Abstract
Background
The emergence of insecticide resistance and outdoor transmission in malaria-endemic areas underlines the urgent need to develop innovative tools, such as spatial repellents (SR), that may circumvent this residual transmission. With limited options for effective insecticides, regular resistance monitoring is warranted for selecting and using appropriate tools. This study evaluates the pyrethroid knockdown resistance (kdr) allele before and after implementing a transfluthrin-based spatial repellent (SR) intervention in placebo-treated clusters.
Methods
This study looks at the frequency distribution of the kdr allele in Sumba Island from June 2015 to August 2018. Insecticide susceptibility tests were carried out on female Anopheles sp. aged 3–5 days against permethrin 21.5 μg/ml, deltamethrin 12.5 μg/ml, and transfluthrin 10 μg/ml using CDC bottle assay. PCR sequencing of representative samples from adult mosquito collections and insecticide tests revealed the presence of kdr mutations (L1014F and L1014S) in the VGSC gene.
Results
A total of 12 Anopheles species, Anopheles tesselatus, Anopheles. aconitus, Anopheles barbirostris, Anopheles kochi, Anopheles annularis, Anopheles maculatus, Anopheles sundaicus, Anopheles flavirostris, Anopheles balabacensis, Anopheles indefinitus, Anopheles subpictus, and Anopheles vagus were analysed. Anopheles vagus and An. sundaicus predominated in the larval populations. Susceptibility assays for all insecticides identified fully susceptible phenotypes in all species examined. Anopheles increasing frequency of kdr mutant alleles during the 3 year SR deployment was observed in both SR-treated and placebo areas, a statistically significant increase occurred in each arm. However, it is unclear how significant SR is in causing the increase in mutant alleles. The L1014S, knockdown resistance east type (kdr-e) allele was detected for the first time among the mosquito samples in this study. The L1014F, knockdown resistance west type (kdr-w) allele and heteroduplex form (wild-type—mutant) were found in almost all Anopheles species examined, including An. vagus, An. aconitus, An. subpictus, An. tesselatus, An. annularis, An. flavirostris and An. sundaicus.
Conclusion
The presence of fully susceptible phenotypes over time, along with an increase in the frequency distribution of the L1014F/S mutations post-intervention, suggest drivers of resistance external to the study, including pyrethroid use in agriculture and long-lasting insecticidal nets (LLINs). However, this does not negate possible SR impacts that support resistance. More studies that enable the comprehension of possible SR-based drivers of resistance in mosquitoes need to be conducted.
Similar content being viewed by others
Background
Malaria is still endemic in nine of the 11 WHO Southeast Asia Region countries, including Indonesia. The number of malaria cases, as indicated by the Annual Parasite Incidence (API), decreased to less than one from 2015 to 2020 but increased to 1.1 in 2021, with a positive trend of malaria cases seen in Eastern Indonesia, including Papua Province, West Papua Province, and East Nusa Tenggara Province [1]. The malaria control and elimination programme now relies mainly on two vector intervention tools, long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), that use insecticides to mitigate malaria transmission. The emergence of Anopheles strains resistant to currently used insecticides poses a critical challenge to achieving malaria elimination by 2030 [2, 3]. Vector control is an essential aspect of the program to combat malaria transmitted by Anopheles. In Indonesia, insecticide use has been the mainstay in controlling many vector-borne diseases and other agricultural pests [4]. The effectiveness of mosquito control programmes relies heavily on the efficacy of insecticides used either on LLINs or IRS, bionomics of the target vector, and human behaviour [5, 6]. Spatial repellents (SR), which repel mosquitoes from humans, thereby preventing infectious bites, also diminish the emergence and spread of resistance since effective concentrations are sublethal and may not select for resistance alleles [7]. Historically, the SR paradigm has been widely used in Indonesian communities, with active ingredients (AIs) limited to pyrethroid compounds, such as metofluthrin and transfluthrin [8,9,10].
