Abstract
Background
Highly active antiretroviral therapy (HAART) has been able to improve the immune system function and survival of human immunodeficiency virus (HIV) patients. However, Patients coinfected with HIV and hepatitis B virus (HBV) are more likely to develop end-stage liver disease (ESLD) than those infected with HBV alone. Consequently, liver transplantation is often required for these patients. This study evaluates the outcomes of orthotopic liver transplantation (OLT) of HIV-HBV coinfected patients in China.
Methods
We conducted a retrospective analysis on all HIV-HBV coinfected patients that underwent OLT from April 1, 2019 to December 31, 2021 and their outcomes were compared to all HBV monoinfected patients undergoing OLT during the same period. Patient outcomes were determined, including cumulative survival, viral load, CD4 T-cell count and postoperative complications.
Results
The median follow-up of HIV recipients was 36 months after OLT (interquartile range 12–39 months). Almost all patients had stable CD4 T-cell count (> 200 copies/ul), undetectable HBV DNA levels, and undetectable HIV RNA load during follow-up. The 1-, 2-, and 3-year posttransplant survival rates were 85.7% for the HIV group (unchanged from 1 to 3 years) versus 82.2%, 81.2%, and 78.8% for the non-HIV group. Cumulative survival among HIV-HBV coinfected recipients was not significantly different from the HBV monoinfected recipients (log-rank test P = 0.692). The percentage of deaths attributed to infection was comparable between the HIV and non-HIV groups (14.3% vs. 9.32%, P = 0.665). Post OLT, there was no significant difference in acute rejection, cytomegalovirus infection, bacteremia, pulmonary infection, acute kidney injury, de novo tumor and vascular and biliary complications.
Conclusions
Liver transplantation in patients with HIV-HBV coinfection yields excellent outcomes in terms of intermediate- or long-term survival rate and low incidence of postoperative complications in China. These findings suggest that OLT is safe and feasible for HIV-HBV coinfected patients with ESLD.
Trial registration
Chinese Clinical Trial Registry (ChiCTR2300067631), registered 11 January 2023.
Similar content being viewed by others
Background
Highly active antiretroviral therapy (HAART), introduced in 1996, has significantly improved the survival of patients infected with the human immunodeficiency virus (HIV) [1]. It is well-established that HAART can suppress HIV replication, enhance immune function, and reduce opportunistic infections. With effective antiretroviral therapy, HIV infection has become a chronic disease, and the clinical comorbidities are increasing [2]. Among HIV-infected patients in China, HBV coinfection rates range between 9.5 and 14.5%, and the prevalence of (hepatitis B virus) HBV surface antigen (HBsAg) is estimated to be 13.7% [3, 4]. Liver-related mortality in HIV patients with viral hepatitis has become a major cause of death in many countries. Thus, end-stage liver disease (ESLD) has become the leading cause of death in HIV patients induced by HBV coinfection [5]. Besides, it is widely thought that HIV coinfection accelerates the course of liver disease and increases mortality. Despite recent advances in treating chronic hepatitis B, liver transplantation (LT) remains the last resort for patients with ESLD [6].
HIV infection has long been considered an absolute contraindication to liver transplantation due to this patient population’s relatively shorter life expectancy. Although the past decade has witnessed significant research progress, the clinical efficacy of LT has not been established. HIV infection and rejection-resistant immunosuppression after LT expose HIV recipients to serious complications, especially opportunistic infections. However, the advent of HAART has improved the prognosis for HIV-infected patients and encouraged many transplant centers to accept HIV-positive candidates. Several studies on outcomes of HIV-positive patients after LT have demonstrated stable HIV infection, survival, and complication rates comparable to HIV-negative patients [7,8,9].
To our knowledge, this is the first retrospective analysis of HIV-infected Chinese patients with HBV-related ESLD who underwent liver transplantation in the HAART era. Importantly, we evaluated the outcomes of all liver transplantations in HIV-positive patients and compared them with HIV-negative ones.
