Abstract
Background
Asthma is characterized by chronic inflammation and airway remodeling. However, limited study is conducted on the gene expression profiles of ovalbumin (OVA) induced asthma in mice. Here, we explored the gene expression profiles in lung tissues from mice with OVA-induced asthma using microarray and bioinformatics analysis.
Methods
For establishment of OVA-induced asthma model, mice first received intraperitoneal sensitization with OVA on day 0, 7 and 14, followed by atomizing inhalation of OVA 3 times a week for 8 weeks. The lung tissues were collected and subjected to microarray analysis, bioinformatics analysis and expression validation.
Results
Microarray data of lung tissues suggested that 3754 lncRNAs and 2976 mRNAs were differentially expressed in lung tissues between control and asthmatic mice, including 1647 up-regulated and 2106 down-regulated lncRNAs, and 1201 up-regulated and 1766 down-regulated mRNAs. GO analysis displayed that the up-regulated genes were enriched in inflammatory response, leukocyte migration involved in inflammatory response, and Notch signaling pathway. KEGG pathway analysis indicated that the enriched pathway terms of the up-regulated gene included Toll-like receptor signaling pathway and Th17 cell differentiation signaling pathway. Additionally, based on the previously published literatures on asthma and inflammation, we screened out down-regulated genes, such as Smg7, Sumo2, and Stat5a, and up-regulated genes, such as Myl9, Fos and Tlr4. According to the mRNA-lncRNA co-expression network, we selected lncRNAs associated with above genes, including the down-regulated lncRNAs of NONMMUT032848, NONMMUT008873, NONMMUT009478, and NONMMUT006807, and the up-regulated lncRNAs of NONMMUT052633, NONMMUT05340 and NONMMUT042325. The expression changes of the above genes were validated in lung tissues by real-time quantitaive PCR and Western blot.
Conclusions
Overall, we performed gene microarray on lung samples from OVA-induced asthmatic mice and summarized core mRNAs and their related lncRNAs. This study may provide evidence for further research on the therapeutic targets of asthma.
Similar content being viewed by others
Background
Bronchial asthma is a common and frequently-occurring disease of the respiratory system, characterized by airway inflammation and airway hyperreactivity (AHR) [1]. It is generally accepted that chronic airway inflammation is one of the important mechanisms leading to AHR, and airway remodeling is the pathological basis causing irreversible AHR and airway obstruction. Ovalbumin (OVA) is one of the main asthmatic allergens. It is commonly used to establish asthma models in animals, models of airway inflammation and remodeling in, which is characterized by airway inflammation, intra-epithelial eosinophil infiltration, TH2 immune response, airway remodeling (such as goblet cell hyperplasia, airway smooth muscle cell (ASMC) hypertrophy and/or hyperplasia, basal membrane thickening, subepithelial fibrosis, bronchial angiogenesis, and collagen deposition), and AHR [2]. However, despite treatment following guidelines, patients with asthma still undergo exacerbations, which may finally lead to the higher disease morbidity and mortality [3, 4]. Overall, asthma remains an important health care problem and a significant financial burden to patients and society. Hence, there is an urgent need to develop better strategies and identify new therapeutic targets for asthma.
Long non-coding RNAs (lncRNAs) are non-protein-coding RNAs with the length of > 200 nt [5]. They can play functions of epigenetic regulation, act as a sequence-specific tether for protein complexes, specify subcellular compartments or localization, and further regulate development, differentiation and disease pathogenesis [6]. Recently, lncRNAs are reported to exert regulatory roles in the airway inflammation of asthmatic subjects [7]. LncRNA BCYRN1 could facilitate the proliferation and migration of ASMCs of asthmatic rat through up-regulation of transient receptor potential [8]. Down-regulating lncRNA-AK149641 in OVA-induced asthmatic mice could alleviate airway inflammation, mucus secretions, decrease expression of interleukin-6 and tumor necrosis factor alpha [9]. LncRNA Malat1 could inhibit the platelet-derived growth factor BB mediated ASMCs proliferation and migration through miR-150-eIF4E/Akt Signaling in the airway remodeling in asthma [10]. Besides, the expression profiles of lncRNAs in CD8 positive T cells were different in different stages of proliferation, demonstrating the association with peripheral CD8 positive T cells in severe asthma [11]. Qiu et al. [12] reported that the lncRNA MEG3 regulated Treg/Th17 balance via targeting miR-17/RORγt in the asthma patients.