Currently used insecticides for vector control include pyrethroids (alpha-cypermethrin, bifenthrin, cyfluthrin, deltamethrin, and lambda-cyhalothrin), carbamates (bendiocarb, propoxur), and organophosphates (fenitrothion, malathion, pirimiphos–methyl) [4]. The use of pyrethroid is not only limited to vector control in public health but is also used to control agricultural pests. The widespread use of pyrethroids in LLINs has led to increasing resistance of the Anopheles population to these compounds, with insecticide resistance (IR) being reported from several significant malaria vectors in Indonesia, including Anopheles sundaicus, Anopheles aconitus, Anopheles subpictus, and Anopheles vagus [10,11,12].
Insecticide resistance in mosquitoes is often mediated by two broad mechanisms: gene target-site mutations and enhanced metabolic detoxification of insecticides [13, 14]. Knockdown resistance (kdr) is a mechanism where target site insensitivity due to point mutations in the insect voltage-gated sodium channel (VGSC) regulatory protein, which blocks pyrethroid and Dichloro-Diphenyl–Trichloroethane (DDT) action, is associated with resistance [15]. The Anopheles kdr resistance allele, first detected in Anopheles gambiae populations from West Africa [16], is due to a single amino acid substitution (leucine to phenylalanine) at nucleotide position 1014 of the gene. An alternate substitution from leucine to serine at the same position was also detected from Anopheles in East African Kenya [17]. West African L1014F (kdr-w) and East African L1014S (kdr-e), respectively, have been found in other geographies since their discovery [18,19,20].
IRS and LLINs kill susceptible mosquitoes on contact [21, 22] while SRs are designed to repel mosquitoes away from human hosts [23, 24]. Since low doses of the SR AI may not result in mortality, selection for resistance in the mosquito should be diminished [24,25,26,27]. The evaluation of a transfluthrin-based SR product in Sumba, Indonesia, for protective efficacy against malaria [28, 29] enabled this temporal evaluation of the frequency of the kdr insecticide resistance allele in Anopheles before and after the implementation of the trial. The results of this study are the first report regarding the existence of kdr allele in Anopheles species in Sumba, Indonesia.
Methods
Ethic statement
This study was approved by the Ethics Committee of Research in Health, Medical Faculty of Hasanuddin University, Makassar, Indonesia No: 01641/H4.8.4.5.31/PP36-KOMETIK/2014; 0424/H4.8.4.5.31/PP36-KOMETIK/2015; 225/H4.8.4.5.31/PP36-KOMETIK/2016 AND 923/H4.8.4.5.31/PP36-KOMETIK/2017.
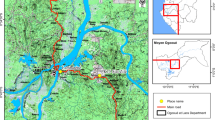
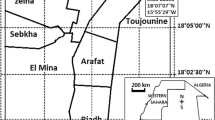
Study area, study design, and survey periods
The study was conducted in West Sumba and Southwest Sumba Districts, East Nusa Tenggara. The population in the study villages mostly work in agriculture and live in traditional houses made of bamboo. In Southwest Sumba, out of 52.8% from total land area used for agriculture with 6.2% of total land area is used for paddy field, while 46.7% is used for cropland. Meanwhile in West Sumba, out of the 86% of the area used for agriculture included 10.6% for paddyfield and 75.6% for cropland. These agricultural lands are dispersed throughout the clusters area [30, 31].
The parent spatial repellent efficacy study was a cluster-randomized, double-blinded, placebo-controlled trial involving with a total 24 clusters divided into 12 cluster per treatment arms (treated or placebo) [28]. Entomologic survey was performed in 3 periods: baseline, intervention, and post-intervention. The baseline is the phase before SR product distribution (June 2015–March 2016); the intervention was during SR product deployment (April 2016–April 2018), and post-intervention was the phase after intervention where no SR was used. Adult mosquito diversity and densities were measured using the Human Landing Catch (HLCs) every 2 weeks from the start of the baseline through the end of the follow-up intervention period. The intervention was launched simultaneously in all clusters and study personnel was distributed the SR product; transfluthrin-based passive emanator produced by S.C. Johnson & Son, Inc. (SCJ). Spatial repellent products were positioned indoor by hung on two metal hooks specially attached to walls. Research staff placed, removed, and replaced SR products in households every 2 weeks.
Susceptibility assays were conducted using CDC bottle assay. Three different insecticides were evaluated using adult F0 anopheline species collected as immatures from larval collection in each cluster in baseline, intervention, and post–intervention periods. Representative samples from susceptibility assay (intervention and post–intervention) and HLCs collection (baseline and intervention) were analysed for the kdr allele [28].