Materials and methods
Study design and participants
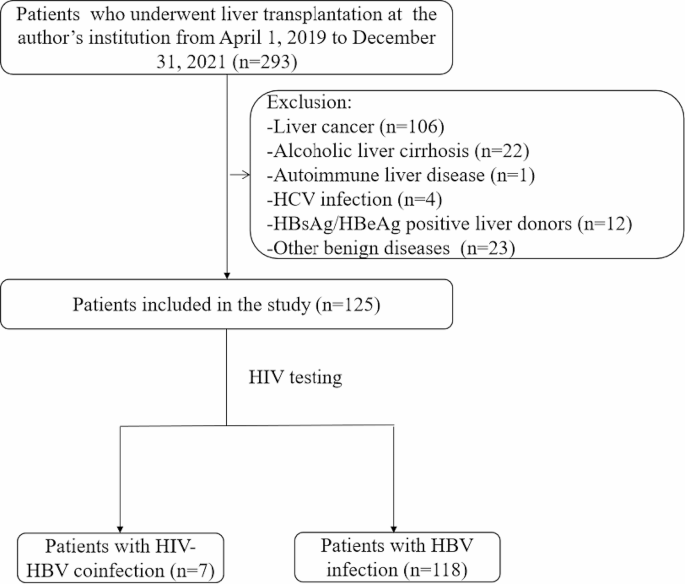
A retrospective, analytical and unicentric study was performed, and the design scheme is shown in Fig. 1. The study protocol followed the principles of the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Shenzhen Third People’s Hospital (No. 2022-038-02). The study has been registered in Chinese Clinical Trial Registry (ChiCTR2300067631). All patients signed an informed consent form before liver transplantation.
We identified all patients (n = 293) who underwent a total orthotopic liver transplantation (OLT) for ESLD between April 1, 2019 to December 31, 2021. We excluded patients younger than 18 years, and those underwent combined transplantation (liver-kidney) or transplantation for primary liver cancer, alcoholic liver cirrhosis, HCV, autoimmune liver disease, other benign diseases or without known ESLD. Clinical data were collected, including sex, age, etiology of liver disease, HIV viral load, CD4 T cell counts, and model of end-stage liver disease (MELD) score. HIV patients that were placed on the waiting list for OLT had similar characteristics to non-HIV ones. In addition, the criteria for efficacy in HIV patients included a stable CD4 T-cell count and serum HIV RNA levels < 500 copies/mL. Our multidisciplinary team, including infectious disease specialists, hepatologists and transplant surgeons, was in charge of selecting patients on the waiting list.
Liver transplantation and immunosuppressive therapy
Liver grafts were obtained from cadaveric donors. All donors were HIV and HBV/HCV-negative. The surgical technique of LT was a modified piggyback technique with triangulation of the hepatic veins. The liver-transplantation procedure and immunosuppressive regimen have been described in our previous published articles [10]. Besides, 2 patients with ABO-incompatible liver transplantation received additional induction therapy, including plasmapheresis twice, and rituximab 375 mg/m2 in two divided doses was administered intravenously before surgery. The immunosuppressive regimen used after liver transplantation was documented in all HIV patients.
HAART Therapy
All patients receiving HAART had documented treatment before and after LT. Instead of using a standard antiretroviral regimen, each patient received an individualized treatment regimen pattern based on tolerability, genotypic sensitivity of the HIV, and physician preference. However, treating HBV infection in a coinfected patient with lamivudine or tenofovir alone can result in HIV resistance to these drugs, which may affect anti-HIV treatment options. All changes in HAART treatment after LT were recorded. Subsequently, some post-LT patients with HIV were switched to albuvirtide and dolutegravir, which have low hepatorenal toxicity and are not CYP3A4 enzyme inhibitors, reducing the impact of calcineurin inhibitor-type immunosuppressive drugs [11–12].
Infection prophylaxis
Given the immunodeficiency from HIV and the antirejection medication, infection prophylaxis is critical. Preoperative prophylaxis was based on a regimen of third-generation cephalosporin plus lactamase inhibitor (3 g/day) from induction to POD 14. Caspofungin (50 mg/day), which has a low effect on tacrolimus concentrations, was administered as an antifungal prophylaxis on the first postoperative day until 2 weeks after surgery. Oral co-trimoxazole for the first 3 months to prevent Pneumocystis jirovecii prophylaxis. For cytomegalovirus prophylaxis, ganciclovir 5 mg/kg daily was given for 2 weeks after liver transplantation. When cytomegalovirus antigen level > 10 was positive, ganciclovir 10 mg/kg/day was administered intravenously (IV) until cytomegalovirus antigen negativity. The antibiotic treatment plan was changed according to the postoperative infection etiological detection and drug susceptibility test.