At present, microarray analysis on asthma pathologies is emerging. Chen et al. [7] indicated that through the microarray and RNA-sequencing conducted on healthy donor and patients with severe asthma, miR-133a-3p-EFHD2/CNN2-AC144831.1 interactions and miR-3613-3p-CD44/BCL11B-LINC00158/CTA-217C2.1/AC010976.2/RP11-641A6.2 interactions were speculated to involve with the development of severe asthma. Kim et al. [13] demonstrated four interconnected hub genes, FOXO1, RUNX1, SP1 and APP from DNA methylation and gene expression networks in lung tissues of OVA-induced asthma model (15 days) of mice. He et al. [14] performed microarray analysis on endobronchial epithelial brushing samples from asthma patients and conducted validation experiments using house dust mite (HDM)-(14 days) and OVA-induced (26 days) allergic inflammatory asthma model. They suggested that ANO7, PYCR1 and UBE2C might play a central roles in the HDM- and OVA-induced allergic inflammatory asthma. Zhang et al. [9] performed gene microarray analysis on lung tissues of OVA-induced asthmatic mice (30 days), and indicated that the lncRNA-AK149641 may serve as a promising target for the treatment of asthma. However, the microarray analysis on lung tissues from the OVA-induced chronic airway remodeling (8 weeks) mouse model is lacking.
Herein, the purpose of this work was to determine the differentially expressed mRNAs and lncRNAs in lung tissues of OVA-induced asthmatic mice. Gene microarray and bioinformatics analysis, including Gene Ontology (GO) analysis, the Kyoto Encyclopedia of Genes and Genomes (KEGG) database analysis, and co-expression network analysis of lncRNA and mRNA, were performed. Our findings may provide evidence on differentially expressed mRNAs and lncRNAs for asthma.
Methods
Animals
Female BALB/c mice (4–8 week; weight 20–22 g; n = 32) were obtained from Experimental Animal Center of Yanbian University (Yanji, China). All mice were housed in the specific pathogen-free conditions with 22 ± 2 °C of room temperature and 50–60% of relative humidity. All methods were performed in accordance with the relevant guidelines and regulations. The experimental protocol was approved by the Ethics Committee of Science and Technology Department of Jilin Province (SYXK (JI) 2020-0009). The study is reported in accordance with ARRIVE guidelines.
Establishment of OVA induced asthma model00
Mice were divided into control and asthmatic group (n = 11 per group). To construct the asthmatic model, OVA (10 µg, Sigma-Aldrich, USA) and aluminium hydroxide adjuvant (1 mg, InvivoGen, USA) were dissolved in 200 µL of sterile PBS, and then intraperitoneally (i.p.) injected on day 0, 7 and 14 for sensitization. In control group, mice were injected with equal volume of PBS. After 3 days after sensitization, the mice were excited by ultrasonic atomizing inhalation with 5% OVA for 30 min and 3 times a week for 8 weeks [2]. During excitation, the mice were under asthmatic attack and showed some symptoms of agitation, cyanosis, tachypnea and bucking. At 48 h after the final inhalation, mice were sacrificed using 100 mg/kg of pentobarbital sodium.
Sample collection
At 48 h after the final inhalation, mice were sacrificed using 100 mg/kg of pentobarbital sodium. The bronchoalveolar lavage fluid was collected to analyze cytokine levels. Then, the left and right lung tissues were collected. The left lung tissues were subjected to HE staining and Masson staining. The right lung tissues from 3 mice of each group were used for gene microarray analysis and those from 8 mice of each group were used for validation of gene and protein expressions.
Verification of asthma model establishment
The asthma model establishment was verified by HE and Masson staining of left lung tissues, as well as cytokine levels (IL-4, IL-5, IL-13 and IFN-γ) in bronchoalveolar lavage fluid (n = 5). Lung tissues were fixed, dehydrated, embedded in paraffin, and cut into 4 μm sections. The lung sections were stained with HE staining kit (#G1120, Solarbio, Beijing, China) and Masson staining kit (#G1345, Solarbio, Beijing, China), according to the instructions. Levels of IL-4, IL-5, IL-13, and IFN-γ were measured with corresponding ELISA Kits (#D4050, M5000, D1300B, and QK285; R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNAs from the right lung tissues were extracted with RNA isolation kit (Tiangen, DP451, Beijing, China). The cDNA samples were obtained by reverse transcription with Reverse Transcription kit (Tiangen, KR118-02). The qRT-PCR was performed with SYBR Green qRT-PCR kit (Tiangen, KR123). The primer sequences for mRNA and lncRNAs were listed in Table 1. Expression levels were calculated by methods of 2–△△Ct, and all values were normalized to GAPDH.