Mosquito larval sampling
All potential Anopheles breeding sites within the target cluster were sampled, and coordinates were recorded. Early stages (1st and 2nd instars) were discarded, and late-stage larvae (3rd and 4th instars) and pupae were counted, recorded, and transported to the field insectary. Larvae were fed daily with a mixture of finely ground fishmeal and yeast and reared to adults. Adult females were transferred to cages (40 cm3 metal frame covered with untreated mosquito nets), held for 3–5 days, and fed a sugar solution until use in bioassays.
Adult mosquito collection
Adult female mosquitoes were collected using HLCs and collections were conducted in 12 spatially distributed geographic clusters, using paired (indoors and outdoors) volunteer collectors in four selected houses at each collection site. Host–seeking mosquitoes landing on exposed feet and legs were caught using an aspirator for 50 min each hour from 18.00 to 06.00 h. Mosquitoes were held in individual paper cups labeled for each hour, location (indoor or outdoor), and household. Mosquito specimens were morphologically identified to species using taxonomic keys [32].
Insecticide susceptibility assay
The standard dosages or concentrations that were applied were recommended by the Centers for Disease Control (CDC) and the World Health Organization (WHO), i.e. deltamethrin 12.5 μg/ml, and permethrin 21.5 μg/ml, with a knockdown (KD) time of 30 min. Unlike the other two insecticides, previously transfluthrin was tested on wild-type An. aconitus laboratory strain at various concentrations (10, 12, 14, 16 μg/ml) before reaching a dose of 10 μg/ml with a KD time of 35 min [33]. A CDC bottle assay was performed by coating glass bottles with insecticide technical grade solution, exposing mosquitoes, and observed for 2 h. The assays were conducted using non–bloodfed, 3–5 day old females according to established guidelines [33]. Resistance status is determined by the percentage of mortality rate after 2 h observation. After each test period, all specimens were stored individually over silica gel for molecular analysis.
DNA extraction
Homogenate and mosquito DNA isolation from individual Anopheline were prepared following cetyl trimethyl ammonium bromide (CTAB) 2% reagent protocol. The CTAB technique was performed according to described protocols [34, 35]. Briefly, mosquitoes were ground with pestles in 1.5 ml microtubes containing 200 μl 2% CTAB and vortexed for 15 s, and then incubated in a heating block at 65 °C for 20 min, after which 200 μl of chloroform was added to each sample and mixed by vortex for 15 s and centrifuged at 12,000 RPM for 5 min. The aqueous phase was transferred to new vials (1.5 ml), 100 μl of cold isopropanol (− 12 °C) was added, and then samples were stored at − 30 °C for 15 min. Following incubation, the samples were centrifuged at 13,000 RPM for 5 min. The supernatant was then decanted, followed by adding 100 μl of cold 70% ethanol (− 12 °C) and centrifugation at 12,000 RPM for 5 min. The ethanol was then decanted, and the DNA pellets were dried in the vial. Finally, the pellets were resuspended by adding 20 μl of water. DNA was used immediately for a polymerase chain reaction (PCR) or stored at − 20 °C for later analysis.
Gene amplification with PCR and sequencing of kdr loci
The kdr gene was amplified using three primers in a semi-nested PCR [11]. Amplified products were cleaned using Exosap and sequenced using Sanger technology with an ABI BigdyeTM terminator per the manufacturer’s recommendation.
Analysis
Sequences were submitted to the National Center for Biotechnology Information’s Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to blast and confirm that the correct loci were amplified. Sequences were then aligned in locus target to identify kdr L1014F or L1014S mutations based on a reference sequence. Hardy–Weinberg equilibrium for observed genotyped frequencies for each kdr mutation was calculated using GenAlEx 6.5 [36]. Statistical analysis using the Pearson's Chi-squared test was conducted to compared kdr allele frequency in clusters with placebo and treated in the baseline and intervention periods.