Prophylaxis against hepatitis B virus recurrence
All patients received long-term passive immunization to prevent hepatitis B virus recurrence after LT. The regimen for prevention of HBV recurrence after LT was as follows: intraoperative administration of 4,000 IU of hepatitis B immune globulin (HBIG) intravenously during the anhepatic phase of liver transplantation, followed by 2,000 IU daily for the first 7 postoperative days. Subsequently, the titer of hepatitis B surface antibody (HBsAb) was maintained above 500 IU/ml within the first postoperative month. Immunoprophylaxis was continued subsequently and throughout follow-up, with monthly intramuscular injections of HBIG 400 to 600 IU to keep the titer of HBsAb greater than 100 IU/ml. From 2019, all patients who underwent LT due to HBV infection received dual immunization prophylaxis with HBIG and nucleoside (acid) analogs. Resumption of oral antiretroviral drugs occurred on day 2 after LT.
Monitoring of graft liver
Regular graft liver biopsies were performed in all patients after intraoperative donor liver blood recirculation, 6 months after liver transplantation, and then annually after liver transplantation. If the result of the liver function examination was abnormal, an additional liver biopsy was required. Each liver biopsy tissue was fixed and paraffin-embedded for histological examination. Diagnosis of acute or chronic rejection was based on the Banff classification.
Postoperative follow-up
Postoperative follow-up schedule: Patients were followed 3 times weekly for the first 2 weeks, then once a week for the 1st month, every 2 weeks for the 3rd month, monthly for the 6th month, every 2 months at the end of the 1st year, and every 3 months for the second and third years. The follow-up plan was adjusted according to the patient’s condition. Post-transplant data collection included liver and kidney function tests, blood cell analysis, blood coagulation function, CD4 T cell counts, HIV-RNA, HBV-DNA, HBsAb levels, immunosuppressive doses and all clinically relevant events such as rejection and infectious complications.
Statistical analysis
All statistical analyses were performed using SPSS 24.0 statistical software. Categorical variables were displayed as frequency (%), continuous variables as mean ± SD or median (interquartile range) used for the descriptive statistics, as appropriate. Group comparisons for categorical variables were performed using the χ²-test and for metric variables using the Mann-Whitney U test. Survival curves were drawn using the Kaplan-Meier method and compared with the log-rank test. A two-sided p-value < 0.05 was statistically significant.
Results
General clinical data
The study included 125 patients with HBV cirrhosis decompensation or liver failure who underwent OLT between April 1, 2019 to December 31, 2021. Seven were HIV/HBV coinfected patients (all male) with a median age of 52 years (interquartile range 46–61 years), including 2 patients with ABO-incompatible liver transplantation. 118 patients without HIV infection that underwent OLT during the same period were also included, and the clinical data are summarized in Table 1. There was no statistically significant difference in charateristic variables (age, sex, indication, HBV DNA load, and MELD score) between the HIV and non-HIV groups.
Patient and graft survival
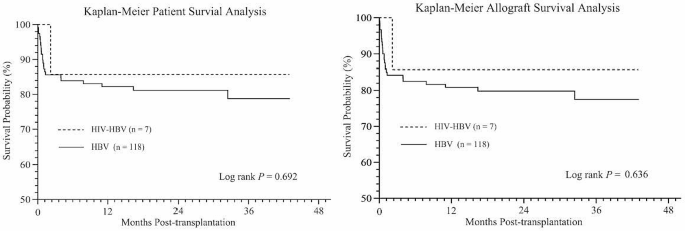
All HIV patients survived beyond 30 days following LT. The median follow-up of HIV recipients was 36 months after LT (interquartile range 12–39 months). As shown in Fig. 2A, the actual 1-, 2-, and 3-year survival rates were 85.7%, 85.7%, and 85.7% for the HIV group versus 82.2%, 81.2%, and 78.8% for the non-HIV group, respectively (log-rank test P > 0.05). The graft 1-, 2-, and 3-year survival rates were 85.7%, 85.7%, and 85.7% for the HIV group versus 80.8%, 79.8%, and 77.5% for the non-HIV group, respectively (P > 0.05, log-rank test) (Fig. 2B). The observed all-cause mortality was 14.3% (n = 1/7) in the HIV group compared to 19.5% in the non-HIV group (n = 23/118). One HIV recipient developed severe septicemia with progressive multiorgan failure and died 2 months after LT. In the non-HIV group, 23 deaths were due to septicemia (34.78%, n = 8), severe pneumonia (13.04%, n = 3), cardiovascular events (8.70%, n = 2), neurological events (13.04%, n = 3), gastrointestinal bleeding (13.04%, n = 3), de novo lung cancer (4.35%, n = 1), graft versus host disease (4.35%, n = 1), early allograft dysfunction (4.35%, n = 1) and disseminated intravascular coagulation (4.35%, n = 1). Of the 23 deaths in the non-HIV group, 17 (73.91%) occurred within 2 months after OLT.
