Abstract
Background
Malaria vector control activities in Sudan rely largely on Long-Lasting Insecticidal Nets (LLINs), Indoor Residual Spray (IRS) and Larval Source Management (LSM). The present study attempted to determine cost effectiveness of inputs and operations of vector control interventions applied in different environmental settings in central and eastern Sudan, as well as their impact.
Methods
The inputs utilized and cost of each vector control activity, operational achievements and impact of the applied malaria vector control activities; IRS, LLINs and LSM were determined for eight sites in Al Gazira state (central Sudan) and Al Gadarif state (eastern Sudan). Operational costs were obtained from data of the National Malaria Control Program in 2017. Impact was measured using entomological indicators for Anopheles mosquitoes.
Results
The total cost per person per year was $1.6, $0.85, and $0.32 for IRS, LLINs and LSM, respectively. Coverage of vector control operations was 97%, 95.2% and 25–50% in IRS, LLINs and LSM, respectively. Vectorial capacity of malaria vectors showed statistically significant variations (P < 0.034) and ranged 0.294–0.65 in areas implemented LSM in comparison to 0.097–0.248 in areas applied IRS and LLINs, respectively. Both indoor and outdoor biting Anopheles mosquitoes showed noticeable increase that reached 3–12 folds in areas implemented LSM in comparison to areas implemented IRS and LLINs. Annual malaria prevalence was 13.1–21.1% in areas implemented LSM in comparison to 3.20%, 4.77% in areas implemented IRS and LLINs, respectively.
Conclusion
IRS and LLINs are cost effective control measures due to adequate inputs and organized process. However, the unit cost of LSM intervention per outcome and subsequently the impact is hugely affected by the low coverage. The very weak support for implementation of LSM which includes inputs resulted in weakness of its process and consequently its impact. Implementation of LSM by local government in urban settings is challenged by many factors the most important are maintenance of adequate stable level of funding, un-adequate number of well trained health workers, unstable political and administrative conditions and weak infrastructure. These challenges are critical for proper implementation of LSM and control of malaria in urban settings in Sudan.
Similar content being viewed by others
Background
Sudan is one of the countries with a high malaria burden in sub-Saharan Africa, with an estimated annual malaria incidence of 27.4 cases per 1000 population and a case fatality rate of about 2 deaths per 100,000 [1]. It is the third largest country by area in Africa and includes a wide range of ecological strata. Therefore, matrix of malaria eco-epidemiology included desert fringe, low stable endemic control, hypo and mesoendemic classes, urban class, irrigated schemes and major dams in addition to emergency and complex situations [2]. Malaria transmission shows a seasonal and unstable pattern and depends on rainfall except in urban settings and irrigated schemes where the transmission is around the year.
Sudan adopted multiple prevention vector control strategies which included indoor residual spraying (IRS) in targeted areas with irrigation schemes, long-lasting insecticidal nets (LLINs), larval source management (LSM) using temephos in urban settings. In addition, environmental management (EM) is carried in urban settings and include different activities, such as intermittent irrigation, repair of broken water pipes and open of rain drain canals [3, 4].
Considerable investments have been put into the scaling-up of malaria control interventions following Global Fund to Fight AIDS, Tuberculosis and Malaria resources since 2002 and the global movement for Scale Up For Impact (SUFI). This was reflected in many efforts which included the support of the LSM in urban settings by the government [5].
Monitoring and evaluation are essential components of malaria vector control activities and enables assessment of the efficacy of its vector control operations and adjust its policies to make the most efficient use of scarce resources [6, 7]. It has two inter-related components: (1) monitoring of programmatic implementation (process), and (2) evaluation of interventions (outcome and impact). Impact measures the reduction observed in transmission of the disease through defined indicators whose calculation is based on epidemiological and entomological surveillance [8, 9].
This study attempted to cost the input and operations of vector control interventions adopted in urban and rural settings in central and eastern Sudan as well as its impact.
Methods
Study area
The present study was carried out in eight sites located et al. Gazira state at central Sudan and Al Gadarif state at eastern Sudan.
