Abstract
Background
Anopheles vagus (subgenus Cellia) has been identified as a vector for malaria, filariasis, and Japanese encephalitis in Asia. Sporozoites of Plasmodium falciparum and Plasmodium vivax have been found in this zoophilic mosquito in Asia and Indonesia. This study systematically reviews publications regarding An. vagus species, variation, bio-ecology, and malaria transmission in various localities in Asia, especially Indonesia, to determine whether the current data support An. vagus as a species complex.
Methods
The databases Pubmed, Scopus, Europe PMC, and Proquest were searched to identify information regarding the morphology, karyotypes, polytene chromosome, cross-mating, ecology, and molecular identification of An. vagus was then evaluated to determine whether there were possible species complexes.
Results
Of the 1326 articles identified, 15 studies were considered for synthesis. The Anopheles spp. samples for this study came from Asia. Eleven studies used morphology to identify An. vagus, with singular studies using each of karyotype identification, chromosomal polytene identification, and cross-breeding experiments. Ten studies used molecular techniques to identify Anopheles spp., including An. vagus. Most studies discovered morphological variations of An. vagus either in the same or different areas and ecological settings. In this review, the members of An. vagus sensu lato grouped based on morphology (An. vagus, An. vagus vagus, An. vagus limosus, and An. limosus), karyotyping (form A and B), and molecular (An. vagus genotype A and B, An. vagus AN4 and AN5). Genetic analysis revealed a high conservation of the ITS2 fragment among members except for the An. vagus genotype B, which was, in fact, Anopheles sundaicus. This review also identified that An. vagus limosus and An. vagus vagus were nearly identical to the ITS2 sequence.
Conclusion
Literature review studies revealed that An. vagus is conspecific despite the distinct morphological characteristic of An. vagus and An. limosus. Further information using another barcoding tool, such as mitochondrial COI and ND6 and experimental cross-mating between the An. vagus and An. limosus may provide additional evidence for the status of An. vagus as a species complex.
Similar content being viewed by others
Background
Malaria is an infectious disease caused by Plasmodium spp. transmitted by female Anopheles mosquitoes. Southeast Asia has the second highest malaria incidence after Africa, with an estimated eight million cases and 11,600 malaria deaths [1]. Globally, a total of 465 Anopheles malaria vectors have been identified morphologically, with 70 species from the four subgenera Anopheles, Cellia, Kerteszia, and Nyssorhynchus transmitting the malaria parasite to humans [2]. Anopheles species are often found in morphologically identical sibling species complexes, with approximately thirty species complexes identified in various parts of the world [3,4,5,6]. Each complex has a different number of sibling species, and 145 species have been identified [2]. The accuracy of morphological identification depends on the known ability to differentiate species and those held within species complexes cannot usually be differentiated morphologically. Cross-mating tests, mitotic and meiotic karyotypes, and molecular procedures are applied to identify sibling species within complexes [3,4,5]. Sibling species held within complexes often express different behaviours and bionomics, therefore, it is crucial to utilize molecular techniques to identify the mosquito specimens to species level, when examining the mosquito geography, ecology, and biology [3, 6].
More than eighty Anopheles species were identified in Indonesia, of which twenty-six are malaria vectors. So far only a few species have been studied as Anopheles species complex in Indonesia, including Anopheles sundaicus, Anopheles maculatus, Anopheles barbirostris and Anopheles punctulatus [7,8,9,10,11,12,13]. This does not rule out the possibility of other complexes that have not been discovered yet.
Anopheles vagus (sub-genus Cellia) was discovered in 1902 by Doenitz in Indonesia [14], and its subspecies, An. vagus limosus was initially reported in the Philippines [14, 15]. In Indonesia, An. vagus is a vector of malaria, filariasis, and Japanese encephalitis [16,17,18]. Except for Papua, practically all Indonesian Islands have An. vagus populations [19], which typically feed on cattle and other animals. These primary topographic zones comprise a diverse habitat, including brackish water, coastal plains, inland, hills, and mountains [20]. Anopheles vagus larvae are generally found in locations with calm or light water flow, such as puddles, on the beach, springs, the edges of rice fields, muddy ponds, animal tracks, and artificial containers such as old tires, drums, and on boats [20,21,22].