Results
Larval samples
Habitat description and species composition
Larval collections in the study sites (Fig. 1) were conducted across all three time points. Natural larval sites were more prevalent than artificial sites. Anopheles larvae have been discovered in paddy fields, streams, seepage, ground pools, and estuaries (Additional file 1). Morphologically identified samples included 12 Anopheles species, including Anopheles tesselatus, Anopheles aconitus, Anopheles barbirostris, Anopheles kochi, Anopheles vagus, Anopheles annularis, Anopheles maculatus Anopheles sundaicus, Anopheles flavirostris, Anopheles balabacensis, Anopheles indefinitus and Anopheles subpictus. Anopheles vagus was the most abundant species in all larval sites studied and, therefore, proportionally tested in insecticide assays, whereas An. sundaicus was dominant in estuaries.
Insecticide susceptibility bioassays
In total, 674 female Anopheles mosquitoes reared from larvae were evaluated for insecticide susceptibility with CDC bottled assay. Due to variations in larval numbers, as well as time to pupation and emergence, the number of replicates per insecticide and the number of mosquitoes per assay varied. The number of individuals used in any single assay ranged from 10 to 25, and the minimum number of replicates was four. The results from the test using deltamethrin (baseline), transfluthrin (intervention), and permethrin (post-intervention) demonstrated that Anopheline mosquitoes were susceptible to all three pyrethroids, with a 100% mortality rate (Table 1).
Allele and genotype frequencies of the kdr 1014 mutation
Mosquitoes (intervention and post-intervention period) from the susceptibility assay (n = 890) were analyzed by sequencing the kdr allele. The kdr allele—wild type (TTA/TTA, TTA/TTG and TTG/TTG), mutant allele L1014F (TTT/TTA, TTT/TTG, TTT/TTT) and L1014S (TCA/TTA and TCA/TCA) were identified. The L1014F (kdr-w) allele was detected in An. sundaicus, An. subpictus, An. tesselatus, An. vagus and An. annularis. The L1014S (kdr-e) allele was detected in An. sundaicus, An. vagus and An. annularis. In addition to these alleles, a heteroduplex two amino acid substitution at 1014 position was documented in An. sundaicus, An. vagus, and An. tesselatus. The kdr genotype frequency increased from 0.059 to 0.203 with An. tesselatus as the spesies that saw an increase in the kdr allele (Table 2).
Adult samples
kdr baseline and intervention phase
Overall, 3520 adult female Anopheles mosquitoes from the six treated and six placebo clusters were analyzed from the baseline and intervention phases. The kdr allele (L1014F/S) were detected in 11 morphologically and molecularly identified species, namely An. aconitus, An. maculatus, An. sundaicus, An. vagus, An. subpictus, An. barbirostris, An. tesselatus, An. annularis, An. flavirostris, An. indefinitus, and An. kochi. In general, all species showed an increase in the kdr allele during the intervention, except An. subpictus from treated arm, and An. sundaicus from placebo arm. However, Anopheles species from placebo arm have a higher kdr frequency (Table 3).
Pearson's Chi-squared test with Yates' continuity correction revealed there was a significant difference meaning between mutant and wildtype numbers in the treated cluster (p = 0.0126), placebo cluster (p = 2.105e-10) and treated vs placebo clusters (p-value < 2.2e-16) during baseline and intervention. The intervention period samples had a higher frequency of all mutant alleles than baseline samples. The frequency of mutant alleles increased 1.1 times from 0.070 to 0.146 (Table 4).
The mean of kdr allele frequency in the placebo cluster, baseline (0.056 ± 0.086), and intervention (0.116 ± 0.104) were higher than SR treated clusters, placebo (0.049 ± 0.04) and intervention (0.072 ± 0.059).
Discussion
A growing body of evidence has demonstrated the potential use of SRs to control malaria and other mosquito-borne diseases [28, 37]. With resistance to active ingredients (AIs) used on intervention products being a significant factor that compromises intervention effectiveness, surveillance for IR is a vital component of any evaluation of an intervention—especially novel paradigms such as SRs. Surveillance for phenotypic IR and kdr alleles was conducted before, during, and after the parent study that evaluated the impact of a pyrethroid (transfluthrin)-based SR product on malaria incidence in which clusters of households were treated with either a transfluthrin-based SR or a placebo.
Phenotypic IR surveillance for IR demonstrated that all Anopheles species in the study clusters remained fully susceptible to multiple pyrethroids following the 2 year SR intervention in Sumba, Indonesia. Molecular analysis revealed that the kdr resistance allele was already present in the population at baseline. However, various alleles increased in frequency over the study sites throughout the primary study.