Al Gazira State lies in the rich Savanna region between latitude 13°–15° and the longitude 32.5°–34°. It is administratively divided into seven localities. The climate of Wad Madani locality, which includes the capital city of Al Gazira state, is considered as hot and dry most of the year. Maximum temperature exceeds 40 °C on average during summer season (April to May) and drops to below 15 °C during winter season (December to February). Most of the rainfall is received during July to October and the mean annual rainfall may exceeds 350 mm.
Al Gadarif State is located between longitudes 33°–36° East, and latitudes 12º–15º North. It shares an international border with Ethiopia to its east. It is admistratively divided into five localities. It is characterized by semi-tropical and dry savannah climate, with a maximum temperature of 41° C in April and May and the mean annual rainfall is about 400 mm per year (July–October).
Selection of study sites
According to the malaria control strategy in 2017 LSM is conducted in urban areas and LLINs and IRS were conducted in rural areas. Four urban sites (Maringan Helat Hassan, Hantoub, Maringan Msane and Albehos district allocated in Wad Madani city/Al Gazira state) were chosen to represent LSM activity. According to their proximity to River Maringan Helat Hassan and Hantoub districts (both applied LSM only) were combined together and Maringan Msane and Albehos districts (both applied LSM plus EM) were also combined together to represent control activities in urban settings supplemented with EM and referred to as LSM and LSM + EM, respectively. Another two rural areas (Alshigab and Altalha villages allocated in South Al Gazira locality) were combined together to represent IRS activity. These two rural sites are part of Al Gazira agricultural Scheme which is the largest agricultural Scheme in Sudan and the majority of the inhabitants work in agriculture and animal husbandry. In addition, two rural areas (Alfaw17 and Alfaw18 villages) which are located in Alfaw locality et al. Gadarif state were combined together to represent LLINs activity. The most important economic activities of the population of these two sites are agriculture and animal husbandry. Figure 1 shows geographical locations of study sites.
Estimation of operational costs
Input utilized and their cost and outcomes in terms of operational achievements (i.e. coverage and quality, timing, frequency, pesticides used, dosage, quantity, equipment, performance and days need) were obtained from monitoring and evaluation data of National Malaria Control Programme (NMCP) and Malaria Vector Control Unit (MVCU) in the local government after their permission. The collected financial data covered the campaigning of IRS, LLINs and LSM activities during 2017.
Ingredient approach was used, and inputs of intervention were identified, valued and classified for each vector control activity. The cost of IRS was divided into two categories, capital costs and recurrent costs according to Creese & Parker [10], and Yukich et al. [11], then the share of each village/district was calculated from total cost of locality. The costs of LLINs and LSM activities was adopted the same way used for estimation of operational costs of IRS All costs converted to US-$ according to exchange rate in 2017.
In addition, costs of one of the most common EM activities (environmental modification through drainage of rain drain canals by the beginning of the rainy season) in urban areas was estimated. This was estimated in Maringan Msane and Albehos districts (El Gazira State). The costs of IRS, LLINs, LSM and EM are provided in Additional files 1, 2 and 3.
Output of vector control interventions
Entomological indicators
Because of the limitation of the MCP data in terms of estimation of all entomological indicators of malaria transmission and the presence of numerous missing data we conducted a cross sectional entomological surveys for Anopheles mosquitoes (adults and immatures) during the rainy season (August to October, 2017) for three days/ month. Entomological surveys were carried out in each of the selected village/district using Pyrethroid Spray Sheet Collection (PSC) method in 20 houses [8, 12], human baited CDC Light Trap collection (LTC) method (indoor and outdoor) in two houses and Exit Window Trap (EWT) method in two houses [12]. Larval collection was performed in 20 stations (10 fixed and 10 spot-check stations) in every village/district according to [12]. Data was collected using designated forms.
Morphological and molecular identification
Adults and larvae were identified using morphological keys [13]. 50 random samples of adult females were identified using species specific identification using PCR technique [14].
Malaria prevalence
Malaria prevalence in 2017 was calculated from the documents of health centres in each village/district.
Data analysis
The entomological indicators of malaria transmission included indoor resting density (IRD), man-biting rate (MBR), human blood index (HBI), sporozoite rate (SP), entomological inoculation rate (EIR), parity rate (PR), longevity (Long), sporogony cycle (N) and vectorial capacity (VC) and they were calculated as described in the literature [12, 15,16,17,18,19,20,21].
