Abstract
Background
Studying the characteristics of hospitalized Coronavirus Disease 2019 (COVID-19) patients is vital for understanding the disease and preparing for future outbreaks. The aim of this study was to analyze and describe the clinical profiles and factors associated with mortality among COVID-19 patients admitted to Jimma Medical Center COVID-19 Treatment Center (JMC CTC) in Ethiopia.
Methods
All confirmed COVID-19 patients admitted to JMC CTC between 17 April 2020 and 05 March 2022 were included in this study. Socio-demographic data, clinical information, and outcome variables were collected retrospectively from medical records and COVID-19 database at the hospital. Bivariable and multivariable analyses were performed to determine factors associated with COVID-19 severity and mortality. A P-value < 0.05 was considered statistically significant.
Results
A total of 542 confirmed COVID-19 patients were admitted to JMC CTC, of which 322 (59.4%) were male. Their median age was 48 years (IQR 32–64). About 51% (n = 277) of them had severe COVID-19 upon admission. Patients with hypertension [AOR: 2.8 (95% CI: 1.02–7.7, p = 0.046)], diabetes [AOR: 8.8 (95% CI: 1.2–17.3, p = 0.039)], and underlying respiratory diseases [AOR: 18.8 (95% CI: 2.06–71.51, p = 0.009)] were more likely to present with severe COVID-19 cases. Overall, 129 (23.8%) died in the hospital. Death rate was higher among patients admitted with severe disease [AHR = 5.5 (3.07–9.9) p < 0.001)] and those with comorbidities such as hypertension [AHR = 3.5 (2.28–5.41), p < 0.001], underlying respiratory disease [AHR = 3.4 (1.97–5.94), p < 0.001], cardiovascular disease (CVDs) [AHR = 2.8 (1.73–4.55), p < 0.001], and kidney diseases [AHR = 3.7 (2.3–5.96), p < 0.001].
Conclusion
About half of COVID-19 cases admitted to the hospital had severe disease upon admission. Comorbidities such as hypertension, diabetes, and respiratory diseases were linked to severe illness. COVID-19 admissions were associated with high inpatient mortality, particularly among those with severe disease and comorbidities.
Similar content being viewed by others
Introduction
More than four years after its declaration as a global pandemic, COVID-19 remains a dynamic public health concern, with the emergence of new variants influencing transmission patterns and epidemiological trends [1, 2]. While global vaccination efforts have significantly reduced severe cases and mortality, disparities in vaccine access and uptake persist, especially in low- and middle-income countries [3]. By late 2023, there was a notable resurgence of cases in several regions, including Europe and North America, primarily due to the emergence of more transmissible variants [4]. In sub-Saharan Africa, challenges such as limited healthcare infrastructure and underreporting of cases continue to pose significant obstacles. Many African countries still experience lower testing rates compared to other regions, contributing to an underestimation of the true burden of COVID-19 [5]. The gap between documented cases and estimated seroprevalence rates suggests ongoing underreporting, underscoring the need for improved surveillance and reporting systems to better capture the pandemic’s full impact [6, 7].
Vaccinations have significantly reduced COVID-19 transmission, severe illness, and mortality. However, disparities in vaccine distribution and acceptance, especially in low- and middle-income countries, hinder global herd immunity [8, 9]. Africa faces particular challenges with low vaccination rates due to supply shortages, logistical issues, vaccine hesitancy, and limited healthcare infrastructure [10, 11]. The emergence of new SARS-CoV-2 variants complicates efforts to combat the pandemic, as these variants may affect transmissibility, disease severity, and vaccine efficacy [12,13,14]. Some variants, like Delta, have shown increased transmissibility and can lead to breakthrough infections among vaccinated individuals, partly due to mutations in the spike protein [15].