Kaplan-Meier curves illustrating (A) patient survival and (B) allograft survival in the liver transplant cohort, comparing HIV-HBV coinfected group with HBV monoinfected group. Log rank test P = 0.692 for patient survival and P = 0.636 for allograft survival. HBV, hepatitis B virus; HIV, human immunodeficiency virus
Rejection and immunosuppression
As shown in Tables 1 and 2 of 7 HIV-infected patients (14.29%) experienced acute allograft rejection. Acute rejection was observed in patient 1 at 2 weeks after liver transplantation. The Banff rejection activity index scores were 4 for patient 1, which suggested mild acute rejection. No patient had histological evidence of chronic rejection. A further course of methylprednisolone and an increased dose of tacrolimus and MMF to enhance baseline immunosuppression was required until graft liver function returned to normal. The only HIV-infected patient who died experienced severe pneumonia and infection post-LT. The infection in patient 7, with obvious bone marrow depression and leukopenia, was thought to result from excessive immunosuppression, and the tacrolimus dose was reduced or even stopped. This episode was unrelated to his demise, which occurred 2 months post-LT. The rejection rate was 14.29% in the HIV group and 4.24% in the non-HIV group, which was not statistically significant (P = 0.227).
All 7 HIV-infected patients received immunosuppression with steroids, tacrolimus, and mycophenolate mofetil. During the follow-up, patients 1, 2 and 3 were switched from tacrolimus to sirolimus due to impaired renal function and patient 6 due to neurologic toxicity. As described above, patient 1 received steroid pulses therapy (80 mg methylprednisolone for 3 days, then tapered) and 200 mg rituximab twice approximately 2 weeks after ABO-incompatible LT due to high anti-A IgG and IgM titers (1:64 and 1:16, respectively) and acute cellular rejection. Liver enzymes decreased and subsequently remained in the normal range.
Patient outcomes following HAART
Of the 7 patients with HIV infection, 3 received more than 12 months of HAART prior to LT. Patients 6 and 7, receiving only 1 month of HAART, developed acute liver failure secondary to HBV and were unaware of HIV infection prior to their hospitalization. All patients receiving HAART had documented treatment before and after LT. Instead of using a standard antiretroviral regimen, each patient received an individualized treatment regimen pattern based on tolerability, genotypic sensitivity of the HIV, and physician preference.
All HIV-infected patients continued to receive HAART treatment on the second day after transplantation. Table 2 shows the antiretroviral and CD4 T-cell count and HIV load for each patient after OLT. Patient 1 had a low CD4 T-cell count (42 cells/ul) with an undetectable HIV load before OLT. However, with HAART, the HIV load was undetectable (< 50 copies/ ml), and the CD4 T-cell count was stable (> 200 cells/ ul) 43 months after OLT. Patients 2, 3 and 4 had stable CD4 T-cell count (> 200 cells/ul) and an undetectable HIV load (< 250 copies/ml) 3 years after OLT. The pretransplant antiretroviral therapy was continued until OLT. The HAART regimen was altered to completely suppress persistent low-level HIV replication, depending on the patient’s condition. Patient 1 was switched from FTC/TDF and DTG to 3TC, TAF and DTG 6 months before OLT. For antiretroviral therapy in patients with a low CD4 T-cell count and an undetectable HIV load, TAF combination therapy continued to be added after liver transplantation. Although HIV load could not be detected after liver transplantation, patient 5 had a low CD4 T-cell count, which remained above 200 cells/ul after switching to B/F/TAF. Patient 7 had an extremely low CD4 T cell count, with an undetectable HIV load after OLT. On the second day, the patient was switched to dolutegravir, albuvirtide, and tenofovir alafenamide. Patient 7 developed severe pneumonia 10 days after OLT. At the time of death, the patient had an HIV viral load of < 500 copies/ml and a CD4 T-cell count of 3 cells/ul. The patient exhibited a progressive course of progression on HAART and low-dose immunosuppressive therapy until he died of sepsis and multiple organ failure 66 days after OLT.
Incidence of infection after OLT
CMV viremia was detected in 5 patients and treated with intravenous ganciclovir. There was no significant difference in the percentage of patients with CMV viremia infection between the HIV group (71.43%, 5 of 7) and the non-HIV group (39.83%, 47 of 118). One HIV recipient developed severe septicemia and severe pneumonia 10 days after OLT. Pathogenic culture and next-generation sequencing of alveolar lavage fluid and blood samples yielded carbapenem-resistant Pseudomonas aeruginosa and Aspergillus. According to drug sensitivity results, voriconazole, amphotericin B liposome, ceftazidime and amikacin were used for combined treatment. Unfortunately, the patient died of sepsis with progressive multiorgan failure 2 months after transplantation. The observed percentage of HIV-infected patients who died of infection was 14.3% (n = 1/7) versus only 9.32% (n = 11/118) in the non-HIV group. The mortality rate due to infectious causes was comparable between the two groups (P = 0.665).
