Abstract
Background
Anti-malarial resistance is, and continues to be a significant challenge in the fight against malaria and a threat to achieving malaria elimination. In Zambia, chloroquine (CQ), a safe, affordable and well-tolerated drug, was removed from use in 2003 due to high levels of resistance evidenced with treatment failure. This study sought to investigate the prevalence of chloroquine resistance markers in Southern and Western Provinces of Zambia 14 years after the withdrawal of CQ.
Methods
Data from a cross-sectional, all-age household survey, conducted during the peak malaria transmission season (April–May 2017) was analysed. During the all-age survey, socio-demographic information and coverage of malaria interventions were collected. Consenting individuals were tested for malaria with a rapid diagnostic test and a spot of blood collected on filter paper to create a dried blood spot (DBS). Photo-induced electronic transfer–polymerase chain reaction (PET–PCR) was used to analyse the DBS for the presence of all four malaria species. Plasmodium falciparum positive samples were analysed by high resolution melt (HRM) PCR to detect the presence of genotypic markers of drug resistance in the P. falciparum chloroquine resistance transporter (Pfcrt) and P. falciparum multi-drug resistance (Pfmdr) genes.
Results
A total of 181 P. falciparum positive samples were examined for pfcrt K76T and MDR N86. Of the 181 samples 155 successfully amplified for Pfcrt and 145 for Pfmdr N86. The overall prevalence of CQ drug-resistant parasites was 1.9% (3/155), with no significant difference between the two provinces. No N86Y/F mutations in the Pfmdr gene were observed in any of the sample.
Conclusion
This study reveals the return of CQ sensitive parasites in Southern and Western Provinces of Zambia 14 years after its withdrawal. Surveillance of molecular resistant markers for anti-malarials should be included in the Malaria Elimination Programme so that resistance is monitored country wide.
Similar content being viewed by others
Background
The goal of the Zambia National Malaria Elimination Programme is to eliminate malaria by 2021, through effective and sustained coverage of proven vector control interventions, which include indoor residual spraying (IRS), distribution of long-lasting insecticide-treated nets (LLINs) and larval source management (LSM), combined with prompt effective case management, health promotion, surveillance and research [1]. While the malaria map has been shrinking over the years, there are a number of challenges in maintaining control or progressing to elimination, that include: access to treatment for most at risk and hard to reach populations; insecticide resistance; residual or outdoor transmission and drug resistance, specifically the threat of resistance to artemisinin and artemisinin-based combination therapy [2].
Anti-malarial drug resistance is defined as the ability of Plasmodium parasite strain to survive and/or multiply despite the administration and absorption of a drug in a dose equal to or higher than the recommended dose, but within tolerance of the human subject [3]. Resistance to chloroquine (CQ) in P. falciparum parasites is predominantly linked to a single mutation in the P. falciparum transporter gene (Pfcrt) on chromosome 7, which encodes a protein localized on the parasite digestive vacuole (DV) membrane. The replacement of lysine (K) at position 76 to a threonine (T), i.e. the K76T mutation, has been established as the most important prognostic marker of treatment failure [4, 5].
CQ acts against the intra-erythrocytic (trophozoite and schizont) stages of the parasite that are responsible for the clinical manifestation of the disease. The intra-erythrocytic stages digest erythrocytic haemoglobin in acidic vacuoles, releasing toxic haem as a by-product which is then biocrystallized into non-toxic haemozoin [6]. CQ interrupts this detoxification process [7], thus poisoning the parasite [8]. CQ resistant (CQR) P. falciparum parasites survive by reducing the accumulation of CQ in the food digestive vacuole thus inhibiting haemozoin formation [6, 9].
Another point mutation N86Y in P. falciparum multidrug resistance gene 1 (Pfmdr1), on chromosome 5, that encodes a P-glycoprotein homologue and is located on the parasite DV membrane has also been implicated in CQ resistance [4, 5]. The exact mechanism by which this is achieved remains unknown, it is likely mediated by an ATP dependent transporter [9].
However, the mutation in Pfcrt is required to confer a basic level of resistance before mutations in Pfmdr1 can have an effect [10].