Various risk factors are associated with severe illness and mortality from COVID-19. These include advanced age, underlying health conditions such as hypertension, diabetes, cardiovascular disease, chronic respiratory disease, obesity, and states of immunocompromise [16, 17]. Furthermore, demographic factors such as male gender [18], socioeconomic status, and access to healthcare resources have also been recognized as playing roles in determining the severity and fatality of the disease [19].
Ethiopia faced significant challenges during the COVID-19 pandemic, including limited healthcare infrastructure and economic constraints [20]. The government quickly implemented measures such as lockdowns, travel restrictions, and hygiene promotion [21]. Despite these efforts, the country’s response was hampered by limited testing and medical resources [22]. The Ethiopian Public Health Institute and the Ministry of Health led initiatives for surveillance, contact tracing, and public awareness, with international support, including vaccines through COVAX [23]. The response aimed to balance public health measures with economic and social impacts, highlighting the challenges of managing a pandemic in a developing country [24, 25].
Furthermore, the lack of comprehensive scientific data on COVID-19 outcomes has hindered evidence-based decision-making and the implementation of targeted public health interventions in Ethiopia. Strengthening research infrastructure and fostering international collaborations will not only enhance the current response but also pave the way for more effective future preparedness efforts, ensuring the protection of public health in Ethiopia and beyond [26]. Therefore, this study aimed to assess the clinical profile and outcomes of hospitalized COVID-19 patients at Jimma Medical Center (JMC) in southwest Ethiopia.
Methods
Study design and setting
We reviewed medical records of all patients admitted with COVID-19 to the Jimma Medical Center (JMC) COVID-19 Treatment Center (CTC) between 17 April 2020 and 05 March 2022. The center, established on 13 March 2020, had a capacity of 23 beds and was equipped with mechanical ventilators, oxygen concentrators, and patient monitors. Integrated with JMC, it was the only COVID-19 intensive care facility in southwest Ethiopia. The center consisted of management and operation sections, with the management team including a scientific advisory council and the operation section divided into six sub-teams: [1] Isolation & Case Management, [2] Surveillance, [3] Risk Communication & Community Engagement (RCCE), [4] Water, Sanitation, & Hygiene (WASH) and Infection Prevention Control (IPC), [5] Research, Innovation, and Diagnostics, and [6] Administration, Data Management, and Finance [27].
Inclusion criteria
Patients whose medical records confirmed the presence of the virus through the identification of viral ribonucleic acid (RNA) in nasopharyngeal swab samples using reverse transcription polymerase chain reaction (RT-PCR) either upon admission or during hospitalization, regardless of whether they exhibited symptoms, were included in the study.
Data collection
Patient sociodemographic and clinical characteristics were collected from medical records and the COVID-19 database at JMC-CTC. The collected data included various aspects such as demographics, clinical manifestations, comorbidities, disease severity upon admission, time/date of admission, length of stay, and discharge outcome.
Operational definitions.
-
a.
COVID-19 specific symptoms (classic): Fever, cough, shortness of breath, loss of smell or taste [28].
-
b.
Extended symptoms: Sore throat, runny nose, arthralgia, fatigue, and headache [28].
-
c.
Mild illness: A person has any of the COVID-19 symptoms except for shortness of breath and difficulty breathing [29].
-
d.
Moderate illness: A person may have lower respiratory tract illness with clinical or radiographic evidence. However, their blood oxygen levels remain at 94% or higher [29].
-
e.
Critical COVID: A COVID-19 case requiring mechanical ventilation or hemodynamic support. This includes patients with acute respiratory distress syndrome, multi-organ dysfunction or failure, and shock [29].
-
f.
Non-severe COVID-19: A person with mild to moderate symptoms that do not require hospitalization. This includes individuals with mild symptoms (any COVID-19 symptoms except shortness of breath and difficulty breathing) and moderate symptoms (lower respiratory tract illness with clinical or radiographic evidence but blood oxygen levels at 94% or higher) [29, 30].
-
g.