Anopheles vagus is predominantly a zoophilic, exophilic, and exophagic vector found in Asia [19]. However, in some previous studies, An. vagus was also reported to be slightly more anthropophilic, i.e. feeding on human blood and/or animal blood [23, 24]. This opportunistic behaviour can make these mosquitoes capable of transmitting Plasmodium. Therefore, these mosquitoes are now regarded as secondary malaria vectors, after the detection of Plasmodium spp. both by biological assays and molecular examination, which has been carried out in several regions in India, Bangladesh, Thailand, China, and especially Indonesia [19, 25,26,27,28,29,30,31].
This review evaluated the current evidence to determine whether An. vagus was a cryptic or sibling species, as well as the behavioural and genetic variation of An. vagus in Asia.
Methods
Literature search
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines [32]. The databases Pubmed, Scopus, Europe PMC, and Proquest were searched using the following keywords: “(Anopheles) AND (Anopheles vagus)”; “((species complex) OR (sibling species)) AND (Anopheles vagus)”. The search was conducted from February to November 2022.
Eligibility criteria and study selection
English and Indonesian language full articles concerning An. vagus, various systematic examinations of species complex based on karyotypic identification, cross-mating experiments, morphometric and morphological investigations of palps and wings, and molecular investigations for identifying species and phylogenetic analysis were evaluated. Three authors (DL, DS, CA) independently extracted data regarding authorship, years, country, study populations, and key findings from each study.
Results
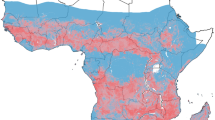
In total, 145 articles were identified in Pubmed, 192 in Scopus, 642 in Europe PMC, and 347 in Proquest, with a further six relevant studies in Google Scholar (Fig. 1). Most studies were about Anopheles spp., focusing on bionomy, survey mosquitoes and their role as a vector, incrimination study, abundance and diversity, and molecular analysis. Only a few articles focused on An. vagus and the systematic study of the possibility of a species complex. Of the fifteen studies reviewed [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] (Table 1), seven studies examined a sample of An. vagus from the Indonesian archipelago (Central Java, East Java, and West Sulawesi Indonesia as well as Dili East Timor), four studies involved samples from India, two from Thailand, one study from the population in 18 areas of mainland Asia and Southeast Asia, and one study from the border regions of Laos and Cambodia (Fig. 2). Of the fifteen articles on An. vagus, all have used different species name in the articles and in the genebank, such as An. vagus. An. limosus, An. vagus vagus and An. vagus limosus [36, 37, 42].
Geographic Distribution Anopheles spp. and Anopheles vagus examination. Map of Country in Mainland Asia and South East Asia was sourced from: https://gadm.org/maps.html
In this review, the members of An. vagus sensu lato (s.l.) grouped based on the morphology (An. vagus, An. vagus vagus, An. vagus limosus, and An. limosus), karyotyping (form A and B), and molecular (An. vagus genotype A and B) and An. vagus AN4 and AN5.
Current grouping of Anopheles vagus
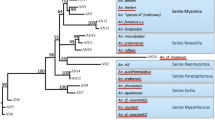
Female An. vagus was initially discovered by Doenitz (1902) in Sumatera, meanwhile the male An. vagus was discovered in Java and other regions in Indonesia [14]. Based on morphological features, An. vagus can be divided into three subspecies: An. vagus vagus, An. vagus limosus and An. vagus albino [48]. Anopheles vagus limosus was found in Luzon Island, Philipines, and An. vagus vagus was found in Lake Lanao, located in Lanao Plateau, Philippines [49]. The status of An. vagus limosus was later promoted as a new species by Ramalingan et al. [50], who collected the two subspecies at the sympatric speciation in Sabah, Malaysia. This separation was reinforced by developing the cladistic classification of the genus Anopheles, which placed An. vagus and An. limosus into different species but still siblings in Pyretophorus series based on morphological and morphometric characters [51,52,53].
