Abstract
Background
Malaria and HIV are two important public health issues. However, evidence on HIV-Plasmodium vivax co-infection (HIV/PvCo) is scarce, with most of the available information related to Plasmodium falciparum on the African continent. It is unclear whether HIV can change the clinical course of vivax malaria and increase the risk of complications. In this study, a systematic review of HIV/PvCo studies was performed, and recent cases from the Brazilian Amazon were included.
Methods
Medical records from a tertiary care centre in the Western Brazilian Amazon (2009–2018) were reviewed to identify HIV/PvCo hospitalized patients. Demographic, clinical and laboratory characteristics and outcomes are reported. Also, a systematic review of published studies on HIV/PvCo was conducted. Metadata, number of HIV/PvCo cases, demographic, clinical, and outcome data were extracted.
Results
A total of 1,048 vivax malaria patients were hospitalized in the 10-year period; 21 (2.0%) were HIV/PvCo cases, of which 9 (42.9%) had AIDS-defining illnesses. This was the first malaria episode in 11 (52.4%) patients. Seven (33.3%) patients were unaware of their HIV status and were diagnosed on hospitalization. Severe malaria was diagnosed in 5 (23.8%) patients. One patient died. The systematic review search provided 17 articles (12 cross-sectional or longitudinal studies and 5 case report studies). A higher prevalence of studies involved cases in African and Asian countries (35.3 and 29.4%, respectively), and the prevalence of reported co-infections ranged from 0.1 to 60%.
Conclusion
Reports of HIV/PvCo are scarce in the literature, with only a few studies describing clinical and laboratory outcomes. Systematic screening for both co-infections is not routinely performed, and therefore the real prevalence of HIV/PvCo is unknown. This study showed a low prevalence of HIV/PvCo despite the high prevalence of malaria and HIV locally. Even though relatively small, this is the largest case series to describe HIV/PvCo.
Similar content being viewed by others
Background
Malaria and human immunodeficiency virus (HIV) infections are two major public health concerns [1]. Given the considerable epidemiological overlap between malaria and HIV, a substantial number of co-infections may occur [1]. In 2019, malaria accounted for 228 million cases and resulted in 405,000 deaths, globally [2]. Of the 5 Plasmodium species that can infect humans, Plasmodium vivax is the most geographically widespread, and is responsible for 75% of malaria-related cases in the Latin American region [2,3,4]. Currently, 37.9 million people are living with HIV worldwide, with 1.7 million newly diagnosed in 2019. An estimated 690,000 deaths were due to AIDS-related diseases in 2019 [5]. Globally, these infections jointly claim the lives of about 2 million people each year [6].
Malaria and HIV infections can interact in a bidirectional and synergistic manner, which may lead to an exponential rise in their deleterious effects [7]. HIV can impair immune responses to malaria parasites, and lead to an inability to control parasite clearance, thus resulting in high parasitic loads, which in turn, can increase malaria transmission rates [1, 8, 9]. Clinically, HIV has been shown to contribute to a higher incidence of falciparum malaria [10], including its severe form, which is characterized by anaemia, cerebral malaria and increased risk of congenital infections [11,12,13]. The impact of HIV on the severity of malaria appears to be restricted to patients with CD4 + T cell counts < 350 cells/μL [10].
Meanwhile, malaria infection is associated with strong CD4 + T cell activation and increased levels of pro-inflammatory cytokines, which provides an ideal micro-environment for HIV viral replication. This potentially worsens the clinical picture, increasing HIV progression to AIDS and maintaining the viral cycle [1, 14,15,16,17,18]. The immunosuppression caused by HIV infection can reduce the control of the Plasmodium infection. Moreover, HIV therapy can impair malaria treatment, with a significant increase in adverse events, as well as potential selection of treatment-resistant parasites [19,20,21,22,23]. Plasmodium co-infection has also been shown to increase HIV viral load and transiently decrease CD4 + T cell count [24,25,26,27]. However, these interactions are mostly described for Plasmodium falciparum.
Studies reporting HIV-P. vivax co-infection (HIV/PvCo) are scarce. Therefore, a clear understanding of the interaction between these two diseases is necessary for more effective control measures, especially in co-endemic areas of vivax malaria and HIV. In this study, clinical and laboratory outcomes in a case series of HIV/PvCo patients admitted to a tertiary care centre in the Western Brazilian Amazon is described. Also, evidence regarding HIV/PvCo in the literature is provided through a systematic review of published articles.
Methods
Case series
All medical records from patients admitted to the Fundação de Medicina Tropical Dr Heitor Vieira Dourado (FMT-HVD) for suspected vivax malaria infection, from March 2009 to December 2018, were screened for eligibility for this study. The FMT-HVD is a tertiary reference health care centre for infectious diseases in Manaus, Western Brazilian Amazon and receives patients directly seeking care and those referred by public and private care networks in Manaus and the surrounding cities. Around 30% of all malaria cases in the municipality are diagnosed in the FMT-HVD and 90% of people living with HIV are followed up at this health care unit. Manaus is the capital of the Amazonas state, has a total area of 11,401 sq km and a population of 2.2 million habitants (population density of 194.7 hab/sq km). The FMT-HVD is part of the Brazilian free public health system (Sistema Único de Saúde—SUS) and adopts all Brazilian guidelines for the management of sexually transmitted infections [28], including HIV infection in adults [29], as well as malaria treatment [30].