Severe illness: A person has blood oxygen levels that are less than 94%, a high breathing rate (≥ 30 breaths/min), and signs of severe lung disease (lung infiltrates > 50%) [29].
Symptom categorization
-
1.
Category 1: One or more classic symptom without extended symptoms.
-
2.
Category 2: One or more classic symptom with extended symptom.
-
3.
Category 3: One or more extended symptom without classic symptoms.
Data analysis
The original data collected in Microsoft Excel was reviewed for completeness and consistency before being exported to SPSS® version 26 (IBM®, New York, USA) for analysis. Normality tests were conducted using visual inspections of histograms and Q-Q plots, as well as the Kolmogorov-Smirnov and Shapiro-Wilk tests. For the bivariate analysis, independent variables with a p-value less than 0.25 were selected as candidates for the multivariable logistic regression analysis. A binary logistic regression model was then used to explore risk factors for the severity of COVID-19 infection. Additionally, a Cox regression analysis was employed to identify predictors of mortality in COVID-19 patients. The equality of survival distributions for different severity levels was tested using Log Rank (Mantel-Cox), Breslow (Generalized Wilcoxon), and Tarone-Ware tests. A p-value of less than 0.05 was used as the threshold for statistical significance.
Result
Socio-demographic and clinical characteristics
From April 2020 to March 2022, a total of 542 COVID-19 patients were admitted to JMC CTC, of which 322 (59.4%) were male. The median age was 48 years (IQR 32–64), with a range of 3 to 102 years. More than half (50.8%) of the cases were younger than 50 years of age. The most frequently reported symptoms were dyspnea (60%) and cough (57.6%). Among those with severe disease, 57.4% and 54% of patients exhibited cough and dyspnea, respectively. Comorbidities were reported in 21.8% of the admitted patients. Among those who died, the majority had comorbidities (80.6%), with hypertension being the most common at 44.2% (Table 1).
Trends in COVID-19 admissions by severity
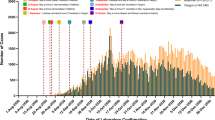
Until November 2020, most admissions were due to non-severe cases. Subsequently, the non-severe admission declined, while admissions due to severe cases gradually increased reaching peak of 49 in April 2021. From June to August 2021, only two cases, both of which were severe, were admitted to the center. The number of admissions, primarily due to severe cases, increased after September 2021 (Fig. 1).
Factors associated with COVID-19 severity
Univariate binary logistic regression analyses were conducted to assess the individual factors associated with severe COVID-19 cases. Subsequently, candidate variables for the final multivariate analysis were selected based on their statistical significance in the univariate analyses. Accordingly, age, admission symptoms, and comorbidities such as hypertension, diabetes, respiratory disease, cardiovascular diseases (CVDs), and kidney diseases, were selected for inclusion in the multivariate logistic regression analysis.
The analysis indicated that with each additional year of age, the odds of experiencing a more severe form of the disease increased by 4% (AOR: 1.04, 95% CI: 1.03–1.05, p < 0.001).
Additionally, individual comorbidities were independently analyzed after adjusting for the presence and absence of comorbidities. Hypertensive patients showed a nearly threefold increased odds of more severe disease compared to non-hypertensive patients [AOR: 2.8 (95% CI: 1.02–7.7, p = 0.046)]. Diabetic patients had approximately nine times higher odds of experiencing severe disease compared to non-diabetic patients [AOR: 8.8 (95% CI: 1.2–17.3, p = 0.039)]. Patients with respiratory diseases exhibited the highest odds of severe disease, with an 18.8-fold increase compared to those without respiratory conditions [AOR: 18.8 (95% CI: 2.06–71.51, p = 0.009)] (Table 2).
Mortality and discharge outcomes
A total of 129 (23.8%) patients died during their hospitalization. The median length of hospital stay was 9.5 days (IQR: 6–15), with duration ranging from one to 40 days. Most of the deceased patients (n = 81, 62.8%) died within the first 7 days of admission. Additionally, 120 (22.1%) patients were transferred to home-based care or nearby facilities for further treatment and follow-up.