Western blot
For extraction of total proteins, the right lung tissues were homogenized using Automatic sample crusher (Tissuelyser II, Qiagen, Germany), and subjected to lysis with RIPA and Protease Inhibitor K (Beyotime, Jiangsu, China). Proteins (20 µg per lane) were separated on 12% SDS-PAGE, and transferred onto PVDF membrane with semi-dry transfer. After blocking with 5% non-fat milk (#1172GR500, BioFroxx, Germany) for 2 h, the membrane was successively incubated with primary antibodies overnight at 4 °C, and secondary antibodies for 1 h at room temperature. Primary antibodies were as follows: Fos antibody (#222699, 1:1000, abcam), SMG7 (suppressor with morphological defects in genitalia 7) (#254610, 1:1000, abcam), Sumo2 (small ubiquitin-like modifier 2) (#233222, 1:1000, abcam), Stat5a (Signal transducer and activator of transcription 5a) (#32043, 1:1000, abcam), Myl9 (myosin regulatory light chain 9) (#191393, 1:1000, abcam), and TLR4 (Toll-like receptors 4) (#13867, 1:1000, abcam). Secondary antibody was Goat Anti-Rabbit IgG H&L (HRP) (#7090, 1:5000, abcam).
Microarray analysis
Microarray analysis was performed according to the Affymetrix manufacture’s protocol. Briefly, obtained cDNAs were hybridized with GeneChip® Mouse Transcriptome Array 1.0 (44,699 genes; 22,829 lnc RNA) for 16 h at 45 °C. After washing and staining by Affymetrix Fluidics Station 450 (Affymetrix, California, USA), the gene chips were scanned using GeneChip® Scanner 3000 7G (eBioscince, California, USA). Data were analyzed using Affymetrix default analysis settings by Robust Multichip Analysis algorithm. Data was filtered by p value of < 0.05 and fold change of > 1.2.
Bioinformatics analysis
Differentially expressed genes were identified using the Limma package. The hierarchical Clustering analysis of differentially expressed lncRNAs was analyzed with Cluster_Treeview software from Stanford University (Palo Alto, CA, USA). The relationship of up-regulated genes and down-regulated genes were visualized by scatter plot. Pathway enrichment was performed with the KEGG database (https://www.genome.jp/kegg/) [15]. Additionally, the differentially expressed mRNAs were analyzed by GO database (http://geneontology.org).
A co-expression network of mRNAs and lncRNAs was constructed according to the normalized signal intensity. Briefly, associations between lncRNAs and mRNAs were predicted by calculating the Pearson co-expression coefficient using the R function cor.test. The significant mRNA-lncRNA pairs were screened to construct the co-expression network of mRNAs and lncRNAs, and the correlation coefficient cut off value was 0.99. Cytoscape (vension: 3.6.0) was used for the visualization of the co-expression network. The degree was calculated to represent the centrality of gene or lncRNA in a network.
Statistical analysis
In the GO and KEGG pathway enrichment analysis, Fisher’s exact test was used to calculate p value, and the p value was adjusted with Benjamini–Hochberg method to obtain p-adjusted value. Experimental data are represented as mean ± SD of at least three experiments. Significant differences were assessed by Student’s t-test. p < 0.05 was considered as statistically significant.
Results
Hierarchical clustering analysis of LncRNA and mRNA expression in OVA-induced asthmatic mice
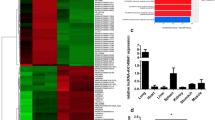
We first assessed asthma model establishment by analyzing the histopathological changes of the lung tissues. HE and Masson staining showed that the OVA group had more inflammatory cell infiltration, thicker smooth muscle layer and subepithelial basement membrane and more collagen deposition around the airway than the control group (Fig. 1 A). Then, we evaluated asthma model establishment by measuring cytokine levels in bronchoalveolar lavage fluid. As shown in Fig. 1B, there were significantly higher levels of TH2-phenotype cytokines (IL-4, IL-5 and IL-13), but significantly lower levels of TH1-phenotype cytokine (IFN-γ) in OVA group than control group (p < 0.05). These results indicate the successful establishment of asthma model in mice.
Verification of asthma model establishment and analysis of differentially expressed lncRNAs and mRNAs in lung tissues. Asthma model was established in mice (n = 11). A Asthma model establishment was assessed by HE staining and Masson staining. Scale bar = 50 μm. Black arrows indicate inflammatory cell infiltration and collagen deposition. Stars indicate smooth muscle layer, and triangles indicate subepithelial basement membrane, respectively. B Asthma model establishment was evaluated by measuring cytokine levels of IL-4, IL-5, IL-13 and IFN-γ in BALF (bronchoalveolar lavage fluid). C Hierarchical clustering was performed for differentially expressed lncRNAs and mRNAs in lung tissues (n = 3). Red indicates the up-regulated lncRNAs or mRNAs and blue indicates down-regulated lncRNAs or mRNAs. lncRNA = long noncoding RNA. M indicates model group, Z indicates control group. D Scatter plot showing the correlation of mRNAs and lncRNAs between asthma model and control mice
To investigate the differentially expressed lncRNAs and mRNAs in the lungs of control and asthma model mice, we performed microarray and hierarchical clustering analysis. There were approximately 3754 differentially expressed lncRNAs and 2967 differentially expressed mRNAs. Among them, there were 1647 up-regulated lncRNAs and 2106 down-regulated lncRNAs, and 1201 up-regulated mRNAs and 1766 down-regulated mRNAs (fold change > 1.2, p < 0.05) (Fig. 1 C). Then, we analyzed the correlation between up-regulated gene and down-regulated genes as shown by the scatterplots (Fig. 1D).