Recurrence of viral hepatitis
Four HIV-infected patients had detectable HBV viral loads at the time of transplant. All four patients that underwent OLT for HBV-related liver disease had undetectable HBV DNA by polymerase chain reaction after surgery. The HBV-DNA levels were less than 100 copies/ml in serum samples from all 7 patients after liver transplantation and remained undetectable during the follow-up period. HBsAg became undetectable within 2 days of liver transplantation. All patients were HBeAg negative after liver transplantation to the end of follow-up. Details of HBV prophylaxis with hepatitis B immunoglobulin in combination with nucleoside or nucleotide analogs are shown in Table 3.
Discussion
In the HAART era, liver transplantation for HIV-infected patients is considered a reasonable choice. However, in the past, most transplant centers refused to accept HIV- infected patients for liver transplantation due to organ shortage and insufficient data. Liver transplantation has been performed in HIV-infected patients in several transplant centers in recent years [13]. Consistent with the literature, we substantiated that liver transplantation is feasible in this patient population; indeed, postoperative HAART therapy can suppress the viral load, stabilize CD4 T-cell count, and lead to no significant increase in opportunistic infections [14,15,16]. Few reports have involved liver transplantation outcomes in HIV/HBV coinfected patients. About 74 million people in China are carriers of the hepatitis B virus, which represents a serious public health issue [17]. Herein, we summarize the results of liver transplantation in HIV-positive patients with HBV from China.
The overall mortality rate for liver transplantation in HIV/HBV coinfected patients at our transplant center was 14.3% (1/7), lower than the mortality rate reported by a Spanish transplant center (38%) and in HIV-infected patients transplanted in Germany (41%) [18, 19]. Our study found 85.7% survival of both patient and graft after a mean follow-up of 36 months (with a maximum follow-up of 43 months), demonstrating the applicability of liver transplantation in this subgroup of patients. Compared with previous studies, the lower mortality rate in HIV/HBV coinfected patients is closely related to the development of efficient antiretroviral therapy, which has low drug resistance, high efficacy, and minimal interaction with commonly used immunosuppressive drugs. In our study, HIV patients were treated with INSTIs after OLT, especially second-generation INSTIs. In addition, the low mortality rate is also influenced by factors such as improvements in liver transplant surgery techniques, perioperative management, and antibiotic upgrades. HIV monoinfection or opportunistic infection does not appear to be a significant risk factor for patient survival after transplantation [20]. Current evidence suggests bacterial infection and sepsis are the leading causes of death after liver transplantation, especially in the early post-transplantation period [21, 22]. Patient 7, who had an HIV load < 50 copies/ml but a low CD4 T-cell count before LT, died of multiple organ failure from bacterial infection and sepsis 66 days after LT. In contrast, the CD4 T-cell count of patient 1 was less than 50 cells/ ul before LT, but the HIV disease was stable, and the patient remained alive after LT. He had an undetectable HIV load (< 50 copies/ ml) and a stable CD4 T-cell count (> 200 cells/ ul), with no opportunistic infection 43 months after LT. This indicates that a T-cell count of fewer than 100 cells/ ul is not an absolute contraindication of liver transplantation without definite infection. These discrepancies may reflect differences in postoperative infection prophylaxis and immunosuppressive management and underline the importance of aggressive infection-prevention therapy and avoidance of excessive immunosuppression early after transplantation in HIV/HBV coinfected patients.
HCV coinfection remains a key factor in the mortality of HIV-positive patients after liver transplantation in European and American HIV-positive patients [23, 24]. HCV recurrence in HIV patients is more aggressive, and liver fibrosis is more rapid due to HAART toxicity [25]. An increasing body of evidence suggests that treatment for HCV recurrence positively affects graft survival and mortality [26, 27]. Unlike HCV coinfection, HIV-infected patients with HBV appear to have better outcomes after OLT when HBV reinfection prophylaxis is properly provided [28]. Notably, there was no significant difference in survival between HIV/HBV coinfected patients and HBV monoinfected patients after liver transplantation. Anadol et al. showed that the 5-year survival rate of HIV/HBV-coinfected patients was 80%, and none of the HIV/HBV-coinfected patients developed clinically relevant HBV-related end-stage liver disease after liver transplantation [19]. The best option for preventing recurrent HBV infection in HIV/HBV coinfected patients appears to be combining pretransplantation and posttransplantation antiviral therapy with HBIG administration [29]. In our study, all patients received prophylaxis in combination with HBIG and antiviral therapy. Consistent with findings reported by Tateo et al. [30], all HBV/HIV coinfected patients were HBsAg-negative and HBV-DNA below 100 IU/ml. Interestingly, despite the successful prevention of recurrent hepatitis B, low levels of HBV-DNA were detected in approximately 50% of HBV/HIV coinfected patients treated with this combination regimen, and an 85% patient survival rate was achieved at 4 years of follow-up [31].