Trend of mortality with severe case admission
During the first five months, there were no admissions of severe cases and death at the center. However, as time progressed, there was a gradual increase in severe cases, culminating in the highest number of deaths during three peak periods: April 2021 (n = 18), October 2021 (n = 19), and January 2022 (n = 16) (Fig. 2).
Time to mortality and hazard factors in COVID-19 patients
The clinical severity status at admission was significantly associated with survival outcomes (p-value < 0.001) (Supplementary Table 1). Patients who were severely ill at the time of admission had poorer survival rates and a shorter time to death (Fig. 3).
Univariate Cox regression analyses assessed individual factors associated with the hazard of death among COVID-19 patients. Variables with fewer than 5 occurrences were excluded due to insufficient sample size for reliable estimates. Factors significant in univariate analyses, including age, clinical severity, and comorbidities (hypertension, diabetes, respiratory disease, cardiovascular disease, and kidney disease), were selected for the multivariate Cox regression analysis.
Severe disease was associated with a 5.5-fold increased hazard of death compared to non-severe cases (AHR: 5.5; 95% CI: 3.07–9.9, p < 0.001). Additionally, individual comorbidities were independently analyzed after adjusting for the presence and absence of comorbidities. Hypertension, respiratory disease, cardiovascular disease, and kidney disease were linked to increased COVID-19 mortality risk, with the following AHR and 95% CI: hypertension [3.5(2.28–5.41), p < 0.001], respiratory disease [3.4(1.97–5.94), p < 0.001], cardiovascular disease [2.8(1.73–4.55), p < 0.001], and kidney disease [3.7(2.3–5.96), p < 0.001] (Table 3).
Discussion
A total of 542 patients were admitted to JMC CTC between April 2020 and March 2022. Cough and dyspnea were the most frequently reported symptoms. Approximately 51% of the patients were classified as having severe COVID-19 at the time of hospitalization. The mortality rate was 23.8%, with a significant majority of the deceased patients (80.6%) having comorbidities, particularly hypertension. Conditions such as hypertension, respiratory disease, and cardiovascular disease were strongly associated with severe outcomes and increased risk of mortality.
During the first few months of the COVID-19 pandemic, Ethiopia did not experience a major outbreak with very low mortality rate [31]. However, by August 2020, the country began active case finding through a large-scale community-based activity and testing (CoMBaT) strategy [32]. This resulted in an increase in admissions for both severe and non-severe cases. Our study also revealed that there were no admissions due to severe COVID-19 or COVID-19 related deaths at JMC until August 2020. The low number of cases during that period may have been a result of limited testing and low disease outbreak. However, starting in September 2020, non-severe admissions decreased as Ethiopia revised its strategy of blanket admission of all confirmed COVID-19 cases to only severe cases and high-risk patients.
Since February 2021, there has been a notable increase in severe cases. The highest number of overall admissions occurred in April 2021, with a majority being severe cases (n = 49) out of a total of 69 admissions. Following this peak, there was a substantial decline over the next four months: May (n = 29), June (n = 0), July (n = 0), and August (n = 2). This pattern can be attributed to the second wave, which began in late January 2021 and persisted until the end of May 2021 [33]. During this period, the Alpha variant, known for its increased transmissibility [34] and higher hospitalization rates compared to earlier strains [35], predominated COVID-19 cases in Ethiopia [33]. These findings align with a study conducted from publicly available data on COVID-19 admissions in Ethiopia [36]. Our study also revealed that admissions due to severe COVID-19 and deaths peaked in October 2021 and January 2022. These peaks corresponded with the third and the fourth waves of COVID-19 outbreak in Ethiopia, which were dominated by the Delta and Omicron variants, respectively [37].