CQ was introduced in 1940 and by 1950 it had become the most widely used anti-malarial. It was the mainstay for presumptive treatment and mass drug administration during the era of malaria eradication campaigns; while in tropical Africa where there was no systematic campaign it effectively replaced quinine in the 1970s [11, 12]. From the early 1980s, there had been observed decreases in CQ efficacy, and increased number of clinical recrudescence and treatment failures [13,14,15,16]. Therapeutic efficacy studies in Zambia [17] and other neighbouring countries [12] also provided evidence for the resistance. This led to Zambia becoming the first African country to abandon CQ and adopt artemether–lumefantrine (AL) as the first-line treatment nationwide in 2002 [18].
Over the years following the withdrawal of CQ, a number of countries, e.g. China in 2017 [19], Malawi in 2014 [20] and parts of Zambia in 2016 [21] have reported the return of CQ susceptibility, potentially raising the prospect of its reintroduction in the future. This study, therefore, aimed to assess the status of CQ resistance marker in Western and Southern Provinces, Zambia to determine whether CQ sensitivity has continued to return.
Methods
Study area
Western and Southern Provinces cover areas of approximately 126,000 km2 and 86,000 km2, respectively, representing ~ 28% of the total Zambia landmass. They are home to approximately 0.9 and (Western) 1.6 (Southern) million people according to the 2010 population census. The Zambezi River flows through both provinces and the plains cover an area of about 10% of the total area of the Western Province. The predominant ethnic groups are the Tonga speaking people in Southern Province and the Lozi speaking people in Western Province [22, 23].
Study design
This study aimed to assess the levels of CQ resistance in selected parts of Western and Southern Provinces using samples taken from an all age household cross-sectional survey. The survey was conducted in the two Provinces of Zambia during the peak malaria transmission season in April and May 2017. The Ministry of Health, PATH-Malaria Control and Elimination Partnership in Africa (MACEPA), and other partners carried out this survey as part of the ongoing efforts to evaluate malaria elimination efforts. From the survey, a subset of known malaria test RDT positive samples were analysed for Pfcrt and Pfmdr markers. In the two provinces sampled, malaria transmission varies greatly, with traditionally higher transmission intensity in Southern Province along Lake Kariba and in areas of Western Province around the swamps and wetland in Luampa, Kaoma and Nkeyema districts and along the Zambezi River basin [24,25,26]. The rest of the areas away from water bodies generally have low transmission.
Selected households were visited by field teams consisting of trained survey data collectors using standardized questionnaires based on the 2015 Malaria Indicator Survey (MIS) questionnaire to collect socio-demographic characteristics of household members and information on coverage of malaria interventions. In addition, malaria testing using malaria rapid diagnostic tests (SD Bioline Pf) was done on household members of all ages and dried blood spots were collected on Whatmann No 3 filter paper. Informed consent/assent was obtained from the participants that were included in the study [26, 27]. Samples were analysed at the National Malaria Elimination Centre (NMEC) Laboratory.
Sampling
The survey sampling methods in each province were different due to historical studies and enumeration in the two provinces. In Southern Province, sampling of households was done randomly based on a pre-existing sampling frame used during a previously-implemented mass drug administration (MDA) trial from the 10 districts along Lake Kariba. This involved the random selection of 52 households from each of the 60 health facility catchment areas used during the trial [25]. For Western Province, with no pre-existing household sampling frame, a 2-stage cluster sampling method using probability proportional to their size (PPS) was employed. Using standardized methods from the MIS and national census data [25, 26], 25 households were selected from 24 standard enumeration areas. Across both surveys, a total sample size of 6977 people was attained. For this study, after excluding clusters with zero prevalence, 13 clusters were randomly selected and all the1567 (22% of total samples collected in the 13 clusters) samples screened for Plasmodium species by PET–PCR. A total of 266/1567 (16.9%) were P. falciparum positive. Due to cost of processing, 181 of these positives were randomly selected for the presence of Pfcrt K76T and Pfmdr N86Y/F.
Laboratory methods
DNA extraction
DNA was extracted either individually or in pools using Qiagen DNA mini kit (Qiagen, Germany) using a 6 mm punch (~ 13.8 µl whole blood) taken from each DBS according to the manufacturer’s instructions with modifications. All RDT positive samples were extracted individually, while RDT negatives were pooled in groups of 10 and the positive pools were deconvoluted.