All patients are registered in the hospital’s electronic medical records (EMR). Data used in this study were obtained after a thorough examination of the hospital’s database. All information regarding patient demographics, malaria symptoms, previous history of HIV infection, laboratory examinations, and outcome status (survival or death) was anonymously retrieved from individual medical records. HIV infection was previously determined by two positive rapid diagnostic tests (RDTs) and confirmed by an immunoassay test, as defined by the Brazil Ministry of Health [31]. Diagnosis was extracted from the EMR system. Plasmodium vivax infection was confirmed by a positive thick blood smear, as defined by the Brazil Ministry of Health guidelines [30], and diagnostic information was subsequently recorded in the hospital’s EMR and/or the Malaria Epidemiological Surveillance Information System (SIVEP-Malaria) [31]. Patients were classified with severe malaria according to the World Health Organization (WHO) guidelines [32].
Systematic review
A systematic review regarding studies of HIV/PvCo was conducted in conformity with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [33, 34]. Studies reporting HIV/PvCo were systematically identified from multiple electronic databases (Medline/PubMed, Lilacs and Scielo), using the following keywords as the search strategy: (HIV AND malaria) OR (AIDS AND malaria) OR (HIV AND vivax) OR (AIDS AND vivax) OR (HIV AND Plasmodium) OR (AIDS AND Plasmodium). The last search was performed in February 2020. No date or language restrictions were applied. All types of study design, with primary clinical data, were included (cross-sectional, longitudinal, case reports, and case series). All duplicates were removed. Additional studies were obtained by an additional search of the references from the included studies.
Study titles and abstracts were reviewed to confirm the inclusion of data on HIV/PvCo and P. vivax mono-infections. Included studies were assessed for eligibility by a full-text review and excluded when an inconclusive diagnosis of Plasmodium species and/or a doubtful HIV-positive co-infection was reported. Two independent authors of the study conducted the systematic review process. Disagreements were resolved by consensus.
For cross-sectional and longitudinal studies, the following data were retrieved: author, year of publication, country, total of malaria cases, total of vivax malaria cases, vivax malaria cases with prior HIV, mean age of population, co-morbidities and co-infections, and clinical outcomes. From the reports and case series, demographic, clinical and laboratory data were retrieved. Baseline patient characteristics were summarized as medians, with interquartile range (IQR) or means with standard deviation (SD).
Ethical considerations
The FMT-HVD Ethics Review Board approved this study as per the guidelines and standards for regulating research on human subjects established in Resolution 466/12, of the National Health Council of the Brazilian Ministry of Health. A waiver of informed consent was obtained due to the retrospective nature of the study. Patient anonymity was preserved throughout data extraction and analysis.
Results
Case series
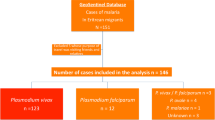
A total of 1,144 patients (5.5% of all FMT-HVD hospitalizations) were admitted with a malaria diagnosis during the study period. Of these, 1,048 (91.6%) were diagnosed with P. vivax mono-infection, out of which 21 (2.0%) were also HIV-positive (Fig. 1). Nineteen patients (90.6%) resided in Manaus’ urban area, one (4.7%) in the peri-urban area and one (4.7%) in Presidente Figueiredo, a municipality 107 km north of Manaus. None of the patients was declared as indigenous.
Table 1 shows a summary of HIV/PvCo patients. Fourteen (66.7%) were men. The mean age was 33 (± 14.2 years), with the youngest patient being a 14-year-old boy (case 6). According to HIV status, 12 (57.1%) patients were living with HIV; 9 (42.9%) presented an AIDS-defining disease; 7 (33.3%) were diagnosed with HIV at hospital admission. Prior HIV viral load and CD4 + T cell counts were reported in 12 (57.1%) patients, with an average of 32.188 (± 74,514) copies/mL and 386 (± 306.4) cells/μL, respectively. A CD4 + T cell count of < 200 cells/μL was recorded in 8 (42.1%) patients. The use of anti-retroviral treatment (ART) was registered in 14 (66.7%) patients; however, only 3 (14.3%) showed adherence to treatment in the 6 months before hospital admission.