Morphology-based grouping of Anopheles vagus
One of the distinctive characteristics of the genus Anopheles is its morphological variety. Anopheles vagus differs from other species in the Pyretophorus series, such An. indefinitus and An. subpictus from its pale apical band on its proboscis. It also differs from An. limosus from it lacks spots on its femur and tibia. Anopheles vagus has three or more palpi with pale or dark bands, a pale band at the proboscis's tip, and a pale band between the two dark bands on the humeral wing [54]. The species of An. vagus and An. limosus are distinguished by the proboscis and prehumeral region variations, which may be a combination of traits from two species specimens from Java, Indonesia [45]
The current study from India found that the An. vagus palp variation is discernible from the uneven and black tip palp [47]. The size and number of the collected branches, hairs, and filaments may vary depending on whether the collected An. vagus specimens were from freshwater or brackish water or due to the season or geography [41, 43, 47]. However, in Bangsring, East Java, the sample population taken from an area near a brackish water pond showed that the two subspecies of An. vagus do not have a combination variation in the proboscis and prehumeral wings. Anopheles vagus vagus is characterized by unspotted legs, a proboscis with pale bands, and prehumeral wings with pale bands between two dark bands, while An. vagus limosus has characteristic unspotted legs, a dark overall proboscis, and prehumeral wings with dark bands [42].
Karyotype grouping of Anopheles vagus
Several mosquitoes of the subgenus Cellia, including a few An. vagus mosquitoes from Thailand were used in the initial studies on metaphase analysis of karyotypes [33]. Two distinct An. vagus karyotypes, form A and form B, were identified with a different length of the chromosomal arm. However, it has yet to be determined if the two metaphase karyotypes represent intra- or interspecies variations [33].
Experimental intraspecies cross-mating was conducted to identify the post-mating barriers between isolines of An. vagus types A and B from the districts of San Sai and San Kamphaeng, Chiang Mai Province, Northern Thailand. The results revealed that the An. vagus eggs are homogeneous with both forms, proving that the An. vagus species has two cytological forms that are polymorphic races [34]. In addition to karyotyping, there is only one study that analyses polytene chromosomes in An. vagus. Therefore, the result must be different from other studies. In that study, it was revealed that distinct polytene chromosomal arms variation in banding and puffing patterns with tetramers, pentamers, and polymers present but no tandem repeats in the ITS2 sequences [40].
Molecular grouping of Anopheles vagus
The advent of molecular technologies in the early 1990s has provided new insights into mosquito taxonomy and further strengthened the taxonomic classification mainly based on morphological features. Molecular barcoding using either the internal transcribed spacer 2 (ITS2) fragment of the nuclear ribosomal gene or the mitochondrial cytochrome oxidase I (COI), and NADH dehydrogenase subunit 6 (ND6) gene offered complementary evidence to the current taxonomic classification and identification of a cryptic species [55,56,57,58].
Anopheles vagus appears dominant in mainland and Southeast Asia populations comprising a molecularly highly diverse yet monospecific, widely dispersed taxon. Interestingly, specimens from Java and East Timor in the Indonesia Archipelago region had a unique genetic lineage to other An. vagus in Asia [45]. Anopheles vagus had a highly conserved ITS2 region containing over 500 bp and flanked by 5.8S rDNA in the upstream and large rDNA in the downstream (Fig. 3). A recent study by An. vagus ITS2 revealed no genetic variations among the An. vagus populations from Bangsring, East Java, Indonesia [39]. Likewise, there were no genetically distinct An. vagus-like species in twelve specimens from the border between Laos and Cambodia [38]. In contrast, studies conducted in East Timor [44] reported two groups of An. vagus based on ITS2 sequence; Genotype A perfect match with sequence of An. vagus from East Timor and East Java (GenBank accession number FJ654649), whereas Genotype B was morphologically identical to An. vagus, but the ITS2 sequence was nearly identical to An. sundaicus (Fig. 4). The An. vagus genotype B was reportedly more anthropophilic than the An. vagus genotype A as it was more frequently collected through the HLC technique.