IRD = Number of mosquitoes collected using PSC/Number of houses surveyed using PSC, HBR = F/W (M: Is the man- biting rate, F: Is the total number of freshly fed (FF) mosquitoes of the particular species, W: Is the total number of human occupants in houses used for collection.)
Resting habit = KHD/NPM (k: a correction value of 1.16, H: human blood index, D: indoor resting density (total number of females Anopheles gambiae collected divided by number of houses used from the spray-sheet collection) and N: average number of persons per house (household size)),
HBI = Number of female mosquitoes positive for human blodd/Total number of mosquitoes fed analysed. In this study the sources of mosquito blood meals was estimated as 0.6 for An. gambiae depending on the previous studies conducted in Sudan and Ethiopia [15, 16].
SR = Number of mosquitoes with Plasmodium sporozoites/Total number of mosquitoes examined × 100,
EIR = Sporozoite rate × man-bitinng (%)/100,
proportion parous = FF + HG+ G/UF + FF + HG + G, P= 3√ proportion parous, longevity = 1/−lnp, = T/(t–t min) (n: duration of sporogony cycle; T: 111, 105, 114 for rPlasmodium falciparum, Plasmodium vivax and Plasmodium malariae, respectively; t: actual average temperature in degrees centigrade and t min = 16 for P. falciparum and P. malariae and 14.5 for P. vivax),
VC = ma2pn/ − lnp (m: density of vector in relation to man (bites/person/night); a: number of blood meals taken on man per vector per day (human blood index multiplied by 0.5, if a gonotrophic cycle of three days is assumed); p: daily survival rate and n: incubation period) Metrological data needed for estimation of certain entomological indicators was obtained from the Sudanese Metrological Authorities (SMA).
The data of costs, inputs utilized and operational achievements and entomological indicators of malaria vectors (mean values of entomological parameters) was calculated using Windows Excel and SPSS programs (Version 16). One way analysis of variance was applied to estimate p values of mean entomological indicators across different vector control activities.
Results
Inputs utilized and operational achievements
Table 1 shows inputs utilized and operational achievements of IRS, LLINs, LSM1 and LSM2 activities. IRS Spray operations were conducted according to the guidelines adapted from the WHO protocols [22]. The LLINs, LSM and EM operations were conducted according to the [23, 24] respectively. Coverage of EM was estimated based on the fact environmental modification includes drainage, filling, land levelling and transformation and impoundment margins. However, only one activity was carried out by responsible authorities (cleaning of rain drain canals) in addition to the fact that most rain drain canals are not tightly covered to prevent breeding of mosquitoes.
Financial costs
The highest cost per person per year was 1.6 US$ for IRS activity followed by 0.85 US$ for LLINs while the lowest cost per person per year was 0.32 US$ for LSM activity (Fig. 2). Environmental management activity conducted before the rainy season included only cleaning of rain drains canals and cost per person per year was 0.57 US$.
Entomological indicators of malaria transmission
A total of 9,679 adult mosquitoes were collected over three months during the rainy season (August to October 2017) by three methods of collection (PSC, LTC, EWT) in eight study sites. 66.3% of the total collection was An. gambiae complex, 1.6% Anopheles rufipes and 0.1% Anopheles pharoensis. 50 random females of An. gambiae complex from all study sites were identified using species specific PCR techniques and results showed that 66% of An. gambiae complex were Anopheles arabiensis.
Table 2 shows entomological indicators of An. arabiensis in areas implemented different vector control interventions. Relatively highest values of IRD and MBR were in areas implemented LSM. Statistically significant variations (P < 0.034) were in VC with the lowest value (0.09) in areas implemented LLINs activity while high values (0.650) was in areas implemented LSM activity. For immature, the higher densities of Anopheles larvae were found in areas implemented LSM activity (8.9 ± 1.4–10.9 ± 6.9) compared to other areas implemented IRS or LLINs (7.4 ± 0.69, 5.7 ± 2.01, respectively).
