre F, Martin-Herrou H, Milesi P, Boussari O, et al. Behavioral cost & overdominance in Anopheles gambiae. PLoS ONE. 2015;10: e0121755.
Kientega M, Clarkson CS, Traoré N, Hui T-YJ, O’Loughlin S, Millogo A, et al. Whole-genome sequencing of major malaria vectors reveals the evolution of new insecticide resistance variants in a longitudinal study in Burkina Faso. bioRxiv. 2023;11(20):567800.
Kouamé RMA, Lynd A, Kouamé JKI, Vavassori L, Abo K, Donnelly MJ, et al. Widespread occurrence of copy number variants and fixation of pyrethroid target site resistance in Anopheles gambiae (s.l.) from southern Côte d’Ivoire. Curr Res Parasit Vector-Borne Dis. 2023;3:100117.
Toé KH, N’Falé S, Dabiré RK, Ranson H, Jones CM. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16:146.
Mosqueira B, Soma DD, Namountougou M, Poda S, Diabaté A, Ali O, et al. Pilot study on the combination of an organophosphate-based insecticide paint and pyrethroid-treated long lasting nets against pyrethroid resistant malaria vectors in Burkina Faso. Acta Trop. 2015;148:162–9.
U.S. President’s Malaria Initiative (PMI). Burkina Faso Malaria Operational Plan FY 2020. 2020. www.pmi.gov. Accessed 20 Feb 2024
The PMI VectorLink. Burkina Faso Entomological Monitoring Annual Report, January-December 2022. Rockville, MD.; 2023.
The PMI VectorLink Project. Burkina Faso Entomological Monitoring Annual Report, January-December 2020. Rockville, MD.; 2021.
Burton MJ, Mellor IR, Duce IR, Davies TGE, Field LM, Williamson MS. Differential resistance of insect sodium channels with kdr mutations to deltamethrin, permethrin and DDT. Insect Biochem Mol Biol. 2011;41(9):723–32.
Reimer L, Fondjo E, Patchoké S, Diallo B, Lee Y, Ng A, et al. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J Med Entomol. 2008;45:260–6.
Vontas J, Katsavou E, Mavridis K. Cytochrome P450-based metabolic insecticide resistance in Anopheles and Aedes mosquito vectors: Muddying the waters. Pestic Biochem Physiol. 2020;170: 104666.
Ndiath MO, Cailleau A, Orlandi-Pradines E, Bessell P, Pagès F, Trape J-F, et al. Emerging knock-down resistance in Anopheles arabiensis populations of Dakar, Senegal: first evidence of a high prevalence of kdr-e mutation in West African urban area. Malar J. 2015;14:364.
Mawejje HD, Weetman D, Epstein A, Lynd A, Opigo J, Maiteki-Sebuguzi C, et al. Characterizing pyrethroid resistance and mechanisms in Anopheles gambiae (s.s.) and Anopheles arabiensis from 11 districts in Uganda. Curr Res Parasitol Vector-Borne Dis. 2023;3:100106.
Acknowledgements
We are grateful to the inhabitants of Goden village and CNRFP technicians for their collaboration during the field work.
Funding
The study was supported by Sapienza University of Rome, Projects RM11916B7AFEA99E to MP; AR11916B4CC76BF1, AR120172B8125A08 and AR22117A76DC0132 to EP.
Author information
Authors and Affiliations
Contributions
EP, EM, VP, AdT and MP conceived the study. WMG organized and supervised field collections. EP, SM and MM carried out molecular analyses. EP, VP, CP and MP analysed the entomological data. EP, EM, VP, AdT, HR and MP drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The entomological collections exploited for this study were conducted upon the ethical approval of the committee “Comité d’éthique pour la recherché en santé”, in agreement with Ministry of Health and Ministry of Research approval n. 2013-7-057, issued on 11 July 2013 and approval no. 2020-7-134, issued on 1 July 2020. Volunteers chosen for mosquito samplings were local collaborators trained by CNRFP in performing landing collections in the village for several years. After the study, medical follow-up was conducted on the volunteers for 2 weeks. None showed any malaria symptoms. To ensure empty houses during HLC, the inhabitants of those houses were moved to another safe place and rewarded for their time. All collectors and inhabitants involved as household representatives were informed about the details of the study and signed informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Perugini, E., Pichler, V., Guelbeogo, W.M. et al. Longitudinal survey of insecticide resistance in a village of central region of Burkina Faso reveals co-occurrence of 1014F, 1014S and 402L mutations in Anopheles coluzzii and Anopheles arabiensis. Malar J 23, 250 (2024). https://doi.org/10.1186/s12936-024-05069-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-05069-9