Of the included patients, 11 (52.4%) reported a first malaria episode. A previous history of co-morbidities and co-infections was present in 14 (66.7%) patients. Of these, 7 (33.3%) reported a diagnosis of HIV, vivax malaria and tuberculosis (TB) co-infection. One (4.8%) female patient was pregnant. She was treated solely with chloroquine for 3 days. According to WHO guidelines [35], severe malaria criteria (significant bleeding, respiratory distress, severe anaemia, pulmonary oedema, metabolic acidosis, and hyperbilirubinaemia, associated with organ dysfunction) were present in 5 (23.8%) patients (cases 5, 10, 14, 19, and 21) (Table 1).
One (4.8%) patient with severe malaria, who was treated with intravenous artesunate, died on the day following hospitalization (case 21). Two (9.5%) patients (cases 5 and 14) returned to the hospital after discharge with signs of haemolysis and were diagnosed with G6PD deficiency (G6PDd). In both cases, treatment with primaquine was stopped. The remaining patients completed anti-malarial treatment with chloroquine and primaquine. Malaria treatment was performed according to the Brazilian Ministry of Health guidelines [30].
Anaemia (haemoglobin level < 12 g/dL) was present in 16 (76.2%) patients, one of whom was severely anaemic (Hb < 7 g/dL). Low platelet levels (< 150,000/cu mm) were recorded in 18 (85.7%) patients, while severe thrombocytopenia (< 50,000/cu mm) was observed in 10 (47.6%) patients, with a single subject presenting significant bleeding (case 5) (Table 1).
Systematic review
The original search yielded 8,460 studies. After the exclusion of duplicates, screening and the use of predefined inclusion criteria, only 11 studies were included for further analysis (Fig. 2). Subsequently, 6 other studies were added after a reference search of the included studies. The selected studies were reviewed and thereafter stratified into 2 main groups; the first group comprised 12 cross-sectional or longitudinal studies (Table 2) and the second group was composed of 5 case report studies (Table 3).
The highest prevalence of studies reporting HIV/PvCo was found in the African and Asian regions, with 35.3 and 29.4%, respectively. The prevalence of reported co-infections ranged from 0.1 to 60%; patient ages ranged from 10 to 60 years. No cases of severe malaria, according to WHO guidelines [32], were reported. Data from the 6 case reports are presented in Table 3. The mean age of these patients was 51.6 (± 13.6 years). Prior and recent HIV viral load and CD4 T-cell count tests were reported in 3 (50.0%) patients and the use of antiretroviral therapy (ART) was described in 2 (33.3%) cases, with one patient adhering to treatment. Pneumocystis sp. pneumonia and TB were reported in three cases (50.0%) [36, 37]. Only 2 (33.3%) patients presented severe malaria, according to WHO guidelines [38, 39].
Discussion
Both malaria and HIV are highly prevalent in tropical and sub-tropical regions of the world, which may result in an increased prevalence of such co-infection [40]. Several studies have reported on HIV and P. falciparum co-infection, mainly in Africa [40,41,42], but only a few studies have described cases of HIV/PvCo patients. Indeed, from the initial search, only 2% of studies dealt with HIV/PvCo. This could be due to several factors, such as low co-infection rates, low prevalence of severe cases and therefore lack of reporting, and most importantly, lack of systematic HIV screening in vivax malaria-positive patients.
HIV and malaria affect millions of people in overlapping geographical areas. Traditional risk factors for HIV and vivax malaria may apparently be dissociated, which could possibly explain the HIV/PvCo low prevalence reported here compared to similar studies from other vivax endemic regions [43,44,45,46,47,48,49,50,51,52,53]. Local characteristics may account for the predisposition of HIV/PvCo. About 90% of all malaria episodes in Brazil is caused by P. vivax and these are concentrated in the Amazon region [54]. The clinical spectrum varies from asymptomatic cases to mild clinical symptomatology, and complications may occur. Male subjects present a higher incidence of malaria with younger individuals predisposed to a higher risk of clinical complications [55]. Malaria transmission in the Amazon region occurs mainly in peri-urban and rural areas, with an increase in the number of cases occurring in the dry season and after public holidays and regional festivities [56]. On the other hand, HIV infection, which is prevalent in 0.4% of the general population in Brazil, occurs mainly in key groups, such as in men who have sex with men, sex workers and transgender individuals [57]. For instance, in 2019, the AIDS detection rate was 29.1 cases/100,000 habitants in the state of Amazonas, while in the capital, Manaus, 46.9 cases/100,000 habitants, which is significantly higher when compared to the rest of the state, and in comparison with the Brazilian national rate (17.8 cases/100,000 habitants) [57]. HIV transmission occurs mainly in urban environments [58]. In the state of Amazonas, Manaus, Parintins and Tabatinga cities are some of the major HIV hotspots [59]. In most municipalities in the interior of the state of Amazonas, there is not a clear delimitation between urban, peri-urban and rural regions. Constant population displacement between such regions and between municipalities is very common. This may partially explain the exposure of people living with HIV to malaria. In addition, it is uncertain to what extent cultural and social characteristics significantly overlap between both diseases to produce a low prevalence as seen in this study. However, since the systematic screening of HIV infection is not done in acute malaria cases in Brazil, the real HIV/PvCo burden is unknown. Furthermore, it is important to mention that HIV-positive cases have been increasing in recent years in the northern region of Brazil [57].