Biting pattern of malaria vectors
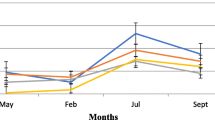
Table 3 shows the biting pattern of Anopheles spp during the rainy season (August to October, 2017) from 6 pm to 6 am in areas implemented different control activities. The peak of both indoor and outdoor Anopheles bites in all areas was between 2 and 4 am.
While lower numbers of indoor and outdoor biting Anopheles mosquitoes were recorded in areas implemented IRS (3.7% and 3.6% respectively) and LLINs (4.0% and 3.1% respectively). Both indoor and outdoor biting Anopheles mosquitoes showed noticeable increase that reached 3–12 folds in areas implemented LSM in comparison to areas implemented IRS and LLINs.
Prevalence of malaria disease
Areas implemented IRS or LLINs showed the lowest prevalence of malaria (4.77% and 3.20%, respectively) however, malaria prevalence was 3–7 folds higher in areas implemented LSM (13.12% and 21.1%, respectively) (Table 4).
Discussion
Vector control is critical for the reduction and interruption of malaria transmission as it breaks the transmission cycle, thereby reducing morbidity and mortality. Both IRS and LLINs can dramatically reduce the burden of malaria by killing adult female mosquitoes when they come to take human blood meals [25]. Although the primary goal of such interventions is the reduction of human/vector contact rather than reduction of the vector population, these control methods may also suppress the local vector population under certain circumstances [26]. In contrast, larval control measures are intended to reduce malaria transmission indirectly by reducing the vector population density near human habitations [27].
In 2017, Sudan adopted multiple prevention vector control strategies which included) IRS in targeted areas with irrigation schemes with seven targeted states, LLINs in twelve states, LSM using temephos on weekly basis and function in 110 urban settings in 18 states [2]. This study attempted to determine cost effectiveness of on-going different malaria vector control activities taking place in Sudan by NMCP in 2017. Both IRS and LLINs control interventions are completely funded by the Global Fund, WHO and other international agencies, and LSM is funded by the local government. The operational achievements of both IRS and LLINs activities showed 95.2% -97% coverage. Cost of LLINs reported in this study (0.85 US$) was greater than cost per person protected/year (0.32 US$) reported in Ghana [28] and less than the cost per individual protected per year (0.87 US$) reported in Sri Lanka [29]. IRS activity costs per person per year was ($ 1.6 US$) and it is less than the value of 3.86 US$ reported from southern Mozambique [30] and sub-Saharan Africa [11] and greater than the cost reported from Kenya valued at 0.86 US$ [31]. Both LLINs and IRS activities were the most cost-effective compared to LSM according to epidemiological (prevalence of malaria) and entomological indicators.
LSM activity had low cost (cost per person per year of 0.32 US$ and accordingly the lowest outcome of operational achievements in comparison to IRS and LLINs activities. However, the unit costs of intervention per outcome in low coverage hugely vary for the cost at high coverage (which was the case for both IRS and LLINs). The calculation of unit cost of LSM intervention is only valid for its context including the reported low level of coverage and higher cost of LSM in high coverage (which may reach double the unit price) is expected. In terms of financial costs this result is less than the cost per person protected per year in Tanzania—Dar el Salaam (0.94 US$) and west Kenya high lands ($1.50) [32] and approximately equivalent to the value (0.49 US$) reported in Sri Lanka [29]. The reported low cost of LSM may be due to the reported low coverage 25–50% which was accompanied with high density of immature malaria vectors (9.8–10.9 larvae/dip) during the rainy season. It is well known that LSM is intrinsically reliant upon careful, continuous performance management of large implementation teams based on feedback from monitoring systems for adult mosquito densities [33].
Low coverage of LSM highlights the challenges in applying it in Madani locality and other similar urban settings in Sudan. Madani locality as well as most urban settings in Sudan suffers from unplanned urbanization. These settings has numerous uncovered rain drain canals, water storage practices of inhabitants and aggregation of other indoor/outdoor water bodies suitable for breeding of malaria vectors might complicate application of LSM. Assessed the impact of urbanization on malaria transmission in sub-Saharan Africa and presented evidence that urbanization allows for increased rates of disease transmission, large numbers of larval development sites due to construction of new settlements, water storage practices and limited methods for disposal of wastewater and refuse. All previously listed factors are well known in Madani locality as well as other urban settings in Sudan [34, 35].