PET–PCR
This was performed as previously described by Lucchi et al. in 2013 [28]. Briefly, 5 µl of DNA template, 2X TaqMan Environmental Master Mix 2.0 (Applied BioSystems, Life Technology LTD, Warrington, UK) and 250 nM each forward and reverse primers were amplified in a 20 µl reaction volume as follows; initial hot-start at 95 °C for 15 min, followed by 45 cycles of denaturation at 95 °C for 20 s, annealing and extension at 60 °C for 40 s. Samples were tested in duplicate and scored positive if both duplicates had a critical threshold (CT) value < 40 [29, 30].
Pre-amplification
Plasmodium falciparum positive samples were pre-amplified using a Pre-amplification master mix (Life Technologies, Inc, Grand Island, NY, USA) and a mixture of the pooled primers in a 10 µl (CT > 35) or 20 µl (CT < 35) reaction volume to enhance the template concentration. The following amplification conditions were used: pre-incubation at 95 °C for 2 min, amplification at 95 °C for 15 s and 60 °C for 4 min and final extension 60 °C for 15 s.
Parasite genotyping
pfcrt K76T and pfmdr N86Y mutations were assessed as described previously with minor modifications [31, 32]. Briefly, samples were prepared in a 5 µl reaction volume consisting of 2µl Lightscanner Master Mix (BioFire Diagnostics, Salt city, UT, U.S.A), 2.5 µl pre-amplified template, and 0.5 µl of primers and probes. The primers were in an asymmetric forward to reverse ratio of 1:5 (0.5 µM forward excess primer-, 0.1 µM reverse limiting primer, and 0.4 µM of the mismatched oligonucleotide at the end as a probe), and then amplified as follows amplification conditions 95 °C denaturation for 2 min, 50 cycles of 94 °C for 5 s and 66 °C for 30 s, and a pre-melt cycle of 5 s each at 95 °C and 37 °C. The change in fluorescence was recorded over a temperature range from 40 °C to 90°C on the Light Cycler 480 system (Roche Diagnostics, Applied Science, Germany) and analysed using the Call-It module within the Light Cycler software.
Statistical analysis
Demographic and laboratory data of participants records were analysed using Stata version 13 (College Station, Texas, USA).
Ethical clearance
Ethical clearance was obtained from the Regional Committee for Medical and Health Research Ethics (REC Western Norway) Ref no. 2016/1393/REK Vest and from the University of Zambia Biomedical Research Ethics Committee (UNZABREC) Ref no. 010-05-16. As this analysis was part of a larger study, ethical clearance for the larger study was also obtained from UNZABREC Ref no. 007-03-14. Permission to use data was obtained from the Ministry of Health. All data analysed were anonymized.
Results
Of the 181 samples, 155 (85.6%) were successfully amplified. Table 1 shows the demographic characteristics of the study participants associated with these samples. There were 72 (46.5%) males and 83 (53.5%) females and the mean age was 20.8 years with a range from 1 year to 93 years.
Prevalence of Pfcrt K76T and Pfmdr N86Y
For Pfcrt, only 1 (0.6% 95% CI 0.02, 3.5) of the samples had the resistant allele with 2 (1.3% 95% CI 0.2, 4.6) samples having both resistant and sensitive alleles. Resistance, described, in this paper, as any sample containing a resistant alleles, was found in 3 samples [1.9% (95% CI 0.4, 0.6)]. For Pfmdr N86Y/F, all the 145 (100%) samples that successfully amplified had the sensitive allele (Table 2).
Prevalence of Pfcrt K76T by province
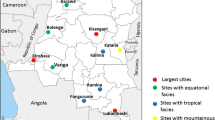
CQ resistance markers prevalence in Western Province was 2/122 [1.6% (0.2–5.7%)] while in Southern Province it was 1/29 [3.4% (0.08–17.2)] (Fig. 1). The sample from Southern Province only contained the resistant allele, while the two from Western Province were mixed, i.e. containing a copy of both sensitive and resistant alleles. Figure 2 shows the district where these samples carrying resistant markers came from.