Sequences Alignment rDNA ITS2 fragment sample An. vagus members from Indonesia and East Timor. An. vagus (GenBank accession number FJ654649) is the reference sequence from all studies from Indonesia and East Timor. An. vagus (OM974188) is newest sequence sample from East Java Indonesia. GenBank accession number GQ500122 for An. vagus genotype A, GQ480824 and GQ480823 for An. vagus Genotype B, MT740902 and MT740903 for An. vagus AN4 dan AN5, MW314227 and OL437110 for An. vagus vagus (1) and (2), MW319822 for An. vagus limosus. OL437109 for An. limosus
Interestingly, two groups of sympatrically distributed An. vagus was also reported in the remote inland village of Karama, West Sulawesi, Indonesia; one was closely related to An. sundaicus-like, namely AN4, while the other was related to An. subpictus like (AN5) [35]. However, no further information as to whether the two An. vagus subgroup was reproductively isolated [47]. Both these studies used the genetic marker internal transcribed spacer 2 (ITS2). These studies also used COI sequences to identify their phylogenetic relationship.
Anopheles vagus vagus and An. vagus limosus from Bangsring, East Java, Indonesia are morphologically different, but their phylogenetic relationship was very close with ITS2 sequence similarity of more than 99%. Both An. vagus vagus and An. vagus limosus ITS2 sequence resembles with the previously published An. vagus ITS2 sequence (GenBank accession number FJ654649) [20, 23].
Discussion
The biological and ecological notion of allopatric speciation which enables the Anopheles species to be separated in an ecological niche, involves ecosystem patterns, geographic barriers, and varied terrain that result in physical separation and reproductive isolation [3, 6, 59, 60]. Initially, it was believed that it was unusual for gene flow to remain uninhabited; however, it is now widely acknowledged that sympatric speciation has occurred possibly involving interbreeding speciation and assortative mating by habitat or secondary gene flow [61,62,63].
Anopheles vagus currently possesses several sub-species based on morphological characteristics and karyotype. Karyotype forms A and B are distributed in allopatric and thus possess geographical barriers to mating but have been shown through laboratory cross-mating to produce viable offspring [35] thus, the finding they concluded that An. vagus form A and form B from allopatric speciation are likely sibling species rather than different species entities[34]
Morphological characteristics divide An. vagus into two subspecies; An. vagus vagus and An. vagus limosus [14, 15]. The latter subspecies was later promoted into a new species, An. limosus (based on Harbach classification for Anopheles) [2, 51,52,53]. Anopheles vagus and An. limosus were distributed sympatricly in several parts of Southeast Asia [42, 45, 50].
Analysis of ITS2 sequences of all members of An. vagus s.l. revealed that An. vagus, An. vagus vagus, An. vagus limosus and An. limosus share almost identical ITS2 sequences except for An. vagus genotype B is, in fact, a member of An. sundaicus [23]. Therefore, despite the distinct morphological variation, all members of An. vagus are possibly conspecific. Regarding An. limosus that has been given new species status, further species confirmation using the other barcoding marker such as COI and ND6 may support this new status. The other issue that is also important is whether the An. vagus and An. limosus naturally mate with each other. So far, despite their close relationship, there is no evidence that they could mate naturally or in a laboratory setting that produces viable offspring.