Challenges for proper application of LSM include: (1) insufficient financial support from state governments that adequately covers all operational activities, (2) severe lack of well-trained health workers, (3) unstable political conditions that accordingly results in unstable administrative process, (4) very weak infrastructure, (5) weak inter and intra-sectoral collaboration between health authorities and other related sectors, (6) absence of involvement of local communities, (7) long use of single larvicide with some reports from urban settings documenting the appearance of resistance to temephos larvicide in urban cities [34]. These listed factors are critical for the control of malaria in urban settings in Sudan.
EM activities are carried out before the beginning of the rainy season in urban settings in Sudan. All these settings are characterized by unplanned urbanization. The estimated cost of the only EM activity carried out (cleaning of rain drain canals) was $0.57 per person per year. However, the reported EM cost exceeds the value of ($0.24) reported in Sri Lanka [28]. In addition, it is clear that this estimation is significantly affected by the the fact that other EM activities were not considered by responsible authorities. It is critical that responsible authorities of EM activities consider all possible activities instead of reliance upon one activity (cleaning of rain drain canals) which do not executed perfectly to prevent breeding of mosquitoes.
The vectorial capacity (VC) of mosquito species is an index used to compare actual or potential vectorial importance of particular species with that of another or to evaluate vector control activities within a given area. This study showed that the vectorial capacity of An. arabiensis for the all vector control activities in the four study areas exceeded 0.01, which is considered as the minimum value required for the vector to maintain malaria transmission [36]. The mean value of VC (0.24) for IRS activity, reported in this study is similar to the VC value (0.24) reported from central Sudan [37].
Efficiency and effectiveness of malaria vector control activities specifically IRS and LLINs depending on the biting pattern of malaria vectors [38]. Many studies highlighted the shift in biting behavior of Anopheline mosquitoes during or after the implementation of malaria vector control activities and may further influenced by the availability of potential hosts in different locations [39]. The present study showed that the peak indoor biting of malaria vectors in areas implemented IRS at 2 am to 4 am and the peak of outdoor biting at 12 am to 2 am. This result is in agreement with the biting peak of malaria vector (An. gambiae) outdoor at (12 am to 2 am) reported in Tanzania [40]. For areas implemented LLINs the peak indoor biting of malaria vectors was at 10 pm to 12 am. This result parallel with findings of a study conducted In Equatorial Guinea for the biting peaks of An. gambiae [41]. The shown peak biting rate of malaria vectors out door at (12 am to 2 am), is similar to the biting peak for An. gambiae sensu stricto from 12 am to 2 am (both indoors and outdoors) from Tanzania [39]. The study finding showed that number of malaria vectors caught outdoors (222 and 890) was more than indoor (235 and 330) in areas implemented LSM during the rainy season.
The reported number of malaria cases of total population from routine surveillance was used as core indicators for tracking of malaria vector control activities in all study areas. Malaria prevalence peaked in all study areas during the rainy season. This study presented a mean of malaria prevalence of 13.2 − 21.1% in areas implemented LSM activity. It is less than the mean malaria incidence (40%) and (56.7%) in a multi-country study undertaken in Sudan, Kenya, India, Cameroon and Benin and Ethiopia [42, 43]. Depending on health facilities reports to assess the impact of community interventions is associated with many challenges that may not be accurate to reflect the true situation of the disease burden in that community. However, all health centres use microscopic examination as the diagnostic tool for malaria disease and there are no other health centre facilities in the study sites. The present data showed higher prevalence of malaria cases reported from areas implemented LSM control intervention. Data obtained from health centres highlight the continuous transmission of malaria during the hot dry season (for all interventions) and the urgent need for proper malaria vector control methods during these seasons, specifically for LSM.
Conclusion
IRS and LLINs are cost-effective methods of malaria control. Application of LSM in urban settings in Sudan is challenged by many factors, including financial, political, weak infrastructure and human capacity and administrative instability. The very weak financial and logistic support for implementation of LSM resulted in weakness of process and significantly affects its impact. It is very critical that local government overcome such challenges for the control of malaria disease in urban settings.
Availability of data and materials
The data used or/and analyzed during this study are available from the corresponding author on reasonable request.