GO enrichment analysis of differentially expressed genes in OVA-induced asthmatic mice
To explore the functional effects of differentially expressed genes, GO enrichment analysis performed. The −LogP was considered as p value of each GO term, and the larger −LogP indicates the smaller p value. Resultantly, the up-regulated genes were significantly enriched in 131 GO terms while the down-regulated genes were significantly enriched in 379 GO terms. The top 22 GO terms were illustrated in Fig. 2. The increased GO terms (p < 0.05) consisted of inflammatory response, leukocyte migration, Notch signaling, positive regulation of NF-kappaB nuclear translocation and neutrophil chemotaxis, G-protein coupled receptor signaling, neutrophil chemotaxis, response to molecule of bacterial origin, positive regulation of inflammatory response, lipopolysaccharide-mediated signaling pathway, nitric oxide production, regulation of T cell cytokine production, and mucus secretion. The decreased GO terms (p < 0.05) included Cell Cycle, Cell Division, Mitotic Nuclear Division, Cellular Response to DNA Damage Stimulus, DNA Repair, Regulation of Transcription, DNA-templated, DNA Replication, Covalent Chromatin Modification, Chromosome Segregation, mRNA Processing, DNA Replication Initiation, RNA Splicing, Mitotic Sister Chromatid Segregation, Double-strand Break Repair Via Homologous Recombination, mRNA Transport, DNA Recombination, Protein Ubiquitination, Negative Regulation of Transcription from RNA Polymerase II Promoter, Positive Regulation of Transcription from RNA Polymerase II Promoter, Nucleosome Assembly, Double-strand Break Repair.
Gene ontology (GO) analysis of differentially expressed mRNAs between control and asthma mice. The top 22 enriched GO terms of up-regulated and down-regulated genes were presented, respectively. The larger − LogP indicates a smaller p value. The Y-axis noted the significantly enriched biological process of GO
KEGG pathway enrichment analysis of differentially expressed genes in OVA-induced asthmatic mice
To further understand the key pathways related to these genes, we conducted the pathway analysis using the KEGG database. As shown in Fig. 3A, the KEGG pathway enrichment of up-regulated genes were mainly related to IL-17 signaling pathway, TNF signaling pathway Toll-like receptor signaling pathway, etc. The KEGG pathway of down-regulated genes were mainly enriched in cell cycle, Hepatitis B and so on. Of interest, pathways were mostly involved in cancer, infectious disease, signal transduction and immune system (Fig. 3B). Above all, the results of the GO and KEGG pathway analysis revealed that the differentially expressed mRNAs were mostly involved in the infectious diseases and immune system.
Pathway enrichment analysis of mRNAs between control and asthmatic mice. A KEGG pathway analysis was performed. The X-axis represents − LogP, whose size is proportional to p value, and the Y-axis represents the significantly enriched pathways. B Pie chart of classifications of significant pathways were illustrated
LncRNA–mRNA co-expression network
A gene co-expression network was constructed to reveal the associations between lncRNA and mRNA in the control and asthma group. According to the published literatures on asthma and inflammation, 11 mRNAs and their regulatory 17 lncRNAs were screened out to construct the lncRNA-mRNA co-expression network (Fig. 4). LncRNA NONMMUT032848 was positively associated with Smg7. NONMMUT008873 was positively associated with Sumo2. NONMMUT009478 was positively associated with Stat5a. NONMMUT007974 was positively associated with Cdk1. LncRNA NONMMUT035972 was positively associated with Topbp1. NONMMUT052633 was negatively associated with Myl9. NONMMUT053402 was positively associated with Tlr4. LncRNA NONMMUT006807 was negatively associated with Tlr4. NONMMUT042325 was positively associated with Fos. NONMMUT035277 and NONMMUT007470 were positively associated with Cpsf6. B630019A10Rik and NONMMUT009675 were positively associated with Stat5b. NONMMUT074536, KnowTID_00003505, and NONMMUT074536 were positively associated with Cdc20. NONMMUT005281 was positively associated with Fxyd2 (Fig. 4).