Conclusion
Literature review studies revealed that An. vagus is possibly conspecific despite distinct morphological characteristics of An. vagus and An. limosus. Further information using mitochondrial COI and ND6 barcoding and experimental cross-mating between the An. vagus and An. limosus may provide additional evidence for the status of An. vagus as a species complex.
Availability of data and materials
All data are available.
Abbreviations
- ITS2:
-
Internal transcribed spacer 2
- COI:
-
Cytochrome oxidase I
- HLC:
-
Human landing catch
- ND6:
-
NADH dehydrogenase subunit 6
References
WHO. World Malaria Report: 20 years of global progress and challenges. Geneva: World Health Organization; 2020. https://www.who.int/publications/i/item/9789240015791
Harbach RE. The phylogeny and classification of Anopheles. In: Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors. IntechOpen; 2013.
WHO. Anopheline Species Complexes in South and South-East Asia. SEARO Technical Publication 57. World Health Organization Regional Office for South-East Asia, New Delhi, India, 2007.
Choochote W, Saeung A. Systematic techniques for the recognition of Anopheles species complexes. In: Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors. IntechOpen; 2013.
Saeung A, Baimai V, Thongsahuan S, Otsuka Y, Srisuka W, Taai K, et al. Cytogenetic, cross-mating and molecular evidence of four cytological races of Anopheles crawfordi (Diptera: Culicidae) in Thailand and Cambodia. C R Biol. 2014;337:625–34.
Weeraratne TC, Surendran SN, Walton C, Karunaratne SHPP. Genetic diversity and population structure of malaria vector mosquitoes Anopheles subpictus, Anopheles peditaeniatus, and Anopheles vagus in five districts of Sri Lanka. Malar J. 2018;17:271.
Sukowati S, Baimai V. A standard cytogenetic map for Anopheles sundaicus (Diptera: Culicidae) and evidence for chromosomal differentiation in populations from Thailand and Indonesia. Genome. 1996;39:165–73.
Syafruddin D, Lestari YE, Permana DH, Asih PBS, Laurent BS, Zubaidah S, et al. Anopheles sundaicus complex and the presence of Anopheles epiroticus in Indonesia. PLoS Negl Trop Dis. 2020;14: e0008385.
Garjito TA, Widiastuti U, Mujiyono M, Prihatin MT, Widiarti W, Setyaningsih R, et al. Genetic homogeneity of Anopheles maculatus in Indonesia and origin of a novel species present in Central Java. Parasit Vectors. 2019;12:351.
Ali RSM, Wahid I, Saeung A, Wannasan A, Harbach RE, Somboon P. Genetic and morphological evidence for a new species of the Maculatus Group of Anopheles subgenus Cellia (Diptera: Culicidae) in Java, Indonesia. Parasit Vectors. 2019;12:107.
Sukowati S, Andris H, Sondakh JS. Penelitian Species Sibling Nyamuk Anopheles barbirostris vander wulp di Indonesia. J Ekol Kesehat. 2004;4:172–80.
Townson H, Dyer N, Mcalister E, Satoto TBT, Bangs MJ, Harbach RE. Systematics of Anopheles barbirostris van der Wulp and a sibling species of the Barbirostris Complex (Diptera: Culicidae) in eastern Java. Indonesia Syst Entomol. 2013;38:180–91.
Laurent B, Supratman S, Asih PBS, Bretz D, Mueller J, Miller HC, et al. Behaviour and molecular identification of Anopheles malaria vectors in Jayapura district, Papua province, Indonesia. Malar J. 2016;15:192.
Rueda LM, Pecor JE, Harrison BA. Updated distribution records for Anopheles vagus (Diptera: Culicidae) in the Republic of Philippines, and considerations regarding its secondary vector roles in Southeast Asia. Trop Biomed. 2011;28:181–7.
Colless DH. The Anopheline mosquitoes of North-West Borneo. Proc Linn Soc New South Wales. 1948;73:71–119.