Discussion
Choloroquine (CQ) was a highly effective and cheap anti-malarial whose utility was drastically reduced through the evolution of anti-malarial drug resistance. This study reveals the return of CQ susceptible P. falciparum parasites in Southern and Western provinces of Zambia 14 years following the withdrawal of chloroquine as first line treatment in 2003. In this study the overall genotypic prevalence of resistant parasites was 1.9%, with no difference between Southern (3.4%) and Western (1.6%) provinces. A total of 3 infections (1.9%) contained the resistant genotype, however two of these samples were also carrying a sensitive allele, suggesting that this allele has almost reached fixation in the population. In contrast, there were no Pfmdr N86Y resistant alleles observed in the study. This mutation is a good indicator of treatment outcome as observed in a previous study where 68% of samples containing this mutation were associated with treatment failure [33].
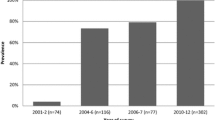
These results mirror those observed in Northern Zambia, Malawi and Kenya [21, 34,35,36]. In Zambia, while not linear, there has been a general reduction in the prevalence of the chloroquine resistance genotypes over time. From a high of 95% resistance in Macha in 2001 (unpublished report as cited by Chileshe et al. [33]). This is seen from the gradual decrease observed from a multi-country study whose site in Zambia was from Ndola, Copperbelt province. It was conducted around 2004–2006 and 2006–2007 and revealed that resistance was around 20–30% [37]. On the other hand, around the same period, samples collected from Lusaka revealed 54% in 2006–2007 [38]. A further reduction to 14.8% was observed from samples collected in Katete and Chipata (unpublished data) and 0% in Nchelenge, Luapula Province, in 2012 [21]. Finally, 2017 samples from Ndola, Copperbelt province identified zero resistance markers [39] (Fig. 3).
The disappearance of CQ resistant parasites appears to be evolving at different rates in the region probably due to co-varying factors such as treatments given; malaria transmission intensity (the rate of recombination among the parasites); and the use of related drugs like Amodiaquine capable of maintaining drug pressure on pfcrt [40]. Amodiaquine has never been used in Zambia, but it has been the second-line treatment for malaria in neighbouring countries like Tanzania, whose nationals often travel to Zambia. As people travel from one place to another they import parasites [41]. In addition, infected individuals may carry resistant parasites; and these travellers could come with drugs from the neighbouring country that could alter the extent of drug pressure [42], these drugs could be shared with the Zambians they are visiting.
This re-emergence of chloroquine sensitive P. falciparum parasites, also called ‘chemo-reversion’, is interpreted as a result of the absence of chloroquine drug pressure leading to the rapid re-expansion of susceptible parasites that had survived in some malaria semi-immune individuals in the population during that period of chloroquine use [34]. Furthermore, it has been suggested that the large-scale use of lumefantrine may have accelerated chemo-reversion by placing a selective pressure towards wild type Pfcrt alleles [40].
This reduction of chloroquine resistant parasites could present a fresh chance to reintroduce chloroquine for malaria prevention especially in key vulnerable populations, as it is safe, well-tolerated in pregnancy at all stages and in children; and long-acting [21]. This could replace sulfadoxine-pyrimethamine and should be introduced as a combination therapy with artesunate or with other short acting drugs to extend the useful lifespan of each of the drugs [43]. In light of the development of drug resistance, a short-term periodical alternate use of combinations with chloroquine may be used to reduce the development of resistance.
Study limitations
These results provide more information of the current state of pfcrtK76T allele in Western and Southern Provinces of Zambia which will be referred to when discussing anti-malarial resistance in Zambia. However, these results should be interpreted with caution: first, the samples size for the low transmission area was small making comparison difficult. Therefore, studies are required with a larger sample size that would provide more accurate estimates and comparisons of low and high transmission areas, especially after a long period of being low transmission areas in a place like Southern Province. Second, survey sampling in the two provinces was done differently making comparisons between the two more challenging. Finally, PCR bias cannot be ruled out completely as 26 samples did not amplify despite undergoing the pre-amplification process, this could have led to an under-or over-estimation of the prevalence.