In the early posttransplant period, graft function and rejection prevention are major determinants of the outcomes of HBV/HIV coinfected patients instead of HIV infection. In addition, although HIV patients are considered immunosuppressed, there is an additional issue in managing these patients that may lead to higher rejection rates [32]. In the present study, 1 case of mild acute rejection was identified in HIV patients (14.3%) compared to 4.24% in non-HIV patients with no significant difference. All rejection episodes were easily treated according to routine protocols as previously described. Patient 7 was transferred to the intensive care unit due to severe pulmonary infection 10 days after the operation, and the concentration of tacrolimus and the dose of MMF were reduced accordingly. Two weeks after the operation, the liver function gradually deteriorated, and a liver biopsy showed acute cholestatic hepatitis with inflammation and necrosis equivalent to G3, moderate intrahepatic cholestasis, and no indication of acute rejection. Due to sepsis and multiple organ dysfunction, the patient was treated with low-dose methylprednisolone antirejection therapy. This episode was unrelated to his demise, which occurred 2 months post-LT. Coffin et al. showed that the acute rejection rates in HIV/HBV coinfected patients and monoinfected HBV patients were comparable (22.7% (n = 5/22) vs. 10% (n = 2/20), p > 0.05) [31].
Postoperative infection of HIV patients is also a key concern in this patient population. Although the prevalence of CMV viremia in patients with advanced HIV infection remains high, good immune recovery by antiretroviral treatment is sufficient to suppress CMV viral levels without increasing the risk of CMV end-organ disease [33]. Based on the medical literature, there has been no increase in the incidence rate of transplant or HIV-related opportunistic infections. The incidence of CMV and other opportunistic infections was not different between the HIV-positive and HIV-negative groups. More importantly, HIV did not appear to progress after liver transplantation in the post-HAART era. CD4 T-cell counts and HIV viral loads were stable in most patients as long as HAART could be administered [34]. In our study, there was no significant difference in the incidence of CMV viremia, bacteremia, and pulmonary infection between the HIV group and the non-HIV group. The comparable mortality rates attributed to infection between the HIV and non-HIV groups suggest that the risk of opportunistic infection after liver transplantation is not increased under HAART treatment.
The optimal management of the HAART regimen after OLT has not yet been determined. Pharmacological interactions between calcineurin inhibitors and HAART regimens containing protease inhibitors have been documented [35, 36]. Potential drug interactions must be considered when considering a specific antiretroviral regimen. Protease inhibitors (PI) are a part of most HAART regimens [37]. It is well known that PIs inhibit CYP3A, a component of the cytochrome P450, which results in markedly prolonged half-lives of the calcineurin inhibitors and sirolimus [38]. Accordingly, we must consider the optimal timing of HAART initiation, drug interactions between HAART and immunosuppressive regimen, and the control of disease recurrence after transplantation. All 7 Chinese patients with HIV/HBV coinfection began to receive HAART treatment early, within 2 days after OLT. The choice of drugs for immunosuppression and antiretroviral therapy is another key factor. Potential hepatotoxicity must be considered in the selection of HAART regimens to reduce liver-related mortality, such as stavudine (D4T) [39], azidothymidine (AZT) [40] or didanosine (ddI) [41]. All Chinese patients did not receive the above HAART drugs. In addition, HAART drugs with minimal interactions with other drugs must be considered. Subsequently, some post-LT patients with HIV were switched to albuvirtide and dolutegravir, which have low hepatorenal toxicity and are not CYP450 enzyme inhibitors, reducing the impact of calcineurin inhibitor-type immunosuppressive drugs. In this study, the HIV RNA load could not be detected during follow-up after LT. However, the adverse effects of HAART combined with immunosuppressive drugs on graft survival, the right time and dose of HAART after LT and the frequency of other secondary complications need to be further evaluated.