Garjito TA, Widiarti, Anggraeni YM, Alfiah S, Tunggul Satoto TB, Farchanny A, et al. Japanese encephalitis in Indonesia: An update on epidemiology and transmission ecology. Acta Trop. 2018;187:240–7.
Lobo V, Laumalay HM. Studi Laboratorium Siklus Hidup Anopheles vagus Pradewasa sebagai Vektor Filariasis dan Malaria di Provinsi Nusa Tenggara Timur. Balaba J Litbang Pengendali Penyakit Bersumber Binatang Banjarnegara. 2019. p. 61–8.
Budiyanto A, Ambarita LP, Salim M. Konfirmasi Anopheles sinensis dan Anopheles vagus sebagai Vektor Malaria di Kabupaten Muara Enim Provinsi Sumatera Selatan. Aspirator J Vector-borne Dis Stud. 2017;9:51–60.
Mahdalena, Vivin, Tri Wurisastuti. Gambaran Distribusi Spesies Anopheles Dan Perannya Sebagai Vektor Malaria Di Provinsi Nusa Tenggara Timur, Papua Dan Papua Barat. Spirakel 2021;l2(1): 46–59. https://doi.org/10.22435/spirakel.v12i1.3441.
Yahya Y, Haryanto D, Pahlevi RI, Budiyanto A. Keanekaragaman Jenis Nyamuk Anopheles di Sembilan Kabupaten (Tahap Pre-Eliminasi Malaria) Di Provinsi Sumatera Selatan. J Vektor dan Reserv Penyakit Badan Penelitian dan Pengembangan Kesehatan. 2020;12:41–52.
Mardiana PD. Habitat Potensial Anopheles vagus di Kecamatan Labuan dan Kecamatan Sumur Kabupaten Pandeglang, Provinsi Banten. J Ekol Kesehat. 2010;9:1139–43.
Dimas Novianto, Alya S, Kesumawati Hadi U, Soviana S. Distribution and the habitat characteristics of Anopheles vagus (Diptera: Culicidae) larvae at paddy fields in the vicinity of Dramaga IPB University Campus Dramaga Bogor West Java. Acta Vet Indones. 2021;137–41. https://doi.org/10.29244/avi...137-141.
Bashar K, Tuno N, Ahmed T, Howlader A. Blood-feeding patterns of Anopheles mosquitoes in a malaria-endemic area of Bangladesh. Parasit Vectors. 2012;5:39.
Laurent B, Burton TA, Zubaidah S, Miller HC, Asih PB, Baharuddin A, et al. Host attraction and biting behaviour of Anopheles mosquitoes in South Halmahera, Indonesia. Malar J. 2017;16:310.
Alam MS, Chakma S, Khan WA, Glass GE, Mohon AN, Elahi R, et al. Diversity of anopheline species and their Plasmodium infection status in rural Bandarban, Bangladesh. Parasit Vectors. 2012;5:150.
Astuti EP, Ipa M, Prasetyowati H, Fuadzy H, Dhewantara PW. Kapasitas Vektor dan Laju Inokulasi Entomologis Anopheles vagus dari Wilayah Endemis Malaria di Provinsi Banten. Vektora J Vektor dan Reserv Penyakit. 2016;8:23–30.
Pinontoan OR, Supadmanaba IGP, Manuaba IBAP, Sukrama IDM, Manuaba IBAP. Local diversity and biting pattern of Anopheles species in Southern Minahasa. Interdiscip Perspect Infect Dis. 2017;2017:6313016.
Bashar K, Tuno N. Seasonal abundance of Anopheles mosquitoes and their association with meteorological factors and malaria incidence in Bangladesh. Parasit Vectors. 2014;7:442.
Permadi IGWDS, Wibowo T, Wigati. Anopheles vagus Sebagai Tersangka Vektor Di Indonesia. Spirakel. 2014;6:33–6.