Conclusion and recommendation
This study suggests CQ sensitive alleles dominate the genetic landscape in Southern (low transmission) and Western (high transmission) Provinces of Zambia 14 years after the withdrawal of CQ. Routine national surveillance of molecular resistant markers for anti-malarial drugs should be included in the National Malaria Elimination Programmes as it strives for elimination. This surveillance should be countrywide and samples from patients visiting health facilities should be included. Armed with this data, the programme can better understand the genetic challenges, and opportunities, for delivering prompt, effective treatment and chemo-protection.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACT:
-
artemisinin-based combination therapy
- AL:
-
artemether–lumefantrine
- Asn:
-
asparagine
- CQ:
-
chloroquine
- DBS:
-
dried blood spot
- DNA:
-
deoxyribonucleic acid
- ITNs:
-
insecticide-treated nets
- IRS:
-
indoor residue spraying
- LLITNs:
-
long-lasting insecticide treated nets
- Lys:
-
lysine
- LSM:
-
larval source management
- NPV:
-
negative predictive value
- NMEC:
-
National Malaria Elimination Centre
- NMCP:
-
National Malaria Control Programme
- MACEPA:
-
Malaria Control and Elimination Partnership in Africa
- MDA:
-
mass drug administration
- MIS:
-
Malaria Indicator Survey
- PCR:
-
polymerase chain reaction
- RDTs:
-
rapid diagnostic tests
- Thr:
-
thyrosine
- SMC:
-
seasonal malaria chemotherapy
References
MOH. National malaria elimination strategic plan 2017–2021. Lusaka: National Malaria Elimination Centre; 2017.
Guyant P, Corbel V, Guerin PJ, Lautissier A, Nosten F, Boyer S, et al. Past and new challenges for malaria control and elimination: the role of operational research for innovation in designing interventions. Malar J. 2015;14:279.
Bloland P. Drug resistance in malaria. Geneva: World Health Organization; 2001.
Djimdé A, Doumbo OK, Steketee RW, Plowe CV. Application of a molecular marker for surveillance of chloroquine-resistant falciparum malaria. Lancet. 2001;358:890–1.
Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–9.
Sanchez CP, Wunsch S, Lanzer M. Identification of a chloroquine importer in Plasmodium falciparum. Differences in import kinetics are genetically linked with the chloroquine-resistant phenotype. J Biol Chem. 1997;272:2652–8.
Chou AC, Chevli R, Fitch CD. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980;19:1543–9.
Dorn A, Vippagunta SR, Matile H, Jaquet C, Vennerstrom JL, Ridley RG. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem Pharmacol. 1998;55:727–36.
Ursos LM, Dzekunov SM, Roepe PD. The effects of chloroquine and verapamil on digestive vacuolar pH of P. falciparum either sensitive or resistant to chloroquine. Mol Biochem Parasitol. 2000;110:125–34.
Warhurst DC. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:299–302.
Nuwaha F. The challenge of chloroquine-resistant malaria in sub-Saharan Africa. Health Policy Plan. 2001;16:1–12.
Bloland PB, Lackritz EM, Kazembe PN, Were JB, Steketee R, Campbell CC. Beyond chloroquine: implications of drug resistance for evaluating malaria therapy efficacy and treatment policy in Africa. J Infect Dis. 1993;167:932–7.
Bruce-Chwatt LJ. Chloroquine resistance in Zambia. BMJ. 1978;2:206.
Bijl HM, Kager J, Koetsier DW, van der Werf TS. Chloroquine- and sulfadoxine-pyrimethamine-resistant Falciparum malaria in vivo—a pilot study in rural Zambia. Trop Med Int Health. 2000;5:692–5.
Ekue J, Ulrich A-M, Njelesani E. Plasmodium malaria resistant to chloroquine in a Zambian living in Zambia. BMJ. 1983;286:1315.
Blom GJ, Baboo KS, Athale UH, van der Werf TS. Plasmodium falciparum malaria in vivo drug sensitivity in Lusaka, Zambia. Cent Afr J Med. 1995;41:6–10.
Hamer DH, MacLeod WB, Addo-Yobo E, Duggan CP, Estrella B, Fawzi WW, et al. Age, temperature, and parasitaemia predict chloroquine treatment failure and anaemia in children with uncomplicated Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2003;97:422–8.