Chemical pesticides, including pyrethroids, are the primary focus for agricultural pest management for fruit and vegetable crops in Sumba Islands including corn as the main crop followed by paddy field. In addition, as an area endemic to malaria, public health insecticide use is often intense. Though the use of insecticides in agriculture is generally regarded as an important driver of insecticide resistance in malaria vectors [38], pyrethroid-based interventions (ITNs and IRS) for controlling Anopheles have been associated with an increase in the frequency of kdr resistance alleles [39, 40]. Here, agricultural use of pyrethroids, along with pyrethroid-based LLINs, likely enabled the selection of baseline kdr resistance alleles seen at baseline. The increased frequency of kdr in placebo clusters is likely due to local selection by agricultural insecticides and chance inclusion in the placebo arm. Mass distribution of LLINs in Sumba occurred in October–December 2014 (Olyset Net, permethrin 2.0%) with reported > 95% coverage of households providing 1–4 nets each. Another mass distribution occurred in February–March 2018 (PermaNet® 3.0, Vestergaard Frandsen SA, Denmark, deltamethrin 180 mg/m2 + piperonyl butoxide synergist) [28]. The increased selection for kdr seen in untreated arms due to factors external to SR implementation is supported by the temporal more significant increase in these alleles from the baseline to the intervention periods in these clusters without SRs being implemented.
These data support the SR paradigm—where sublethal doses of repellant products will diminish the selection of IR phenotypes and genotypes. However, this does not counter the possibility of the emergence of SR-based resistance [23]. The selection of reduced spatial repellency, IR alleles, and reduced spatial repellant sensitivity suggests that long-term use of SRs might temporally impact vector populations. The kdr trait enables resistance to pyrethroid and DDT [41, 42] by reducing neuronal sensitivity [43]. This decreases the irritant and the repellent effects and either cancels or reduces the knock-down effect [44]. Here, increased kdr may result in less repelled mosquitoes and therefore, obtain higher doses of the volatile insecticide, increasing the death rate [45]. Additional secondary impacts of exposure to sublethal amounts of a spatial repellant (reduced feeding, increased mortality [46], combined with the increased lethality based on increased exposure would also support the relatively lower increase in kdr alleles seen in the intervention clusters in a panmictic population of mosquitoes. Unfortunately, adult collections in the post-intervention period, were not conducted, and comparisons of kdr allele frequencies after SR trial conclusion were impossible.
The lack of phenotypic resistance over the three time points suggests that the SR intervention did not select for pyrethroid resistance in 2 years of SR implementation. However, the three insecticides evaluated for resistance may have different insecticidal properties and were not assessed at the same time. Ideally, all three insecticides (deltamethrin at baseline), transfluthrin during the intervention, and permethrin post-intervention) would have been evaluated at all three-time points to enable a comparable evaluation of temporal insecticide susceptibility.
Of the 12 Anopheles species that were found to carry the kdr allele, 11 species are reported as human night-biting mosquitoes in Sumba [47] and confirmed as malaria vectors [10, 28] Homozygous, heterozygous, and heteroduplex forms of kdr alleles were found in these species and likely reflect different molecular evolution dynamics combined with different insecticide exposure levels in each Anopheles species. The dynamics of resistance acquisition and spread may be linked to many factors, including the history of insecticide use, the level of pre-existing susceptibility to the compound, the frequency and heritability of genes related to resistance, and the co-selection of distinct resistance mechanisms in the same population [13].
Heteroduplexes are formed between double-stranded DNA from two gene alleles [48]. The presence of a heteroduplex mutation that contains a sequence mismatch (mutant, wild-type, and wild-type, mutant) can be separated by denaturing high-performance liquid chromatography (HPLC), enzymatic (RNase cleavage assay), electrophoretic methods and chemical cleavage assays [49]. In these studies, mosquito samples with heteroduplex form were not subjected to the further examination but were still included in the resistant heteroduplex criteria carrying the mutant allele.