Ethiopia began COVID-19 vaccination in March 2021 [38]. Although the number of admissions and deaths at the hospital significantly decreased between May and August 2021, it is difficult to attribute this to the vaccination because only less than 5% of the population was vaccinated during this time [39].
Clinical parameters, including symptom category at admission and pre-existing comorbidities, were found to be associated with COVID-19 severity. Patients with one or more classic COVID-19 symptoms were more likely to present with severe illness compared to asymptomatic individuals by 15.9 folds. The odds increased to 17.2 among those with classic symptoms plus extended symptoms. This finding aligns with a retrospective analysis from China, which reported that the likelihood of developing severe illness increased with an increasing number of presenting symptoms at hospital admission [40]. This finding is also consistent with a global systematic review and meta-analysis that involved data from 14 countries [41]. Previous studies have suggested that an aberrant host immune response and cytokine storm may significantly contribute to the severity of COVID-19 [42].
In this study, age was associated with the severity of COVID-19. For each additional year of age, the likelihood of experiencing a more severe form of the disease increased by 4%, which aligns with findings from previous studies [43,44,45]. Additionally, patients with comorbidities such as hypertension, diabetes, and respiratory diseases were more likely to experience severe COVID-19 illness. Several systematic reviews have found similar patterns in both developing and developed countries, indicating that the relationship between comorbidities and the severity of COVID-19 is consistent across different socioeconomic contexts [41, 46, 47].
The median length of hospital stay was 9.5 days (IQR 6–15), with range of one to 40 days. This is consistent with findings from other studies conducted in Ethiopia [48, 49]. However, this finding is lower than studies from China [50], US [51], and Sweden [52]. The shorter median hospital stay observed in our study compared to those from China, the US, and Sweden may reflect variations in treatment protocols, healthcare resources,, and patient demographics across these regions.
Our study also showed that clinical severity and pre-existing comorbidities were significantly associated with the risk of death. Severe disease was associated with a 5.5-fold increased hazard of death compared to non-severe cases. This finding is consistent with systematic reviews and meta-analyses from Sub-Saharan countries [53] as well as Asia, North America, Europe, and South America [54]. Additionally, comorbidities including, hypertension (AHR: 3.5), respiratory disease (AHR: 3.4), cardiovascular disease (AHR: 2.8), and kidney disease (AHR: 3.7) were linked to increased COVID-19 mortality risk. Our findings are consistent with different systematic review meta-analysis studies [53,54,55,56,57,58]. This is because chronic conditions are often associated with a subclinical inflammation, weakened innate immune responses, and increased expression of ACE-2 receptor, which facilitates the entry of SARS-CoV-2 into the host cells and is associated with higher COVID-19 mortality [45].
Strength and limitation
This study covered nearly two years of COVID-19 pandemic, focusing on COVID-19 related hospitalizations to a tertiary teaching hospital in Ethiopia, and provides valuable insights into COVID-19 in African contexts. While the study offers original findings, it is limited by its retrospective design. Retrieving data from medical records was found to be a challenge due to incomplete documentations and lost records. Moreover, the lack of routinely recorded laboratory and imaging results, as well as case management details, prevented their inclusion in this study.
Conclusion
This study found that patients hospitalized with severe symptoms and comorbidities—such as hypertension, respiratory disease, cardiovascular disease, and kidney disease—faced a significantly higher risk of in-hospital mortality. The study also identified distinct patterns in admissions and mortality that corresponded with the pandemic waves and variant prevalence in Ethiopia. Although vaccination efforts began in March 2021, their impact on admissions and mortality was minimal during the study period due to low vaccination coverage. Overall, this research enhances our understanding of COVID-19 outcomes in African contexts and highlights the importance of ongoing monitoring and management, especially with the emergence of new variants and evolving data.
Data availability
All relevant data are within the manuscript.
References
Organization WH. Coronavirus disease (COVID-19) Epidemiological Updates and Monthly Operational Updates. 2024.