References
WHO. Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015.
Noor AM, ElMardi KA, Abdelgader TM, Patil AP, Amine AA, Bakhiet S, et al. Malaria risk mapping for control in the Republic of Sudan. Am J Trop Med Hyg. 2012;87:1012–21.
Kafy HT. 2018. Progress in implementation of integrated vector management in Sudan. 13th RPM- VCWG Meeting February. Geneva, Switzerland
Malik EM, Khalafalla O. Malaria in Sudan: past, present and the future. Gezira J Health Sci. 2004;1:47–53.
Federal Ministry of Health. Sudan malaria indicator survey. Khartoum, Sudan, 2016.
WHO. Guidelines for malaria vector control. Geneva: World Health Organization; 2019.
WHO. Malaria surveillance, monitoring and evaluation: a reference manual. Geneva: World Health Organization; 2018.
WHO. IT intervention: a manual for national central programme managers. Geneva: World Health Organization; 2003.
Githeko AK. Entomological correlates of epidemiological impacts: how do we know it is working? In: Takken W, Martens P, Bogers RJ (eds.); Environmental change and malaria risk. Wageningen UR Frontis Series vol.9. Springer. 2005:215–9.
Creese A, Parker D. Cost analysis in primary health care. A Training Manual for Programme Managers. WHO Publications Center USA, 49 Sheridan Avenue, Albany, NY 12210; 1994.
Yukich JO, Lengeler C, Tediosi F, Brown N, Mulligan JA, Chavasse D, et al. Costs and consequences of large-scale vector control for malaria. Malar J. 2008;7:258.
WHO. Vector control. Guide for participants. Geneva, World Health Organization, 2013.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–43.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Kenea OL. Entomological impact of combined and separate use of indoor residual spraying and long-lasting insecticidal nets for malaria prevention in Adami Tullu district, South-Central Ethiopia. Doctoral dissertation, Addis Ababa University, School of Graduate Studies, 2016.
Osama A. M. Ali. Malaria vectorial capacity of population of Anopheles arabiensis in Khartoum Master degree thesis, University of Khartoum, Faculty of sciences, Sudan 1992.
Beier JC. Vector incrimination and entomological inoculation rates. Malaria Methods and Protocols. 2002:3–11.
Davidson G. Estimation of the survival rate of anopheline mosquitoes in nature. Nature. 1954;174:792–3.
Macdonald G. The epidemiology and control of malaria. Oxford University Press, 1957.
Garrett-Jones C, Shidrawi G. Malaria vectorial capacity of a population of Anopheles gambiae: an exercise in epidemiological entomology. Bull World Health Organ. 1969;40:531–45.
Detinova TS. Age-grouping methods in Diptera of medical importance, with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:13–191.
WHO. Indoor residual spraying: use of indoor residual spraying for scaling up global malaria control and elimination: WHO position statement. Geneva, World Health Organization; 2006.
WHO. Insecticide-treated mosquito nets: a WHO position statement. Geneva: World Health Organization; 2007.
WHO. Manual on environmental management for mosquito control, with special emphasis on malaria vectors. Geneva, World Health Organization; 1982.
Lengeler C, Sharp B. Indoor residual spraying and insecticide-treated nets. Reducing the malaria burden: evidence of effectiveness for decision makers. Global Health Council, Washington DC, 2003.
Gimnig JE, Kolczak MS, Hightower AW, Vulule JM, Schoute E, Kamau L, et al. Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in western Kenya. Am J Trop Med Hyg. 2003;68(Suppl 4):115–20.
Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol. 2007;21:2–21.
Grabowsky M, Nobiya T, Ahun M, Donna R, Lengor M, Zimmerman D, et al. Distributing insecticide-treated bednets during measles vaccination: a low-cost means of achieving high and equitable coverage. Bull World Health Organ. 2005;83:195–201.
Konradsen F, Steele P, Perera D, Van Der Hoek W, Amerasinghe PH, Amerasinghe FP. Cost of malaria control in Sri Lanka. Bull World Health Organ. 1999;77:301–9.