Sipilanyambe N, Simon JL, Chanda P, Olumese P, Snow RW, Hamer DH. From chloroquine to artemether–lumefantrine: the process of drug policy change in Zambia. Malar J. 2008;7:25.
Lu F, Zhang M, Culleton RL, Xu S, Tang J, Zhou H, et al. Return of chloroquine sensitivity to Africa? Surveillance of African Plasmodium falciparum chloroquine resistance through malaria imported to China. Parasit Vectors. 2017;10:355.
Frosch AE, Laufer MK, Mathanga DP, Takala-Harrison S, Skarbinski J, Claassen CW, et al. Return of widespread chloroquine-sensitive Plasmodium falciparum to Malawi. J Infect Dis. 2014;210:1110–4.
Mwanza S, Joshi S, Nambozi M, Chileshe J, Malunga P, Kabuya JB, et al. The return of chloroquine-susceptible Plasmodium falciparum malaria in Zambia. Malar J. 2016;15:584.
CSO. Census of population and housing. Lusaka: Southern Province Analytical Report; 2010. p. 2014.
CSO. Census of Population and Housing. Lusaka: Western Province analytical report; 2010. p. 2014.
Eisele TP, Bennett A, Silumbe K, Finn TP, Chalwe V, Kamuliwo M, et al. Short-term impact of mass drug administration with dihydroartemisinin plus piperaquine on malaria in Southern Province Zambia: a cluster-randomized controlled trial. J Infect Dis. 2016;214:1831–9.
Eisele TP, Silumbe K, Finn T, Chalwe V, Kamuliwo M, Hamainza B, et al. Assessing the effectiveness of household-level focal mass drug administration and community-wide mass drug administration for reducing malaria parasite infection prevalence and incidence in Southern Province, Zambia: study protocol for a community randomized controlled trial. Trials. 2015;16:347.
Ministry of Health. Malaria indicator survey 2015 report. Zambia: Lusaka; 2015.
Roll Back Malaria Monitoring and Evaluation Reference Group WHO, United Nations Children’s Fund, Measure DHS, Measure Evaluation, and U.S. Centers for Disease Control and Prevention. Malaria Indicator Survey: Basic documentation for survey design and implementation. Calverton, Maryland 2005.
Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, et al. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET–PCR. PLoS ONE. 2013;8:e56677.
Akerele D, Ljolje D, Talundzic E, Udhayakumar V, Lucchi NW. Molecular diagnosis of Plasmodium ovale by photo-induced electron transfer fluorogenic primers: PET–PCR. PLoS ONE. 2017;12:e0179178.
Lucchi NW, Karell MA, Journel I, Rogier E, Goldman I, Ljolje D, et al. PET–PCR method for the molecular detection of malaria parasites in a national malaria surveillance study in Haiti, 2011. Malar J. 2014;13:462.
Obaldia N, Baro NK, Calzada JE, Santamaria AM, Daniels R, Wong W, et al. Clonal outbreak of Plasmodium falciparum infection in eastern Panama. J Infect Dis. 2015;211:1087–96.
Daniels R, Ndiaye D, Wall M, McKinney J, Sene PD, Sabeti PC, et al. Rapid, field-deployable method for genotyping and discovery of single-nucleotide polymorphisms associated with drug resistance in Plasmodium falciparum. Antimicrob Agents Chemother. 2012;56:2976–86.
Chileshe J, Mbewe BS. The correlation between chloroquine treatment outcomes and the mutation in Pfcrt and Pfmdr1 genes in Zambia. J Life Sci. 2012;6:268–73.
Laufer MK, Takala-Harrison S, Dzinjalamala FK, Stine OC, Taylor TE, Plowe CV. Return of chloroquine-susceptible falciparum malaria in Malawi was a reexpansion of diverse susceptible parasites. J Infect Dis. 2010;202:801–8.
Mwai L, Ochong E, Abdirahman A, Kiara SM, Ward S, Kokwaro G, et al. Chloroquine resistance before and after its withdrawal in Kenya. Malar J. 2009;8:106.