LncRNAs and mRNAs co-expression network. Network of selected lncRNAs and mRNAs. Circle indicate mRNAs and squares indicate lncRNAs. The betweenness centrality was highlighted by the size of the circle. Red and blue denotes up-regulation and down-regulation, respectively. Lines indicate the relationship between the groups. The positive association was indicated by solid line and the negative association was indicated by dotted line
Validation of genes by qRT-PCR and Western blot analysis
The lung tissues of each group were extracted for gene and protein validation. Analysis by qR-PCR showed that the expressions of Smg7, Sumo2, and Stat5a were significantly reduced and those Myl9, Fos and Tlr4 were significantly elevated in the lungs of OVA-induced asthmatic mouse compared to the control group (p < 0.05) (Fig. 5A). The expressions of Cpsf6, Cdc20, Stat5b, Cdk1, Topbp1 and Fxyd2 were no statistically significant (Fig. 5A). Then, qRT-PCR was used to validate the related lncRNAs of Smg7, Sumo2, Stat5a, Myl9, Fos and Tlr4. As shown in Fig. 5B, the expressions of NONMMUT032848, NONMMUT008873, NONMMUT009478, and NONMMUT006807 were down-regulated in lungs of model mice; in contrast, those of NONMMUT052633, NONMMUT05340 and NONMMUT042325 were significantly up-regulated (p < 0.05). Additionally, Western blot was performed to determine the expression of SMG7, SUMO2, and STAT5, Myl9, Fos and Tlr4. Compared with the control group, the expression of Fos, Myl9 and Tlr4 in the asthma model was increased, while the expression of Smg7, Sumo2 and Stat5a was decreased (Fig. 5C).
Twelve selected mRNAs and seven selected lncRNAs were validated in vivo. Transcriptional and protein levels of the lncRNAs and mRNAs were determined by qRT-PCR and western blot (n = 8). A qRT-PCR results of mRNAs. B qRT-PCR results of lncRNAs. C Western blot results. The original blots are represented in Additional file 1: Figure S1.*p < 0.05, **p < 0.01, ***p < 0.001 compare to control group. ns, not significant
Discussion
The asthma model, which is characterized by airway inflammation, can be induced by OVA stimulation for less than 30 days [9]. In this study, to establish asthma model characterized by airway remodeling, we prolonged OVA stimulation to 8 weeks. The prolonged OVA stimulation may aggravate the inflammatory response and lead to persistent inflammation [16]. The pathological changes in the airway epithelium such as subepithelial basement membrane thickening, smooth muscle cell hyperplasia and hypertrophy may occur under the action of cytokines and inflammatory mediators [2]. Thus, both airway inflammation and airway remodeling characteristics were present in the asthma model induced by prolonged OVA stimulation. The pathological findings in this study suggest that the airway remodeling asthma model was successfully established.
After successful asthma model establishment, we determined the differentially expressed mRNAs and lncRNAs in the lung tissues from control and OVA-induced asthmatic mice using a transcriptomics based approach. There were differentially expressed 3754 lncRNAs (1647 up-regulated and 2106 down-regulated lncRNAs) and 2967 mRNAs (1201 up-regulated and 1766 down-regulated mRNAs). Through bioinformatics analysis, we found that the variety of the differentially expressed mRNAs from lung samples of control and model mice were involved in Cell cycle, Apoptosis, and Toll-like receptor signaling pathway, etc., which are mostly associated with the development of asthma. Among them, 6 mRNAs and 7 of their potential regulator lncRNAs were validated by qRT-PCR and/or Western blot. Most of them were enriched in the cancer, infectious disease and signal transduction. These mRNAs and lncRNAs may enrich the potential therapeutic targets of intervention of allergic inflammatory and airway remodeling asthma.
The mRNAs from our microarray data are those widely reported and have important roles in disease control. The small ubiquitin-like modifier (SUMO) can regulate protein post-translational modification, which consists of 3 SUMO paralogs, including Sumo1, Sumo2 and Sumo3, and the latter two always form the Sumo2/3 complex [17]. Sumo2/3 is mainly involved in the acute stress response, such as inflammation and hypoxia [18]. Brandsma et al. [19] demonstrated that there was markedly decreased Sumo2 in lung tissues of severe chronic obstructive pulmonary disease (COPD) patients by STRING protein–protein interaction network analysis, and hypothesized that it may play a role in attenuating oxidative stress and anti-inflammation in COPD. Here, we validated the up-regulated SUMO2 expression and co-expressed lncRNA NONMMUT008873 in the OVA-induced asthmatic mice. Therefore, we speculate that Sumo2 may also play a role in asthma, and lncRNA NONMMUT008873 may play regulatory role in inflammation and oxidative stress by positively regulating transcriptional level of Sumo2 mRNA during asthma.