Conteh L, Sharp BL, Streat E, Barreto A, Konar S. The cost and cost-effectiveness of malaria vector control by residual insecticide house-spraying in southern Mozambique: a rural and urban analysis. Trop Med Int Health. 2004;9:125–32.
Guyatt H, Kinnear J, Burini M, Snow R. A comparative cost analysis of insecticide-treated nets and indoor residual spraying in highland Kenya. Health Policy Plan. 2002;17:144–53.
Worrall E, Fillinger U. Large-scale use of mosquito larval source management for malaria control in Africa: a cost analysis. Malar J. 2011;10:338.
Fillinger U, Kannady K, William G, Vanek MJ, Dongus S, Nyika D, et al. A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam. Tanzania Malar J. 2008;7:20.
Azrag RS, Mohammed BH. Anopheles arabiensis in Sudan: a noticeable tolerance to urban polluted larval habitats associated with resistance to Temephos. Malar J. 2018;17:204.
Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, et al. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–76.
Dhewantara PW, Prasetyowati H, Hodijah DN, Kusnandar AJ. Vectorial capacity, entomological inoculation rate, and stability index of Anopheles sundaicus (L.) in Sukaresik Village Pangandaran West Java. International Seminar Integrated Vector Management, UNDIP. 2013;231–6.
Ahmed IM. Malaria transmission in Abu Ushar village, Gezira irrigated area, central Sudan. MSc Thesis. Faculty of Science: University of Khartoum, 2015.
Le Menach A, Takala S, McKenzie FE, Perisse A, Harris A, Flahault A, et al. An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets. Malar J. 2007;6(1):10.
Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–53.
Geissbühler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, et al. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam. Tanzania Malar J. 2007;6:126.
Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island. Equatorial Guinea Malar J. 2011;10:184.
Kleinschmidt I, Mnzava AP, Kafy HT, Mbogo C, Bashir AI, Bigoga J, et al. Design of a study to determine the impact of insecticide resistance on malaria vector control: a multi-country investigation. Malar J. 2015;14:282.
Kibret S, Wilson GG, Ryder D, Tekie H, Petros B. Environmental and meteorological factors linked to malaria transmission around large dams at three ecological settings in Ethiopia. Malar J. 2019;18:54.
Acknowledgements
We would like to thank Dr. Hamoda Toto and Mr. Osman Abakar, from the Integrated Vector Management Department/Federal Ministry of Health and the staff members of the IVM unit/state ministries of health at both Algazeira and Al Gadarief States. We appreciate the support of the National Malaria Control Program and the IVM unit at localities of Madani, South Algazira and Al Faw. We would like to thank Blue Nile Institute of Communicable Diseases/University of Al Gazira for logistic support during field surveillances. We would like to thank anonymous reviewer for valuable comments and insights to the manuscript.
Funding
The present study did not received external fund.
Author information
Authors and Affiliations
Contributions
SMH; conceptualization, acquisition, analysis and interpretation of data, drafted the first draft of the manuscript, RSA; conceptualization, scientific design and substantively revised the manuscript, OMA; conceptualization, design, analysis and interpretation of data, substantively revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of this study was approved by the ethical committee of High Institute of Public Health, Alexandria University in Egypt and permission was taken from the Integrated Vector Management Unit, Federal Ministry of Health in Sudan.
Competing interests
Authors declared that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Financial costs included in the analysis of the LLIBN and IRS control activities.
Additional file 2.
Financial costs included in the analysis of the LSM and EM activities.
Additional file 3:
Table S4.5. Expenditure for IRS malaria vector control activity in Alshigab and Altalha Villages in two rounds per year (2017, US$). Table S4.6. Expenditure for LLIBN malaria vector control activity in Alfaw 17 and Alfaw 18 Villages , in one round per 3 years, (2017 US$). Table S4.7. Expenditure for LSM malaria vector control activity in Maringan Helat Hassan and Hantoub Districts per year, (2017 US$). Table S4.8. Expenditure for EM malaria vector control activity in Maringan Msane and Albehoth Districts per year, (2017 US $).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
HasapAla, S.M., Azrag, R.S. & Awad, O.M. Cost effectiveness of malaria vector control activities in Sudan. Malar J 23, 80 (2024). https://doi.org/10.1186/s12936-024-04900-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-04900-7