Separately, HIV, malaria and TB are considered to be the most common and severe infectious diseases in the world [60]. Interestingly, a triple infection with these three diseases was more prevalent in this study when compared with other studies from Africa, although this may be attributed to screening, as previously mentioned [42, 61].
Two patients presented with G6PDd. The lower the G6PD activity, the lower the individual’s ability to tolerate oxidative stress and, when faced with oxidative stressors, such as certain foods, e.g., fava beans and drugs, such as primaquine or sulfonamides, G6PDd individuals may develop acute life-threatening haemolysis [62]. The prevalence of G6PDd applies to both HIV and malaria. HIV infection and antiretroviral therapy (ART) are both associated, separately and together, with increased oxidative stress. The impact of G6PDd on the oxidative stress of people living with HIV (PLHIV) on ART is controversial [63,64,65]. PLHIV have significantly lower levels of antioxidants, haematological parameters and CD4 + T cells compared to healthy subjects. Nonetheless, PLHIV on ART have presented a higher level of antioxidants compared to ART naïve subjects [67]. Also, antioxidant status has been shown to be significantly higher in those with CD4 + T cells ≥ 200/cu mm [66].
The prevalence of G6PDd varies across Latin American and Caribbean countries, with the African variant present in a wide range in this region [67]. Primaquine is a strong oxidative drug and may cause severe acute haemolysis in G6PDd individuals who take primaquine for malaria treatment [68]. G6PDd testing is currently recommended by WHO prior to starting primaquine in the radical cure of P. vivax and Plasmodium ovale malaria [30, 69]. Systematic screening for G6PDd is however not a requirement when starting HIV treatment [29]. Therefore, it was not possible to determine whether G6PD deficiency in HIV/PvCo influenced clinical outcomes.
Separately, malaria and HIV cause significant laboratory abnormalities; a co-infection scenario may intensify such alterations [27, 40]. Anaemia, thrombocytopenia, and leukopaenia, for both malaria and HIV, and in malaria/HIV co-infected patients, have been reported to be strong and independent predictors of morbidity and mortality [52]. The prevalence of anaemia in this case series was high, with most patients presenting mild to moderate anaemia, similar to other studies of HIV/PfCo [26, 70, 71]. Nonetheless, this is higher when compared to P. vivax mono-infected adults from the Amazonas [72].
A high prevalence of thrombocytopenia (85.7%) was also observed in this study. Two studies conducted in patients with P. vivax showed a similar prevalence (62.9 and 72%, respectively) [73]. In a systematic review [74], severe and fatal thrombocytopenia was observed in 10.1% of patients with vivax mono-infection malaria, while severe thrombocytopenia was more prevalent in this study. Anaemia and thrombocytopenia in HIV-malaria co-infections have a multifactorial origin and are a frequent complication that may become clinically important in HIV infection [40, 52, 75].
The impact of HIV on the clinical severity of falciparum malaria seems to be primarily motivated by the inability of the immune system to control the parasitic burden [41, 76, 77]. Severe malaria was observed in approximately 30% of adults with falciparum malaria and HIV in the urban area of Burkina Faso [78]. Studies in areas of low malaria transmission in South Africa and India showed an association between severe malaria and HIV [76, 79,80,81]. For severe vivax malaria, the current study showed a higher prevalence compared to another study with severe vivax malaria in children and adult patients (23.8 vs 12.6%) [82]. Despite the low number of cases, HIV co-infection seems to exacerbate clinical worsening of vivax malaria, as it has a higher prevalence than that found in vivax malaria mono-infection patients treated at FMT-HVD (~ 14%) [55]. Some studies have shown that the risk of malaria severity increases in HIV patients with a CD4 + T cell count < 200 × 106 cells/L or < 350 cells/μL [14, 80]. In this study, 42.1% of patients with malaria infection had CD4 + T cell counts of less than 200 cells/μL, and one of them had severe malaria.
This study had several limitations. Regarding the systematic review, prevalence studies and those exploring severe clinical outcomes may underestimate the HIV/malaria co-infection, since it is rarely screened in vivax malaria-endemic regions. The comparison of clinical disease dynamics and important outcomes was not possible due to the absence of control groups, e.g., P. vivax mono-infection and HIV mono-infection to address associations of laboratory and clinical outcomes with HIV/PvCo, which was mainly due to the lack of systematic screening. Moreover, there is a low prevalence of severe cases of P. vivax, especially when opportunely diagnosed and treated, or in the absence of co-morbidities. Despite the analysis of the present results, it is not possible to assume that malaria increases anaemia and thrombocytopenia in PLHIV, or vice-versa. Finally, an accurate prevalence of HIV/PvCo, and roughly all other co-infections, is significantly hampered by the absence of a systematic screening, which in low- and middle-income countries is performed at the discretion of a clinician upon clinical suspicion.