Signal transducer and activator of transcription (STAT) pathway is a well-known signaling pathway associated with asthma [20]. STAT family contains seven proteins, and among them, STAT5 acts as a transcriptional activator in the immune response [20]. STAT5 consists of two isoforms, STAT5a and STAT5b, which can regulate lymphocyte proliferation, apoptosis, asthma and cancer [21]. Our results showed that STAT5a was decreased in the asthma model of mice, and this is supported by the another published research that the expression of STAT3 and STAT5a genes in the peripheral blood mononuclear lymphocytes were down-regulated in the severe refractory asthma [22]. We also demonstrated that lncRNA NONMMUT009478 was decreased in the lungs of model mice, and had positive regulation on the STAT5a. Hence, the results may provide another regulatory target in asthma for further research.
Previous studies have found an increased expression of Fos in the peripheral blood mononuclear cells, monocytes and T cells from corticosteroid resistant asthma patients [23], and in the various brains regions of asthmatic mice [24]. Furthermore, Fos can be induced by Thymic stromal lymphopoietin and inhibited by Dexamethasone in the peripheral blood mononuclear cell of the patients with severe asthma [25]. Zhang et al. [26] identified that Fos was involved in the PI3K-AKT signaling pathway in patients with colorectal cancer using PPCR array. Liu et al. [27] reported that Fos was downstream of tumor necrosis factor in the KEGG pathway analysis, and played an essential role in cancer migration, proliferation, and invasion; furthermore, Fos was regulated by lncRNA RUNX1-IT1 and RUNX1 in the pancreatic cancer. Consistently, our microarray results showed that Fos and its lncRNA NONMMUT042325 expression was up-regulated in the lungs of asthmatic mice. Hence, inhibition of lncRNA NONMMUT042325 expression may have an important role in regulating the allergic inflammatory asthma.
SMG7 is a nonsense-mediated decay complex protein, and is responsible for the surveillance and degradation of abnormal RNAs [28]. SMG7 may directly interact with p53, regulate p53 stability, and lead to p53-mediated DNA damage response [29]. Yang et al. [30] suggested that SMG7 can protect against TNF-α-induced human cancer cell line apoptosis by regulating Pvt1 and the tumor suppressor CYLD. Our data showed that the gene expression and protein level of SMG7 and co-expressed lncRNA NONMMUT032848 was reduced in the asthma model of mice. These findings may provide new clues for identifying the therapeutic targets of asthma.
Additionally, we found an increased gene expression and protein levels of MYL9 in the OVA-induced asthmatic mice. It has been demonstrated that MYL9 can regulate ASMCs contraction through self-phosphorylation [31]. Xu et al. [32] reported that decrease of Myl9 could attenuate ASMCs abnormal proliferation, migration, apoptosis and contraction, which could further lead to the airway remodeling in response to platelet derived growth factor, a well-known mediator to induce ASMC remodeling. We also found that lncRNA NONMMUT052633 had positive correlation with Myl9, which may be considered as a promising target for the treatment of asthma.
TLR4 is a well-known innate immune regulator and can participate in the development of asthma via interacting with TLR-related genes, such as high mobility group box 1 [33]. Shang et al. [32] suggested that TLR4/MyD88/NF-κB signaling pathway was closely related to asthma pathogenesis. Our microarray data showed that two lncRNAs could regulate the expression of Tlr4. NONMMUT053402 had positive correlation with Tlr4; on the contrary, lncRNA NONMMUT006807 had negative correlation with Tlr4. The expressions of these genes were all validated by qRT-PCR. Therefore, the lncRNAs of NONMMUT053402 and NONMMUT006807 may have functions related to the allergic inflammatory states of asthma.
Besides OVA, HDM, as the most common inhaled human allergen [34], is also commonly used for establishing allergic asthma model in mice [14, 35]. Compared with OVA-induced asthma model, HDM induced asthma model is more responsive to human allergen. HDM can disrupt the tight junctions in the airway, induce the production of cytokines, chemokines and collagens, and enhance the degranulation of eosinophils and mast cells [35]. Our laboratory is now working on the establishment of HDM-induced asthmatic mouse model. We will further conduct comparative studies on OVA- and HDM-induced asthma models.
Conclusion
In summary, our microarray data revealed that when there was allergic airway inflammation, expressions of multiple genes were altered. The core mRNAs and co-expressed lncRNAs were screened out and further validated by RT-PCR and Western blot. The interactions of SMG7-NONMMUT032848, SUMO2-NONMMUT008873, STAT5a-NONMMUT009478, MYL9-NONMMUT052633, Fos-NONMMUT042325 and TLR4-NONMMUT006807/NONMMUT053402 were found to be involved in the allergic inflammatory asthma. Our findings may provide evidence for the development of therapeutic agents for bronchial asthma.
Availability of data and materials
The microarray data presented in the study are publicly available and can be found at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE197090.