CDC COVID Data Tracker. Home [Internet]. [cited 2024 Aug 5]. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
Gozzi N, Chinazzi M, Dean NE, Longini IM Jr, Halloran ME, Perra N, et al. Estimating the impact of COVID-19 vaccine inequities: a modeling study. Nat Commun. 2023;14(1):3272.
Koelle K, Martin MA, Antia R, Lopman B, Dean NE. The changing epidemiology of SARS-CoV-2. Science (80-). 2022;375(6585):1116–21.
Nguimkeu P, Tadadjeu S. Why are the Number of COVID-19 Cases Lower Than Expected in Sub-Saharan Africa? A Cross-Sectional Analysis of the Role of Demographic, Epidemiologic and Environmental Factors. USA: Working Paper, Georgia State University; 2020.
Ngere I, Dawa J, Hunsperger E, Otieno N, Masika M, Amoth P, et al. High seroprevalence of SARS-CoV-2 but low infection fatality ratio eight months after introduction in Nairobi, Kenya. Int J Infect Dis. 2021;112:25–34.
Manabe YC, Sharfstein JS, Armstrong K. The need for more and better testing for COVID-19. JAMA. 2020;324(21):2153–4.
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23.
Onigbinde OA, Ajagbe AO. COVID-19 vaccination and herd immunity In Africa: An incentive-based approach could be the game-changer to vaccine hesitancy. Public Heal Pract (Oxford, England). 2022;4:100282.
Abosede DA, Ajadi A. COVID-19 vaccines in Africa: challenges and implications for the future. Int J Dev Sustain Int J Dev Sustain. (11 1):16–27.
Machingaidze S, Wiysonge CS, Hussey GD. Strengthening the expanded programme on immunization in Africa: looking beyond 2015. PLoS Med. 2013;10(3):e1001405.
Boehm E, Kronig I, Neher RA, Eckerle I, Vetter P, Kaiser L. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect. 2021;27(8):1109–17.
Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54(1):524–40.
Khan A, Khan T, Ali S, Aftab S, Wang Y, Qiankun W, et al. SARS-CoV-2 new variants: characteristic features and impact on the efficacy of different vaccines. Biomed Pharmacother. 2021;143:112176.
Callaway E. Delta coronavirus variant: scientists brace for impact. Nature. 2021;595(7865):17–8.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42.
Galbadage T, Peterson BM, Awada J, Buck AS, Ramirez DA, Wilson J, et al. Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes. Front Med. 2020;7:348.
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6.
First case of COVID-19 confirmed in Ethiopia | WHO |. Regional Office for Africa [Internet]. [cited 2022 Dec 19]. https://www.afro.who.int/news/first-case-covid-19-confirmed-ethiopia
Deressa W, Worku A, Abebe W, Getachew S, Amogne W. Social distancing and preventive practices of government employees in response to COVID-19 in Ethiopia. PLoS ONE. 2021;16(9):e0257112.
Ayele W, Gage A, Kapoor NR, Kassahun Gelaw S, Hensman D, Derseh Mebratie A, et al. Quality of routine health data at the onset of the COVID-19 pandemic in Ethiopia, Haiti, Laos, Nepal, and South Africa. Popul Health Metr. 2023;21(1):1–11.
Dima FG, Girma S. Review on Covid-19 distribution, Socio-Economic Impact and Its Preventive Measures in Ethiopia.
Engidaw AE. Small businesses and their challenges during COVID-19 pandemic in developing countries: in the case of Ethiopia. J Innov Entrep. 2022;11(1):1.
Angaw KW. Policy responses and social solidarity imperatives to respond the COVID-19 pandemic socioeconomic crises in Ethiopia. Clin Outcomes Res. 2021;279–87.
Khatri RB, Endalamaw A, Erku D, Wolka E, Nigatu F, Zewdie A, et al. Preparedness, impacts, and responses of public health emergencies towards health security: qualitative synthesis of evidence. Arch Public Heal. 2023;81(1):208.