Conclusion
Malaria from P. vivax infection appears to have a low prevalence in HIV-infected individuals, with only a few studies describing clinical and laboratory outcomes. This study showed a low prevalence of HIV/PvCo, despite the important local prevalence of vivax malaria and HIV diseases separately. Even though relatively small, thus far this is the largest case series to describe HIV/PvCo. The findings described here shows the need for further research on the interaction between HIV and vivax malaria diseases, due to the potential worsening of both disease conditions in a co-infection scenario, the impact of G6PDd, and the possible epidemiological effects. At present, with a potential increase of severe cases due to the increase of vivax malaria cases and new HIV diagnoses, prospective studies are needed to elucidate aspects related to the pathogenesis of this co-infection, concomitant treatment and drug interactions, severity outcomes, and the possible increased frequency of P. vivax relapses secondary to HIV co-infection. In addition, future studies should address how vivax malaria chronically influences CD4 cells and how this is associated with HIV viral load dynamics.
Abbreviations
- AIDS:
-
Acquired immunodeficiency syndrome
- ART:
-
Antiretroviral treatment for HIV/AIDS
- CD4:
-
CD4 + T-cells
- EMR:
-
Electronic medical record
- G6PD:
-
Glucose-6-phosphate dehydrogenase
- FMT-HVD:
-
Fundação de Medicina Tropical Dr. Heitor Vieira Dourado
- HIV:
-
Human immunodeficiency virus
- HIV/PfCo:
-
HIV-Plasmodium falciparum co-infection
- HIV/PvCo:
-
HIV-Plasmodium vivax co-infection
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RDT:
-
Rapid diagnostic tests
- SIVEP:
-
National epidemiological surveillance system for malaria
- TB:
-
Tuberculosis
- WHO:
-
World Health Organization
References
Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors. 2013;6:8.
WHO. World Malaria Report 2019. Geneva, World Health Organization, 2019.
Gething PW, Elyazar IRF, Moyes CL, Smith DL, Battle KE, Guerra CA, et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814.
Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, et al. Global epidemiology of Plasmodium vivax. Am J Trop Med Hyg. 2016;95(Suppl):15–34.
UNAIDS data 2019; 2019.
WHO. Malaria in HIV/AIDS patients. Geneva, World Health Organization, 2017.
Nyabadza F, Bekele BT, Rúa MA, Malonza DM, Chiduku N, Kgosimore M. The implications of HIV treatment on the HIV-malaria coinfection dynamics: a modeling perspective. Biomed Res Int. 2015;2015:14.
Van Geertruyden J, Mulenga M, Mwananyanda L, Chalwe V, Moerman F, Chilengi R, et al. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J Infect Dis. 2006;194:917–25.
Geertruyden JPV, Mulenga M, Kasongo W, Polman K, Colebunders R, Kestens L, et al. CD4 T-cell count and HIV-1 infection in adults with uncomplicated malaria. J Acquir Immune Defic Syndr. 2006;43:363–7.
Chandramohan D, Greenwood BM. Is there an interaction between human immunodeficiency virus and Plasmodium falciparum? Int J Epidemiol. 1998;27:296–301.
Munyenyembe AU, Gausi K, Nyirenda TS, Hiestand J, Mallewa J, Mandala W. HIV infection has a profound effect on hematological factors but not on electrolyte profile of Malawian adults presenting with uncomplicated malaria and severe malaria. J Blood Med. 2018;9:153–62.
Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in Sub-Saharan Africa. Science. 2006;314:1603–6.
Sourabie YS, Zongo ROS. Coinfection HIV and malaria in Department of Paediatrics of the University Hospital Souro Sanou. J Hematol Thrombo Dis. 2015;3:213.
Chavale H, Santos-Oliveira JR, Da-Cruz AM, Enosse S. Enhanced T cell activation in Plasmodium falciparum malaria-infected human immunodeficiency virus-1 patients from Mozambique. Mem Inst Oswaldo Cruz. 2012;107:985–92.
Ned RM, Moore JM, Chaisavaneeyakorn S, Udhayakumar V. Modulation of immune responses during HIV-malaria co-infection in pregnancy. Trends Parasitol. 2005;21:284–91.
Franke MF, Spiegelman D, Ezeamama A, Aboud S, Msamanga GI, Mehta S, et al. Malaria parasitemia and CD4 T cell count, viral load, and adverse HIV outcomes among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2010;82:556–62.
Tagoe DNA, Boachie J. Assessment of the impact of malaria on cd4 + T Cells and haemoglobin levels of HIV-malaria co-infected patients. J Infect Dev Ctries. 2011;6:660–3.
WHO. Malaria and HIV interactions and their implications for public health policy. Geneva, World Health Organization; 2005.
White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–92.
Byakika-Kibwika P, Ddumba E, Kamya M. Effect of HIV-1 infection on malaria treatment outcome in Ugandan patients. Afr Health Sci. 2007;7:86–92.