Abbreviations
- OVA:
-
Ovalbumin
- AHR:
-
Airway hyperreactivity
- ASMC:
-
Airway smooth muscle cell
- lncRNAs:
-
Long non-coding RNAs
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
References
Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. The Lancet. 2018;391(10122):783–800.
Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367(3):551–69.
Tyler SR, Bunyavanich S. Leveraging-omics for asthma endotyping. J Allergy Clin Immunol. 2019;144(1):13–23.
Castillo JR, Peters SP, Busse WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. 2017;5(4):918–27.
Wu H, Yang L, Chen LL. The diversity of long noncoding RNAs and their generation. Trends Genet. 2017;33(8):540–52.
Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2010;220(2):126–39.
Chen Y, Mao ZD, Shi YJ, Qian Y, Liu ZG, Yin XW, Zhang Q. Comprehensive analysis of miRNA-mRNA-lncRNA networks in severe asthma. Epigenomics. 2019;11(2):115–31.
Zhang XY, Zhang LX, Tian CJ, Tang XY, Zhao LM, Guo YL, Cheng DJ, Chen XL, Ma LJ, Chen ZC. LncRNAs BCYRN1 promoted the proliferation and migration of rat airway smooth muscle cells in asthma via upregulating the expression of transient receptor potential 1. Am J Transl Res. 2016;8(8):3409–18.
Zhang J, Zhou Y, Gu H, Zhang J, Tang H, Rong Q, Gu L, Pan J, Zhao D, Liu F. LncRNA-AK149641 associated with airway inflammation in an OVA-induced asthma mouse model. J Bioenerg Biomembr. 2020;52(5):355–65.
Lin L, Li Q, Hao W, Zhang Y, Zhao L, Han W. Upregulation of LncRNA malat1 induced proliferation and migration of airway smooth muscle cells via miR-150-eIF4E/Akt signaling. Front Physiol. 2019;10:1337.
Tsitsiou E, Williams AE, Moschos SA, Patel K, Rossios C, Jiang X, Adams OD, Macedo P, Booton R, Gibeon D, et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J Allergy Clin Immunol. 2012;129(1):95–103.
Qiu YY, Wu Y, Lin MJ, Bian T, Xiao YL, Qin C. LncRNA-MEG3 functions as a competing endogenous RNA to regulate Treg/Th17 balance in patients with asthma by targeting microRNA-17/ RORgammat. Biomed Pharmacother. 2019;111:386–94.
Feng L, Yang X, Asweto CO, Wu J, Zhang Y, Hu H, Shi Y, Duan J, Sun Z. Genome-wide transcriptional analysis of cardiovascular-related genes and pathways induced by PM2.5 in human myocardial cells. Environ Sci Pollut Res Int. 2017;24(12):11683–93.
He LL, Xu F, Zhan XQ, Chen ZH, Shen HH. Identification of critical genes associated with the development of asthma by co-expression modules construction. Mol Immunol. 2020;123:18–25.
Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Varricchi G, Ferri S, Pepys J, Poto R, Spadaro G, Nappi E, Paoletti G, Virchow JC, Heffler E, Canonica WG. Biologics and airway remodeling in severe asthma. Allergy. 2022.
Liu Y, Li M, Yin Z, Zhou S, Qiu Y. SUMO-modified bone marrow mesenchymal stem cells promoted the repair of articular cartilage in rats. Cell Biol Int. 2020;44(2):560–8.
Zhang L, Liu X, Sheng H, Liu S, Li Y, Zhao JQ, Warner DS, Paschen W, Yang W. Neuron-specific SUMO knockdown suppresses global gene expression response and worsens functional outcome after transient forebrain ischemia in mice. Neuroscience. 2017;343:190–212.
Brandsma CA, Guryev V, Timens W, Ciconelle A, Postma DS, Bischoff R, Johansson M, Ovchinnikova ES, Malm J, Marko-Varga G, et al. Integrated proteogenomic approach identifying a protein signature of COPD and a new splice variant of SORBS1. Thorax. 2020;75(2):180–3.
Wyszomierski SL, Yeh J, Rosen JM. Glucocorticoid receptor/signal transducer and activator of transcription 5 (STAT5) interactions enhance STAT5 activation by prolonging STAT5 DNA binding and tyrosine phosphorylation. Mol Endocrinol (Baltimore, MD). 1999;13(2):330–43.
Szelag M, Wesoly J, Bluyssen HA. Advances in peptidic and peptidomimetic-based approaches to inhibit STAT signaling in human diseases. Curr Protein Pept Sci. 2016;17(2):135–46.
Saeedfar K, Behmanesh M, Mortaz E, Masjedi MR. The expression of STAT3 and STAT5A genes in severe refractory asthma. Tanaffos. 2017;16(1):1–8.