Chala TK, Abera EG, Tukeni KN, Didu GH, Abbagidi FA, Yesuf EA, et al. The need to establish and sustain public health emergency operation centers for managing infectious disease outbreaks: lesson from response to louse-borne relapsing fever outbreak in Jimma, Ethiopia. Disaster Med Public Health Prep. 2023;17:e535.
Antonelli M, Capdevila J, Chaudhari A, Granerod J, Canas LS, Graham MS, et al. Optimal symptom combinations to aid COVID-19 case identification: analysis from a community-based, prospective, observational cohort. J Infect. 2021;82(3):384–90.
Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–66.
Sisay G, Mantefardo B, Beyene A. Time from symptom onset to severe COVID-19 and risk factors among patients in Southern Ethiopia: a survival analysis. J Int Med Res. 2022;50(8):03000605221119366.
Gudina EK, Gobena D, Debela T, Yilma D, Girma T, Mekonnen Z, et al. COVID-19 in Oromia Region of Ethiopia: a review of the first 6 months’ surveillance data. BMJ Open. 2021;11(3):e046764.
WHO Regional Office for Africa, BULLETIN. COVID-19 2020, 05 AUGUST ETHIOPIA. 2020;(August). https://www.afro.who.int/sites/default/files/2020-08/ETHIOPIA_COVID19 response bulletin_05AUG2020%282%29_0.pdf.
Sisay A, Tshiabuila D, van Wyk S, Tesfaye A, Mboowa G, Oyola SO et al. Molecular epidemiology and diversity of SARS-CoV-2 in Ethiopia, 2020–2022. Genes (Basel). 2023;14(3):705.
Pascall DJ, Vink E, Blacow R, Bulteel N, Campbell A, Campbell R, et al. The SARS-CoV-2 alpha variant was associated with increased clinical severity of COVID-19 in Scotland: a genomics-based retrospective cohort analysis. PLoS ONE. 2023;18(4):e0284187.
Paredes MI, Lunn SM, Famulare M, Frisbie LA, Painter I, Burstein R et al. Associations between SARS-CoV-2 variants and risk of COVID-19 hospitalization among confirmed cases in Washington State: a retrospective cohort study. Medrxiv. 2022.
Amhare AF, Tao Y, Li R, Zhang L. Early and subsequent epidemic characteristics of COVID-19 and their impact on the epidemic size in Ethiopia. Front Public Heal. 2022;10:834592.
Hasenauer J, Merkt S, Ali S, Gudina EK, Adissu W, Muenchhoff M et al. Long-term monitoring of SARS-CoV-2 seroprevalence and variants in Ethiopia provides prediction for immunity and cross-immunity. 2023.
Ayele AD, Ayenew NT, Tenaw LA, Kassa BG, Yehuala ED, Aychew EW, et al. Acceptance of COVID-19 vaccine and associated factors among health professionals working in hospitals of South Gondar Zone, Northwest Ethiopia. Hum Vaccin Immunother. 2021;17(12):4925–33.
Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–53.
Wang F, Cao J, Yu Y, Ding J, Eshak ES, Liu K, et al. Epidemiological characteristics of patients with severe COVID-19 infection in Wuhan, China: evidence from a retrospective observational study. Int J Epidemiol. 2020;49(6):1940–50.
Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS ONE. 2021;16(3):e0247461.
Assal HH, Abdel-hamid HM, Magdy S, Salah M, Ali A, Elkaffas RH, et al. Predictors of severity and mortality in COVID-19 patients. Egypt J Bronchol. 2022;16(1):1–9.
Starke KR, Reissig D, Petereit-Haack G, Schmauder S, Nienhaus A, Seidler A. The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Glob Heal. 2021;6(12):e006434.