Kredo T, Mauff K, Van Der Walt JS, Wiesner L, Maartens G, Cohen K, et al. Interaction between artemether–lumefantrine and nevirapine-based antiretroviral therapy in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55:5616–23.
Troye-Blomberg M, Berzins K. Immune interactions in malaria co-infections with other endemic infectious diseases: implications for the development of improved disease interventions. Microbes Infect. 2008;10:948–52.
Byakika-Kibwika P, Lamorde M, Mayito J, Nabukeera L, Namakula R, Mayanja-Kizza H, et al. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012;67:2213–21.
Kublin JG, Patnaik P, Jere CS, Miller WC, Hoffman IF, Chimbiya N, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365:233–40.
Patnaik P, Jere CS, Miller WC, Hoffman IF, Wirima J, Pendame R, et al. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis. 2005;192:984–91.
Tay SCK, Badu K, Mensah AA, Gbedema SY. The prevalence of malaria among HIV seropositive individuals and the impact of the co-infection on their hemoglobin levels. Ann Clin Microbiol Antimicrob. 2015;14:4–11.
Hochman S, Kim K. The impact of HIV and malaria coinfection: what is known and suggested venues for further study. Interdiscip Perspect Infect Dis. 2009;2009:1–8.
Ministério da saúde.Protocolo Clínico e Diretrizes Terapêuticas para Atenção Integral às Pessoas com Infecções Sexualmente Transmissíveis. 2da edição. Brasilia, Brasil, Ministério da Saúde, 2015.
Ministério da saúde. Protocolo clínico e diretrizes terapêuticas para manejo da infecção pelo HIV em adultos. Brasilia, Brasil, Ministério da Saúde, 2018.
Ministério da Saúde. Guia Prático de Tratamento da Malária no Brasil. 1a edição. Brasilia Brasil, Ministério da Saúde, 2010.
Ministério da saúde. Manual técnico para o diagnóstico da infecção pelo HIV em adultos e crianças. 4a Edição. Brasilia, Brasil, Ministério da Saúde, 2018.
WHO. Guidelines for the treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
WHO. Severe malaria section 1: epidemiology of severe falciparum malaria. Trop Med Int Health. 2014;19(Suppl 1):7–131.
Katongole-Mbidde E, Banura C, Kizito A. Blackwater fever caused by Plasmodium vivax infection in the acquired immune deficiency syndrome. BMJ. 1988;296:827.
McIver LJ, Kippin AN, Parish ST, Whitehead OG. HIV, malaria and pneumonia in a Torres Strait Islander male—a case report. Commun Dis Intell Q Rep. 2010;34:448–9.
Ranaweera D, Kanchana Rajapaksha RMJ, Silva P, Hettiarachchi R, de Gunasekera WMKT, AW, Herath H, , et al. Severe Plasmodium vivax malaria, HIV, tuberculosis co-infection in a Sri Lankan traveller: case management and challenges during the prevention of malaria reintroduction phase. Malar J. 2018;17:429.
Montenegro-Idrogo JJ, Vargas-Gonzales R, Sihuincha M. Malaria en pacientes con infección por VIH: serie de casos en un hospital peruano. Rev Peru Med Exp Salud Publi. 2019;36:520.
Kwenti TE. Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies. Res Rep Trop Med. 2018;9:123–36.
Saracino A, Nacarapa EA, Da Costa Massinga ÉA, Martinelli D, Scacchetti M, De Oliveira C, et al. Prevalence and clinical features of HIV and malaria co-infection in hospitalized adults in Beira. Mozambique Malar J. 2012;11:241.
Naing C, Sandhu NK, Wai VN. The effect of malaria and HIV co-infection on anemia. Medicine (Baltimore). 2016;95:e3205.
Volsky DJ, Wu YT, Stevenson M, Dewhurst S, Sinangil F, Merino F, Rodrigues L. Antibodies to HTLV-III/LAV in Venezuelan patients with acute malarial infections. N Engl J Med. 1986;6(314):647–8.
Lo SS, de Andrade JC, Condino ML, Alves MJ, Semeghini MG, Galvão EC. Malária em usuários de drogas de administração endovenosa associada à soropositividade para HIV. Rev Saude Publi. 1991;25:17–22.
Barata LCB, Andriguetti MTM, de Matos MR. Surto de malária induzida entre usuários de drogas injetáveis. Rev Saude Publi. 1993;27:9–14.
Erhabor O, Babatunde S, Uko KE. Some haematological parameters in plasmodial parasitized HIV-infected Nigerians. Niger J Med. 2006;15:52–5.
Bharti AR, Saravanan S, Madhavan V, Smith DM, Sharma J, Balakrishnan P, et al. Correlates of HIV and malaria co-infection in Southern India. Malar J. 2012;11:306.
Wondimeneh Y, Ferede G, Atnafu A, Muluye D. HIVmalaria co-infection and their immunohematological profiles. Eur J Exp Biol. 2013;3:497–502.