Lane SJ, Adcock IM, Richards D, Hawrylowicz C, Barnes PJ, Lee TH. Corticosteroid-resistant bronchial asthma is associated with increased c-fos expression in monocytes and T lymphocytes. J Clin Investig. 1998;102(12):2156–64.
Chen Z, Liu NN, Xiao J, Wang YH, Dong R. The amygdala via the paraventricular nucleus regulates asthma attack in rats. CNS Neurosci Ther. 2020;26(7):730–40.
Liu S, Verma M, Michalec L, Liu W, Sripada A, Rollins D, Good J, Ito Y, Chu H, Gorska MM, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141(1):257-268 e256.
Zhang T, Ma Y, Fang J, Liu C, Chen L. A deregulated PI3K-AKT signaling pathway in patients with colorectal cancer. J Gastrointest Cancer. 2019;50(1):35–41.
Liu S, Zhang J, Yin L, Wang X, Zheng Y, Zhang Y, Gu J, Yang L, Yang J, Zheng P, et al. The lncRNA RUNX1-IT1 regulates C-FOS transcription by interacting with RUNX1 in the process of pancreatic cancer proliferation, migration and invasion. Cell Death Dis. 2020;11(6):412.
Yang L, Kraft VAN, Pfeiffer S, Merl-Pham J, Bao X, An Y, Hauck SM, Schick JA. Nonsense-mediated decay factor SMG7 sensitizes cells to TNFalpha-induced apoptosis via CYLD tumor suppressor and the noncoding oncogene Pvt1. Mol Oncol. 2020;14(10):2420–35.
Cowen LE, Luo H, Tang Y. Characterization of SMG7 14-3-3-like domain reveals phosphoserine binding-independent regulation of p53 and UPF1. Sci Rep. 2019;9(1):13097.
Luo H, Cowen L, Yu G, Jiang W, Tang Y. SMG7 is a critical regulator of p53 stability and function in DNA damage stress response. Cell Discov. 2016;2:15042.
Licht AH, Nübel T, Feldner A, Jurisch-Yaksi N, Marcello M, Demicheva E, Hu JH, Hartenstein B, Augustin HG, Hecker M, et al. Junb regulates arterial contraction capacity, cellular contractility, and motility via its target Myl9 in mice. J Clin Investig. 2010;120(7):2307–18.
Xu W, Hong W, Shao Y, Ning Y, Cai Z, Li Q. Nogo-B regulates migration and contraction of airway smooth muscle cells by decreasing ARPC 2/3 and increasing MYL-9 expression. Respir Res. 2011;12(1):14.
Hwang YH, Lee Y, Paik MJ, Yee ST. Inhibitions of HMGB1 and TLR4 alleviate DINP-induced asthma in mice. Toxicol Res (Camb). 2019;8(5):621–9.
Bracken SJ, Adami AJ, Szczepanek SM, Ehsan M, Natarajan P, Guernsey LA, Shahriari N, Rafti E, Matson AP, Schramm CM, et al. Long-term exposure to house dust mite leads to the suppression of allergic airway disease despite persistent lung inflammation. Int Arch Allergy Immunol. 2015;166(4):243–58.
Youssef M, De Sanctis JB, Kanagaratham C, Tao S, Ahmed E, Radzioch D. Efficacy of optimized treatment protocol using LAU-7b formulation against ovalbumin (OVA) and house dust mite (HDM)-induced allergic asthma in atopic hyperresponsive A/J mice. Pharm Res. 2020;37(2):31.
Acknowledgements
Not applicable.
Funding
This work was supported by a Grant from National Natural Science Foundation of China (Nos. 81970018, 81860729, 82160004 and 81660003), Jilin Province postdoctoral researchers (No. 2020-498), and China Postdoctoral Science Foundation of Pneumonia Epidemic Prevention and Control (No. 2020T130099ZX).
Author information
Authors and Affiliations
Contributions
Study design: YS, JJ, LL, GY; data collection: YS, JJ, QB, SL; statistical analysis: CX; data interpretation: YS, JJ, YZ; manuscript preparation: YS, JJ; literature search: JJ, HP, LL; funds collection: GY. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. The experimental protocol was approved by the Ethics Committee of Science and Technology Department of Jilin Province (SYXK (JI) 2020-0009). The study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Figure S1.
The un-cropped Western Blot images for Figure 5C, which showed the expression levels of Fos, Myl9, Smg7, Stat5a, Sumo2, tlr4 and β-actin in lung tissues of asthmatic mice.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, Y., Jiang, J., Bai, Q. et al. Gene expression profiles and bioinformatics analysis in lung samples from ovalbumin-induced asthmatic mice. BMC Pulm Med 23, 50 (2023). https://doi.org/10.1186/s12890-023-02306-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02306-w