In conclusion, our data suggest that liver transplantation for patients with HIV/HBV coinfection represents the only way to survive from decompensated cirrhosis or liver failure. The outcome of these recipients is highly dependent on the patient’s state at the time of transplantation. Patient 1 in this study had a CD4 T-cell count of less than 100 cells/ul, which is not an absolute contraindication of liver transplantation without an established infection. The acceptable survival rate and control of HIV/HBV replication corroborate that the strategy of providing liver transplantation for HIV/HBV-coinfected patients with acute liver failure and end-stage liver disease is reasonable in China. Additional studies must be performed to determine medium- or long-term survival and improve post-transplant management to balance complex interaction factors, such as HAART and immunosuppressive drug selection, optimal treatment timing and dose adjustment.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available as they contain information that could compromise participant privacy and consent but are available from the corresponding author on reasonable request.
Abbreviations
- 3TC:
-
Lamivudine
- ACLF:
-
Acute-on-chronic liver failure
- B/F/TAF:
-
Bictegravir/emtricitabine/tenofovir alafenamide
- CMV:
-
Cytomegalovirus
- DLC:
-
Decompensated liver cirrhosis
- ESBL:
-
End-stage liver disease
- FTC:
-
Emtricitabine
- HAART:
-
Highly active antiretroviral therapy
- HBV:
-
Hepatitis B virus
- HBeAg:
-
Hepatitis B e-antigen
- HBIG:
-
Hepatitis B immune globulin
- HBsAg:
-
Hepatitis B surface antigen
- HIV:
-
Human immunodeficiency virus
- MELD:
-
Model for end stage liver disease
- MMF:
-
Mycophenolate mofetil
- OLT:
-
Orthotopic liver transplantation
- POD:
-
Postoperative day
- TAF:
-
Tenofovir alafenamide
- TDF:
-
Tenofovir disoproxil fumarate
References
Survival. of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017; 4: e349-e356.
Rowell-Cunsolo TL, Hu G, Bellerose M, Liu J. Trends in Comorbidities among Human Immunodeficiency Virus-Infected Hospital Admissions in New York City from 2006–2016. Clin Infect Dis. 2021;73:e1957–63.
Xie J, Han Y, Qiu Z, Li Y, Li Y, Song X, et al. Prevalence of hepatitis B and C viruses in HIV-positive patients in China: a cross-sectional study. J Int AIDS Soc. 2016;19:20659.
Yu S, Yu C, Li J, Liu S, Wang H, Deng M. Hepatitis B and Hepatitis C prevalence among people living with HIV/AIDS in China: a systematic review and Meta-analysis. Virol J. 2020;17:127.
Yang R, Gui X, Xiong Y, Gao SC, Yan Y. Impact of hepatitis B virus infection on HIV response to antiretroviral therapy in a Chinese antiretroviral therapy center. Int J Infect Dis. 2014;28:29–34.
Lacombe K, Rockstroh J. HIV and viral Hepatitis coinfections: advances and challenges. Gut. 2012;61:i47–58.
Agüero F, Forner A, Manzardo C, Valdivieso A, Blanes M, Barcena R. Human immunodeficiency virus infection does not worsen prognosis of liver transplantation for hepatocellular carcinoma. Hepatology. 2016;63:488–98.
Jacob JS, Shaikh A, Goli K, Rich NE, Benhammou JN, Ahmed A, et al. Improved survival after liver transplantation for patients with HIV and HIV/HCV Coinfection in the INSTI and DAA eras. Clin Infect Dis. 2023;76:592–9.
Peters MG, Kottilil S, Terrault N, Amara D, Husson J, Huprikar S. Retrospective-prospective study of safety and efficacy of sofosbuvir-based direct-acting antivirals in HIV/HCV-coinfected participants with decompensated liver disease pre- or post-liver transplant. Am J Transpl. 2021;21:1780–8.
Tang J, Zhang K, Fang T, Weng R, Liang Z, Yan X, et al. Outcomes of ABO-incompatible liver transplantation in end-stage liver disease patients co-infected with hepatitis B and human immunodeficiency virus. World J Gastroenterol. 2023;29:1745–56.
Yang W, Xiao Q, Wang D, Yao C, Yang J. Evaluation of pharmacokinetic interactions between long-acting HIV-1 fusion inhibitor albuvirtide and lopinavir/ritonavir, in HIV-infected subjects, combined with clinical study and simulation results. Xenobiotica. 2017;47:133–43.
Miller MM, Liedtke MD, Lockhart SM, Rathbun RC. The role of dolutegravir in the management of HIV infection. Infect Drug Resist. 2015;8:19–29.