Srimuang K, Miotto O, Lim P, Fairhurst RM, Kwiatkowski DP, Woodrow CJ, et al. Analysis of anti-malarial resistance markers in pfmdr1 and pfcrt across Southeast Asia in the tracking resistance to Artemisinin Collaboration. Malar J. 2016;15:541.
Sagara I, Oduro AR, Mulenga M, Dieng Y, Ogutu B, Tiono AB, et al. Efficacy and safety of a combination of azithromycin and chloroquine for the treatment of uncomplicated Plasmodium falciparum malaria in two multi-country randomised clinical trials in African adults. Malar J. 2014;13:458.
Chaponda E. The prevalence of Plasmodium falciparum point mutations associated with resistance to chloroquine and artemisinin in Lusaka Urdan District. Dissertation. University of Zambia, Biomedical Sciences; 2008.
Kasonde-Chanshika B, Shimaponda-Mataa NM. Profiling chloroquine resistance-associated Pfcrt-76T and Pfmdr1-86Y mutations in Plasmodium falciparum isolates of Ndola, Zambia. In; Zambia National Health Conference. Lusaka: NHRA; 2018:204.
Thomsen TT, Madsen LB, Hansson HH, Tomás EV, Charlwood D, Bygbjerg IC, et al. Rapid selection of Plasmodium falciparum chloroquine resistance transporter gene and multidrug resistance gene-1 haplotypes associated with past chloroquine and present artemether–lumefantrine use in Inhambane District, southern Mozambique. Am J Trop Med Hyg. 2013;88:536–41.
Lowa M, Sitali L, Siame M, Musonda P. Human mobility and factors associated with malaria importation in Lusaka district, Zambia: a descriptive cross sectional study. Malar J. 2018;17:404.
Bridges DJ, Molyneux M, Nkhoma S. Low level genotypic chloroquine resistance near Malawi’s northern border with Tanzania. Trop Med Int Health. 2009;14:1093–6.
Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimde AA, Kouriba B, Taylor TE, Plowe CV. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–5.
Acknowledgements
We acknowledge the role played by PATH Malaria Control and Elimination Partnership in Africa (MACEPA) in providing reagents and the Ministry of Health through the National Malaria Elimination Centre (NMEC) for allowing us to use the samples, the NMEC molecular laboratory team, Conceptor Mulube, Sandra Chishimba, Brenda Mambwe for the assistance rendered during the running of the samples. Further acknowledgement goes to Rachel Daniel, Sara Volkman and Dyann F. Wirth of Harvard University for their role in the optimization and validation of the HRM assay. We also wish to thank the PATH MACEPA team based in Seattle, USA, Maya Fraser and Travis Porter from Tulane University from for helping with data extraction process, Manny Lewis for proof reading the manuscript PATH MACEPA. We also wish to acknowledge Gillie Cheelo of the University of Zambia and for designing the maps.
Funding
This work was funded by Norwegian Loan Scheme Lånakassen and the Zambian Ministry of Health through PATH MACEPA.
Author information
Authors and Affiliations
Contributions
LS conceived the idea, processed the samples and drafted the manuscript, MCM and DJB assisted in the running of the samples and writing the methods and correcting the manuscript, JMM helped design and undertake of the original study and in the extraction and analysis of the data, and also contributed to the writing and correction of the manuscript, MBH and ECK facilitated the running of the samples at the NMEC laboratory and helped in the writing and correction of the manuscript. BL, JC contributed to the design of the study and manuscript correction. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the Regional Committee for Medical and Health Research Ethics (REC Western Norway) Ref No. 2016/1393/REK Vest and from the University of Zambia Biomedical Research Ethics Committee (UNZABREC) Ref No. 010-05-16. As this analysis was part of a larger study, ethical clearance for the larger study was also obtained from UNZABREC. Permission to use data was obtained from the Ministry of Health. All data analysed were anonymized.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sitali, L., Mwenda, M.C., Miller, J.M. et al. En-route to the ‘elimination’ of genotypic chloroquine resistance in Western and Southern Zambia, 14 years after chloroquine withdrawal. Malar J 18, 391 (2019). https://doi.org/10.1186/s12936-019-3031-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-019-3031-4