This study recognizes several limitations, first, during the study period, a susceptibility test of the same insecticide would be ideal. Furthermore, this resistance status may be restricted by location, species or influenced by the number of samples tested in each location, requiring testing in each cluster. Second, adult mosquito collection in post-intervention should be performed to confirm the frequency of the kdr mutant allele in the absence of SR. Third, detecting metabolic detoxification enzymes will contribute to comprehensively understanding the causes of the high level of mutant allele in Anopheles populations in Sumba. Overall, the concurrent use of pyrethroids in public health and agricultural sectors continues to drive mosquito resistance and alerts to the need to use alternative tools that do not drive resistance. It is also essential to mitigate the emergence of resistance by applying different classes of insecticides for public health and agricultural purposes. In this context, sustainable insecticide resistance monitoring using phenotypic bioassays and molecular tools is necessary to inform policy and to establish an integrated vector and pest control programme. Since the Indonesian malaria control and elimination programme targets elimination by 2030, SRs may be considered for strategy inclusion as the data demonstrate an epidemiological impact [28].
Conclusion
Implementing a transfluthrin-based SR product over 2 years did not select for resistance to pyrethroids tested. Although the kdr resistance allele was present at baseline and increased throughout the study, the data presented cannot explain an association with the SR. The possible external driver of kdr allele increases, the possible lower increase of kdr in intervention clusters, and the actual reduction in malaria in intervention clusters all support SRs as valid intervention in these settings.
Availability of data and materials
All relevant data are within the manuscript.
References
Kementerian Kesehatan RI. Profil Kesehatan Indonesia. 2022. p xxix–288.
WHO. Global malaria programme. Global technical strategy for malaria, 2016–2030. Geneva: World Health Organization; 2015.
Susanna D, Pratiwi D. Current status of insecticide resistance in malaria vectors in the Asian countries: a systematic review. F1000Res. 2021;10:200.
Kementrian Kesehatan RI. Direktorat Jenderal Pengendalian Penyakit dan Penyehatan Lingkungan. Pedoman penggunaan insektisida (pestisida) dalam pengendalian vektor. 2012;viii–26.https://repository.kemkes.go.id/book/485
Paaijmans KP, Lobo NF. Gaps in protection: the actual challenge in malaria elimination. Malar J. 2023;22:46.
Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar J. 2019;18:445.
Achee NL, Perkins TA, Moore SM, Liu F, Sagara I, Van Hulle S, et al. Spatial repellents: the current roadmap to global recommendation of spatial repellents for public health use. Curr Res Parasitol Vector Borne Dis. 2023;3:100107.
Logan J. An expert review of spatial repellents for mosquito control. Innovative Vector Control Consortium, 2020. https://www.ivcc.com/wp-content/uploads/2020/08/An-Expert-Review-of-Spatial-Repellents-for-Mosquito-Control.pdf
Kim DY, Hii J, Chareonviriyaphap T. Transfluthrin and metofluthrin as effective repellents against pyrethroid-susceptible and pyrethroid-resistant Aedes aegypti (L.) (Diptera: Culicidae). Insects. 2023;14:767.
Syafruddin D, Bangs MJ, Sidik D, Elyazar I, Asih PBS, Chan K, et al. Impact of a spatial repellent on malaria incidence in two villages in Sumba, Indonesia. Am J Trop Med Hyg. 2014;91:1079–87.
Syafruddin D, Hidayati AP, Asih PB, Hawley WA, Sukowati S, Lobo NF. Detection of 1014F kdr mutation in four major Anopheline malaria vectors in Indonesia. Malar J. 2010;9:315.
Trapsilowati W, Prihatin MT, Setyaningsih R, Garjito TA, Pujiyanti A, Mulyono A. Receptivity status of malaria transmission toward malaria elimination in Indonesia. In5th Universitas Ahmad Dahlan Public Health Conference (UPHEC 2019) 2020 (pp. 170–173). Atlantis Press.
Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653–65.
Ffrench-Constant RH. The molecular genetics of insecticide resistance. Genetics. 2013;194:807–15.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s.. Insect Mol Biol. 1998;7:179–84.
Salako AS, Ahogni I, Aïkpon R, Sidick A, Dagnon F, Sovi A, et al. Insecticide resistance status, frequency of L1014F Kdr and G119S Ace-1 mutations, and expression of detoxification enzymes in Anopheles gambiae (s.l.) in two regions of northern Benin in preparation for indoor residual spraying. Parasit Vectors. 2018;11:618.