Sumarnrote A, Corbel V, Overgaard HJ, Celhay O, Marasri N, Fustec B, et al. Plasmodium infections in Anopheles mosquitoes in Ubon Ratchathani Province, Northeastern Thailand during a malaria outbreak. J Am Mosq Control Assoc. 2018;34:11–7.
Qin Q, Li Y, Zhong D, Zhou N, Chang X, Li C, et al. Insecticide resistance of Anopheles sinensis and An. vagus in Hainan Island, a malaria-endemic area of China. Parasit Vectors. 2014;7:92.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2020;2021:178–89.
Baimai V, Kijchalao U, Rattanarithikul R. Metaphase karyotypes of Anopheles of Thailand and Southeast Asia. VI. The Pyretophorus and the Neomyzomyia series, subgenus Cellia (Diptera:Culicidae). J Am Mosq Control Assoc. 1996;12:669–75.
Choochote W, Jitpakdi A, Sukontason K, Chaithong U, Wongkamchai S, Pitasawat B, et al. Intraspecific hybridization of two karyotypic forms of Anopheles vagus (Diptera: Culicidae) and the related egg surface topography. Southeast Asian J Trop Med Public Health. 2002;33(Suppl 3):29–35.
Davidson JR, Wahid I, Sudirman R, Small ST, Hendershot AL, Baskin RN, et al. Molecular analysis reveals a high diversity of Anopheles species in Karama, West Sulawesi. Indonesia ParasitVectors. 2020;13:379.
Senjarini K, Hasanah LNU, Septianasari MA, Abdullah MK, Oktarianti R, Wathon S. Karakterisasi Berbasis Marka Molekuler ITS2 Terhadap Sub-Spesies Kompleks Anopheles vagus vagus Dan Anopheles vagus limosus. J Bioteknol Biosains Indones. 2021;8:174–84.
Senjarini K, Abdullah MK, Azizah N. Redesigning primer of ITS2 (Internal Transcribed Spacer 2) for specific molecular characterization of malaria vectors Anopheles species. Med Arch. 2021;75:418–23.
Zhang C, Luo C, Yang R, Yang Y, Guo X, Deng Y, et al. Morphological and molecular identification reveals a high diversity of Anopheles species in the forest region of the Cambodia-Laos border. Parasit Vectors. 2022;15:94.
Hasanah LNU, Masruroh D, Wahyuni I, Oktarianti R, Wathon S, Labes A, et al. Internal transcribed spacer 2 (ITS2) based molecular identification of malaria vectors from Bangsring Banyuwangi-Indonesia. Asia-Pacific J Mol Biol Biotechnol. 2022;30:57–68.
Paul S, Banerjee PK. Cytological and molecular studies based on ovarian nurse cell’s polytene chromosome and ITS-2 sequence of Anopheles vagus in West Bengal. Int J Res Stud Zool. 2016;2:68–73.
Kaur J. Reporting of morphological variations on wings and palpi of Anopheles (Cellia) fluviatilis James and Anopheles (Cellia) vagus Donitz. J Entonol Zool Studies. 2016;4:402–5.
Wahyuni I, Senjarini K, Oktarianti R, Wathon S, Nur Uswatul Hasanah L. Identifikasi Morfologi Spesies Sibling Anopheles vagus vagus dan Anopheles vagus limosus Asal Desa Bangsring, Banyuwangi. Biosf J Biol dan Pendidik Biol. 2018;8:27–31.
Alfiah S, Mujiyono. Variasi Morfologi Anopheles vagus Donitz dari habitat Air Tawar dan Air Payau. Vektora J Vektor dan Reserv Penyakit. 2014;6:61–8.
Cooper RD, Edstein MD, Frances SP, Beebe NW. Malaria vectors of Timor-Leste. Malar J. 2010;9:40.
Zarowiecki M, Walton C, Torres E, Mcalister E, Htun PT, Sumrandee C, et al. Pleistocene genetic connectivity in a widespread, open-habitat-adapted mosquito in the Indo-Oriental region. J Biogeogr. 2011;38:1422–32.