Bellino S, Punzo O, Rota MC, Del Manso M, Urdiales AM, Andrianou X et al. COVID-19 disease severity risk factors for pediatric patients in Italy. Pediatrics. 2020;146(4).
Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Heal. 2020;8(8):e1003–17.
Honardoost M, Janani L, Aghili R, Emami Z, Khamseh ME. The association between presence of comorbidities and COVID-19 severity: a systematic review and meta-analysis. Cerebrovasc Dis. 2021;50(2):132–40.
Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS ONE. 2020;15(12):e0243191.
Memirie ST, Yigezu A, Zewdie SA, Mirkuzie AH, Bolongaita S, Verguet S. Hospitalization costs for COVID-19 in Ethiopia: empirical data and analysis from Addis Ababa’s largest dedicated treatment center. PLoS ONE. 2022;17(1):e0260930.
Kaso AW, Agero G, Hurissa Z, Kaso T, Ewune HA, Hareru HE, et al. Survival analysis of COVID-19 patients in Ethiopia: a hospital-based study. PLoS ONE. 2022;17(5):e0268280.
Wang Z, Liu Y, Wei L, Ji JS, Liu Y, Liu R, et al. What are the risk factors of hospital length of stay in the novel coronavirus pneumonia (COVID-19) patients? A survival analysis in southwest China. PLoS ONE. 2022;17(1):e0261216.
Ohl ME, Miller DR, Lund BC, Kobayashi T, Miell KR, Beck BF, et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw open. 2021;4(7):e2114741–2114741.
Larsson E, Brattström O, Agvald-Öhman C, Grip J, Campoccia Jalde F, Strålin K, et al. Characteristics and outcomes of patients with COVID‐19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiol Scand. 2021;65(1):76–81.
Bepouka B, Mayasi N, Mandina M, Longokolo M, Odio O, Mangala D, et al. Risk factors for mortality in COVID-19 patients in sub-saharan Africa: a systematic review and meta-analysis. PLoS ONE. 2022;17(10):e0276008.
Noor FM, Islam MM. Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis. J Community Health. 2020;45(6):1270–82.
Ghislain MR, Muzumbukilwa WT, Magula N. Risk factors for death in hospitalized COVID-19 patients in Africa: a systematic review and meta-analysis. Med (Baltim). 2023;102(35):e34405.
Sepandi M, Taghdir M, Alimohamadi Y, Afrashteh S, Hosamirudsari H. Factors associated with mortality in COVID-19 patients: a systematic review and meta-analysis. Iran J Public Health. 2020;49(7):1211.
Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS ONE. 2020;15(8):e0238215.
Ng WH, Tipih T, Makoah NA, Vermeulen JG, Goedhals D, Sempa JB et al. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. mBio. 2021; 12 (1): e03647-20. https://doi.org/10.1128/mBio. 03647-20. PMID.
Acknowledgements
We extend our heartfelt gratitude to the Jimma Emergency Operation Center, Jimma Medical Center, and the dedicated study teams for their invaluable support during the intervention and data collection process.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
EGA and EKG conceptualized and designed the study. EGA analyzed the data and wrote the original draft. TKC and KNT involved for data curation. EKG and DY provided supervision for the overall activities. All authors accepted responsibility for all aspects of the research, including writing, reviewing, and editing, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical clearance and consent to participate
Ethical approval for the study was obtained from the Institutional Review Board (IRB) of Jimma University, Institute of Health (Reference number: RPGD/978/2020). The IRB of Jimma University, Institute of Health, also granted a waiver for the requirement of informed consent to participate, as the study involved a retrospective review of anonymized medical records. All data were handled with strict confidentiality to ensure participant anonymity.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abera, E.G., Tukeni, K.N., Chala, T.K. et al. Clinical profiles and mortality predictors of hospitalized patients with COVID-19 in Ethiopia. BMC Infect Dis 24, 908 (2024). https://doi.org/10.1186/s12879-024-09836-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09836-6