Kawada H, Futami K, Komagata O, Kasai S, Tomita T, Sonye G, et al. Distribution of a knockdown resistance mutation (L1014S) in Anopheles gambiae s.s. and Anopheles arabiensis in Western and Southern Kenya. PLoS ONE. 2011;6:e24323.
Namountougou M, Diabaté A, Etang J, Bass C, Sawadogo SP, Gnankinié O, et al. First report of the L1014S kdr mutation in wild populations of Anopheles gambiae M and S molecular forms in Burkina Faso (West Africa). Acta Trop. 2013;125:123–7.
Reimer L, Fondjo E, Patchoké S, Diallo B, Lee Y, Ng A, et al. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J Med Entomol. 2014;45:260–6.
da Cruz DL, Paiva MHS, Guedes DRD, de Souza Gomes EC, Pires SG, Gomez LF, et al. First report of the L1014F kdr mutation in wild populations of Anopheles arabiensis in Cabo Verde, West Africa. Parasit Vectors. 2021;14:582.
Unwin HJT, Sherrard-Smith E, Churcher TS, Ghani AC. Quantifying the direct and indirect protection provided by insecticide treated bed nets against malaria. Nat Commun. 2023;14:676.
Coleman S, Dadzie SK, Seyoum A, Yihdego Y, Mumba P, Dengela D, et al. A reduction in malaria transmission intensity in Northern Ghana after 7 years of indoor residual spraying. Malar J. 2017;16:324.
Wagman JM, Achee NL, Grieco JP. Insensitivity to the spatial repellent action of transfluthrin in Aedes aegypti: a heritable trait associated with decreased insecticide susceptibility. PLoS Negl Trop Dis. 2015;9:e0003726.
Achee NL, Bangs MJ, Farlow R, Killeen GF, Lindsay S, Logan JG, et al. Spatial repellents: from discovery and development to evidence-based validation. Malar J. 2012;11:164.
Ogoma SB, Lorenz LM, Ngonyani H, Sangusangu R, Kitumbukile M, Kilalangongono M, et al. An experimental hut study to quantify the effect of DDT and airborne pyrethroids on entomological parameters of malaria transmission. Malar J. 2014;13:131.
Roberts D. Insecticide repellency in malaria vector control: a position paper. VBC Project report No 81131. 1993.
Chareonviriyaphap T, Bangs MJ, Suwonkerd W, Kongmee M, Corbel V, Ngoen-Klan R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit Vectors. 2013;6:280.
Syafruddin D, Asih PBS, Rozi IE, Permana DH, Hidayati APN, Syahrani L, et al. Efficacy of a spatial repellent for control of malaria in Indonesia: a cluster-randomized controlled trial. Am J Trop Med Hyg. 2020;103:344–58.
Permana DH, Zubaidah S, Syahrani L, Asih PBS, Syafruddin D, Rozi IE, et al. Impact of a spatial repellent product on Anopheles and non-Anopheles mosquitoes in Sumba, Indonesia. Malar J. 2022;21:166.
Statistik Pertanian Kabupaten Sumba Barat Daya 2018. 2019. P x–55.
Statistik Pertanian Kabupaten Sumba Barat 2018. 2019. p iv–29.
Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand IV Anopheles. Southeast Asian J Trop Med Public Health. 2006;37(Suppl 2):1–128.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2nd ed. Geneva: World Health Organization; 2016.
Irfan M, Ting ZT, Yang W, Chunyu Z, Qing M, Lijun Z, Feng L. Modification of CTAB protocol for maize genomic DNA extraction. Res J Biotechnol. 2013;8:41–5.
Tel-Zur N, Abbo S, Myslabodski D, Mizrahi Y. Modified CTAB procedure for DNA isolation from epiphytic cacti of the genera Hylocereus and Selenicereus (Cactaceae). Plant Mol Biol Reporter. 1999;17:249–54.
Peakall R, Smouse PE. GenALEx 6.5: genetic analysis in excel. Population genetic software for teaching and research-an update. Bioinformatics. 2012;28:2537–9.
Morrison AC, Reiner RC Jr, Elson WH, Astete H, Guevara C, Del Aguila C, et al. Efficacy of a spatial repellent for control of Aedes-borne virus transmission: a cluster-randomized trial in Iquitos, Peru. Proc Natl Acad Sci USA. 2022;119:e2118283119.