Zomuanpuii R, Ringngheti L, Brindha S, Gurusubramanian G, Senthil KN. ITS2 characterization and Anopheles species identification of the subgenus Cellia. Acta Trop. 2013;125:309–19.
Paul S, Chattopadhyay A, Banerjee PK. Studies on seasonal abundance & molecular characterization of Anopheles subpictus and Anopheles vagus based on ITS2 sequence variability. Int J Mosq Res. 2015;2:131–5.
Reid JA, Knight KL. Classification within the subgenus Anopheles (Diptera, Culicidae). Ann Trop Med Parasitol. 1961;55:474–88.
Darsie RF Jr, Cagampang-Ramos A. Anopheline mosquitoes of the Lanao Plateau, Philippines, and status of the local malaria vector (Diptera, Culicidae). J Med Entomol. 1971;8:387–90.
Ramalingan S. Some new records of Anopheles from Sabah, Malaysia. Southeast Asian J Trop Med Public Health. 1974;5:147–8.
Anthony TG, Harbach RE, Kitching IJ. Phylogeny of the Pyretophorus Series of Anopheles subgenus Cellia (Diptera: Culicidae). Syst Entomol. 1999;24:193–205.
Harbach RE. The classification of genus Anopheles (Diptera: Culicidae): a working hypothesis of phylogenetic relationships. Bull Entomol Res. 2004;94:537–53.
Harbach RE. Review of the internal classification of the genus Anopheles (Diptera: Culicidae): the foundation for comparative systematics and phylogenetic research. Bull Entomol Res. 1994;84:331–42.
O’Connor CT, Soepanto A. Kunci bergambar nyamuk Anopheles dewasa di Sumatera-Kalimantan. Departemen Kesehatan RI; 2000.
Collins FH, Paskewitz SM. A review of the use of ribosomal DNA (rDNA) to differentiate among cryptic Anopheles species. Insect Mol Biol. 1996;5:1–9.
Lunt D, Zhang D, Szymura J, Hewitt G. The insects and COI gene: evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol Biol. 1996;5:153–65.
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994;87:651–701.
Takano KT, Nguyen NTH, Nguyen BTH, Sunahara T, Yasunami M, Nguyen MD, et al. Partial mitochondrial DNA sequences suggest the existence of a cryptic species within the Leucosphyrus group of the genus Anopheles (Diptera: Culicidae), forest malaria vectors, in northern Vietnam. Parasit Vectors. 2010;3:41.
Schuler H, Hood G, Egan SP. Modes and mechanisms of speciation. Rev Cell Biol Med. 2016;2:60–91.
Hoy MA. Insect molecular genetics: an introduction to principles and applications. 2nd ed. Acad Press; 2003.
Fitzpatrick BM, Fordyce JA, Gavrilets S. What, if anything, is sympatric speciation? J Evol Biol. 2008;21:1452–9.
Bolnick DI, Fitzpatrick BM. Sympatric speciation: models and empirical evidence. Annu Rev Ecol Evol Syst. 2007;38:459–87.
Richards EJ, Servedio MR, Martin CH. Searching for Sympatric speciation in the genomic era. BioEssays. 2019;41: e1900047.
Acknowledgements
The authors wish to thank for Iche Andriyani Liberty from Department of Public Health and Community Medicine, Universitas Sriwijaya and RM Indra, who were helped to set up the literature search strategy for this study protocol.
Funding
None.
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution to the paper as follows: study conception and design DL and DS; data collection: DL; analysis and interpretation of results: DL, DS and CA; draft manuscript preparation: DL, DS, CA, AG, LV, LS, IS. All authors reviewed the results and approved the final version of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dalilah, D., Syafruddin, D., Saleh, I. et al. A systematic review: is Anopheles vagus a species complex?. Malar J 23, 88 (2024). https://doi.org/10.1186/s12936-024-04888-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-04888-0