Douglas NM, Pontororing GJ, Lampah DA, Yeo TW, Kenangalem E, Poespoprodjo J, et al. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua. Indonesia BMC Med. 2014;12:217.
Rattanapunya S, Kuesap J, Chaijaroenkul W, Rueangweerayut R, Na-Bangchang K. Prevalence of malaria and HIV coinfection and influence of HIV infection on malaria disease severity in population residing in malaria endemic area along the Thai-Myanmar border. Acta Trop. 2015;145:55–60.
Mohapatra PK, Pachuau E, Kumar C, Borkakoty B, Zomawia E, Singh A, et al. HIV–malaria interactions in North-East India: a prospective cohort study. Indian J Med Res. 2017;145:387–94.
Sahle T, Yemane T, Gedefaw L. Effect of malaria infection on hematological profiles of people living with human immunodeficiency virus in Gambella, southwest Ethiopia. BMC Hematol. 2017;17:2.
Wondimeneh Y, Gebrecherkos T, Muluye D, Damtie D, Ferede G. HIV and malaria infections and associated risk factors among febrile illness patients in Northwest Ethiopia. Turkiye parazitolojii Derg. 2018;42:180–6.
Siqueira AM, Mesones-Lapouble O, Marchesini P, de Souza SV, Brasil P, Tauil PL, et al. Plasmodium vivax landscape in Brazil: scenario and challenges. Am J Trop Med Hyg. 2016;95:87–96.
Siqueira AM, Lacerda MVG, Magalhães BML, Mourão MPG, Melo GC, Alexandre MAA, et al. Characterization of Plasmodium vivax-associated admissions to reference hospitals in Brazil and India. BMC Med. 2015;13:15.
Sampaio VS, Siqueira AM, Alecrim das MGC, Mourão MPG, Marchesini PB, Albuquerque BC, et al. Malaria in the State of Amazonas: a typical Brazilian tropical disease influenced by waves of economic development. Rev Soc Bras Med Trop. 2015;48:4–11.
Ministério da Saúde. Boletim Epidemiológico HIV/Aids-2019. Brasilia; Brasil. Ministério da Saúde, 2019.
Rothenberg RB, Dai D, Adams MA, Heath JW. The human immunodeficiency virus endemic: maintaining disease transmission in at-risk urban areas. Sex Transm Dis. 2017;44:71–8.
De ORDSM, Benzaken AS, Saraceni V, Sabidó M. HIV/AIDS epidemic in the State of Amazonas: characteristics and trends from 2001 to 2012. Rev Soc Bras Med Trop. 2015;48(Suppl 1):70–8.
Vitoria M, Granich R, Gilks CF, Gunneberg C, Hosseini M, Were W, et al. The global fight against HIV/AIDS, tuberculosis, and malaria: current status and future perspectives. Am J Clin Pathol. 2009;131:844–8.
Irene AA, Enekembe MA, Meriki HD, Fonkeng NF, Nkuo-Akenji T. The effect of malaria/HIV/TB triple infection on malaria parasitaemia, haemoglobin levels, CD4+ cell and acid fast bacilli counts in the South West Region of Cameroon. J Infect Pulm Dis. 2016;2:2–5.
Cappellini M, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74.
Pornprasert S, Panya A, Cheepsunthorn C, Srithep S, Kingkeow D. HAART has no major impact on hematological and plasma bilirubin changes in HIV-infected patients with congenital G-6-PD deficiency. Curr HIV Res. 2013;11:193–7.
Wiesch JS, Wichmann D, Hofer A, Van Lunzen J, Burchard GD, Schmiedel S. Primary HIV infection presenting as haemolytic crisis in a patient with previously undiagnosed glucose 6-phosphate dehydrogenase deficiency. AIDS. 2008;22:1886–8.
Xu JZ, Francis RO, Nadal LE, Shirazi M, Jobanputra V, Hod EA, et al. G6PD deficiency in an HIV clinic setting in the Dominican Republic. Am J Trop Med Hyg. 2015;93:722–9.
Coco-Bassey SB, Asemota EA, Okoroiwu HU, Etura JE, Efiong EE, Inyang IJ, et al. Glutathione, glutathione peroxidase and some hematological parameters of HIV-seropositive subjects attending clinic in University of Calabar teaching hospital, Calabar. Nigeria BMC Infect Dis. 2019;8:944.
Monteiro WM, Val FFA, Siqueira AM, Franca GP, Sampaio VS, Melo GC, et al. G6PD deficiency in Latin America: systematic review on prevalence and variants. Mem Inst Oswaldo Cruz. 2014;109:553–68.
Brito-Sousa JD, Santos TC, Avalos S, Fontecha G, Melo GC, Val F, et al. Clinical spectrum of primaquine-induced hemolysis in G6PD deficiency: a nine-year hospitalization-based study from the Brazilian Amazon. Clin Infect Dis. 2019;69:1440–2.