Reid MC, McKenzie FE. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J. 2016;15:107.
Padonou GG, Sezonlin M, Ossé R, Aizoun N, Oké-Agbo F, Oussou O, et al. Impact of three years of large scale indoor residual spraying (IRS) and insecticide treated nets (ITNs) interventions on insecticide resistance in Anopheles gambiae s.l. in Benin. Parasit Vectors. 2012;5:72.
Temu EA, Maxwell C, Munyekenye G, Howard AFV, Munga S, Avicor SW, et al. Pyrethroid resistance in Anopheles gambiae, in Bomi County, Liberia, compromises malaria vector control. PLoS ONE. 2012;7:e44986.
Oppenoorth FJ. Biochemistry and genetics of insecticide resistance. Annu Rev Entomol. 1965;10:185–206.
Soderlund DM. Pyrethroids, knockdown resistance and sodium channels. Pest Manag Sci. 2008;64:610–6.
Bloomquist JR, Soderlund DM. Pyrethroid insecticides and DDT modify alkaloid-dependent sodium channel activation and its enhancement by sea anemone toxin. Mol Pharmacol. 1988;33:543–50.
Labbé P, Alout H, Djogbénou L, Pasteur N, Weill M. Evolution of resistance to insecticide in disease vectors. In: Tibayrenc M, editor. Genetics and evolution of infectious disease. Elsevier; 2011. p. 363–409.
Hodjati MH, Curtis CF. Dosage differential effects of permethrin impregnated into bednets on pyrethroid resistant and susceptible genotypes of the mosquito Anopheles stephensi. Med Vet Entomol. 1997;11:368–72.
Burton TA, Kabinga LH, Simubali L, Hayre Q, Moore SJ, Stevenson JC, et al. Semi-field evaluation of a volatile transfluthrin-based intervention reveals efficacy as a spatial repellent and evidence of other modes of action. PLoS ONE. 2023;18:e0285501.
Syahrani L, Permana DH, Syafruddin D, Zubaidah S, Asih PBS, Rozi IE, et al. An inventory of human night-biting mosquitoes and their bionomics in Sumba, Indonesia. PLoS Negl Trop Dis. 2022;16:e0010316.
Paniego N, Fusari C, Lia V, Puebla A. SNP genotyping by heteroduplex analysis. In: Batley J, editor. Plant genotyping: methods and protocols. Humana Press; 2015. p. 141–50.
Palais RA, Liew MA, Wittwer CT. Quantitative heteroduplex analysis for single nucleotide polymorphism genotyping. Anal Biochem. 2005;346:167–75.
Acknowledgements
The authors are grateful for the support of the Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Cibinong, Indonesia; The University of Hasanuddin, Makassar, Indonesia; the Ministry of Health Republic of Indonesia; District health departments of Southwest and West Sumba, and East Nusa Tenggara Province. We appreciate the contribution of the entomology teams, local field workers, data entry clerks, and the numerous local volunteers for their dedication and active participation in this study. This publication is dedicated in memory of Ibu Siti Zubaidah and Michael J Bangs, who had a particular interest in this study, where she imparted much experience and knowledge. Molecular analyses of samples in this study were a part of the doctoral program activity for LS.
Funding
The sample collection of this study was funded by an award from the University of Notre Dame (Grant# OPP1081737) (from the Bill and Melinda Gates Foundation—BMGF). Molecular analysis was supported by the Government of Indonesia National Research and Innovation Agency (BRIN) through the Health Research Organization.
Author information
Authors and Affiliations
Contributions
LS and PA have the same contribution as the main contributor. DS, NL, and NA conceived and designed the study; LS, PA, SZ, IR, DP, CB, MB, JG, NA, NL, and DS were responsible for the investigation, design methodology and contributed to data curation and formal analysis. LS, PA, AB, AD, NL, and DS wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Number of Anopheles larvae in breeding sites.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Syahrani, L., Asih, P.B.S., Bowolaksono, A. et al. Impact of a spatial repellent intervention on Anopheles kdr insecticide resistance allele in Sumba, Indonesia. Malar J 23, 31 (2024). https://doi.org/10.1186/s12936-024-04841-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-04841-1