WHO. Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale malaria Global Malaria Programme. Geneva, World Health Organization, 2016.
Berg A, Patel S, Langeland N, Blomberg B. Falciparum malaria and HIV-1 in hospitalized adults in Maputo, Mozambique: does HIV-infection obscure the malaria diagnosis? Malar J. 2008;7:252.
Onyenekwe CC, Ukibe N, Meludu SC, Ilika A, Aboh N, Ofiaeli N, et al. Prevalence of malaria as co-infection in HIV-infected individuals in a malaria endemic area of southeastern Nigeria. J Vector Borne Dis. 2007;44:250–4.
Marques MM, Costa MRF, Santana Filho FS, Vieira JLF, Nascimento MTS, Brasil LW, et al. Plasmodium vivax chloroquine resistance and anemia in the western Brazilian Amazon. Antimicrob Agents Chemother. 2014;58:342–7.
Araujo CF, Lacerda MVG, Abdalla DSP, Lima ES. The role of platelet and plasma markers of antioxidant status and oxidative stress in thrombocytopenia among patients with vivax malaria. Mem Inst Oswaldo Cruz. 2008;103:517–21.
Lacerda MVG, Mourão MPG, Coelho HCC, Santos JB. Thrombocytopenia in malaria: who cares? Mem Inst Oswaldo Cruz. 2011;106:52–63.
Jegede FE, Oyeyi TI, Abdulrahman SA, Mbah HA, Badru T, Agbakwuru C, et al. Effect of HIV and malaria parasites coinfection on immune-hematological profiles among patients attending anti-retroviral treatment (ART) clinic in Infectious Disease Hospital Kano. Nigeria PLoS One. 2017;12:e0174233.
Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. Childhood malaria in a region of unstable transmission and high human immunodeficiency virus prevalence. Pediatr Infect Dis J. 2003;22:1057–63.
Otieno RO, Ouma C, Ongecha JM, Keller CC, Were T, Waindi EN, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20:275–80.
Diallo AH, Ki-Zerbo G, Sawadogo AB, Guiguemde TR. Severe malaria and HIV in adult patients in Bobo-Dioulasso, Burkina Faso (in French). Med Trop (Mars). 2004;64:345–50.
Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS. 2004;18:547–54.
Cohen C, Karstaedt A, Frean J, Thomas J, Govender N, Prentice E, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis. 2005;41:1631–7.
Khasnis AAKD. Human immunodeficiency virus type 1 infection in patients with severe falciparum malaria in urban India. J Postgrad Med. 2003;49:114–7.
Siqueira AM, Cavalcante JA, Vítor-Silva S, Reyes-Lecca RC, Alencar AC, Monteiro WM, et al. Influence of age on the haemoglobin concentration of malaria-infected patients in a reference centre in the Brazilian Amazon. Mem Inst Oswaldo Cruz. 2014;109:569–76.
Ramírez-Olivencia G, Herrero MD, Subirats M, De Juanes JR, Peña JM, Puente S. Paludismo importado e infección por VIH en Madrid. Perfil clínico y epidemiológico. Rev Clin Esp. 2011;212(1):10–7. https://doi.org/10.1016/j.rce.2011.07.016.
Tano ZN, Filho CEK, Breganó RM, Pavanelli WR, Ruzon UG. Hyperreactive malarious splenomegaly and aids: a case report. Brazilian J Infect Dis. 2014;18(5):565–7. https://doi.org/10.1016/j.bjid.2014.04.005.
Acknowledgements
Not applicable.
Funding
This research was funded by the Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEM): Universal Amazonas (#002/2018), Pro-Estado Program (#002/2008, #007/2018, #005/2019) and POSGRAD 2020 Program (#006/2020). WM and ML are CNPq fellows. The funders had no role in study design, data collection, manuscript preparation, or decision to publish.
Author information
Authors and Affiliations
Contributions
PLDT, NCV, CCG and JV were responsible for the data collection from medical records. PLDT and NCV performed the systematic review. PLDT, BMS, DCC, VSS and FV performed the statistical analysis and wrote the first manuscript draft. PLDT, DCC, VSS, AMS, FEME, MVGL, WMM and FV participated in study design, coordination and elaborated the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Review Board (ERB) at the FMT-HVD approved this study, which followed the guidelines and standards for regulating research on human subjects established in Resolution 466/12, of the National Health Council of the Brazilian Ministry of Health. A waiver of informed consent was obtained due to the retrospective nature of the study. Patient anonymity was preserved throughout the analysis.
Consent for publication
All authors have given consent for publication.
Availability of data and material
All data generated from this study are included in the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Del-Tejo, P.L., Cubas-Vega, N., Caraballo-Guerra, C. et al. Should we care about Plasmodium vivax and HIV co-infection? A systematic review and a cases series from the Brazilian Amazon. Malar J 20, 13 (2021). https://doi.org/10.1186/s12936-020-03518-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-020-03518-9