Eman P, Chacon E, Gupta M, Berger JC, Shah MB, El Haddad HE, et al. Long term outcomes of patients transplanted for hepatocellular carcinoma with human immunodeficiency virus infection. HPB (Oxford). 2019;21:1009–16.
Baccarani U, Righi E, Adani GL, Lorenzin D, Pasqualucci A, Bassetti M, et al. Pros and cons of liver transplantation in human immunodeficiency virus infected recipients. World J Gastroenterol. 2014;20:5353–62.
Schreibman I, Gaynor JJ, Jayaweera D, Pyrsopoulos N, Weppler D, Tzakis A, et al. Outcomes after orthotopic liver transplantation in 15 HIV-infected patients. Transplantation. 2007;84:697–705.
Vernadakis S, Sotiropoulos GC, Brokalaki EI, Esser S, Kaiser GM, Cicinnati VR, et al. Long-term outcomes of liver transplant patients with human immunodeficiency virus infection and end-stage-liver-disease: single center experience. Eur J Med Res. 2011;16:342–8.
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55.
Anadol E, Beckebaum S, Radecke K, Paul A, Zoufaly A, Bickel M, et al. Orthotopic liver transplantation in human-immunodeficiency-virus-positive patients in Germany. AIDS Res Treat. 2012;2012:197501.
Testillano M, Fernandez JR, Suarez MJ, Gastaca M, Bustamante J, Pijoan JI, et al. Survival and hepatitis C virus recurrence after liver transplantation in HIV- and hepatitis C virus-coinfected patients: experience in a single center. Transpl Proc. 2009;41:1041–3.
Teicher E, Boufassa F, Vittecoq D, Antonini TM, Tateo MG, Coilly A, et al. Infectious complications after liver transplantation in human immunodeficiency virus-infected recipients. Transpl Infect Dis. 2015;17:662–70.
Miro JM, Agüero F, Duclos-Vallée JC, Mueller NJ, Grossi P, Moreno A. Infections in solid organ transplant HIV-infected patients. Clin Microbiol Infect. 2014;20:119–30.
Moreno A, Cervera C, Fortún J, Blanes M, Montejo E, Abradelo M. Epidemiology and outcome of infections in human immunodeficiency virus/hepatitis C virus-coinfected liver transplant recipients: a FIPSE/GESIDA prospective cohort study. Liver Transpl. 2012;18:70–81.
Campos-Varela I, Dodge JL, Berenguer M, Adam R, Samuel D, Di Benedetto F, et al. Temporal trends and outcomes in Liver Transplantation for recipients with HIV infection in Europe and United States. Transplantation. 2020;104:2078–86.
Dalla E, Bulfoni M, Cesselli D, Pravisani R, Hidaka M, Eguchi S, et al. Reinfection of transplanted livers in HCV- and HCV/HIV-Infected patients is characterized by a different MicroRNA expression Profile. Cells. 2022;11:690.
Gobran ST, Ancuta P, Shoukry NH. A tale of two viruses: immunological insights into HCV/HIV coinfection. Front Immunol. 2021;12:726419.
Agüero F, Forner A, Valdivieso A, Blanes M, Barcena R, Manzardo C. Human immunodeficiency virus-infected liver transplant recipients with incidental hepatocellular carcinoma: a prospective multicenter nationwide cohort study. Liver Transpl. 2017;23:645–51.
Agüero F, Rimola A, Stock P, Grossi P, Rockstroh JK, Agarwal K. Liver retransplantation in patients with HIV-1 infection: an International Multicenter Cohort Study. Am J Transpl. 2016;16:679–87.
Congly SE, Doucette KE, Coffin CS. Outcomes and management of viral hepatitis and human immunodeficiency virus co-infection in liver transplantation. World J Gastroenterol. 2014;20:414–24.
Roche B, Roque-Afonso AM, Nevens F, Samuel D. Rational basis for optimizing short and long-term Hepatitis B Virus Prophylaxis Post Liver transplantation: role of Hepatitis B Immune Globulin. Transplantation. 2015;99:1321–34.
Tateo M, Roque-Afonso AM, Antonini TM, Medja F, Lombes A, Jardel C. Long-term follow-up of liver transplanted HIV/hepatitis B virus coinfected patients: perfect control of hepatitis B virus replication and absence of mitochondrial toxicity. Aids. 2009;23:1069–76.
Coffin CS, Stock PG, Dove LM, Berg CL, Nissen NN, Curry MP, et al. Virologic and clinical outcomes of hepatitis B virus infection in HIV-HBV coinfected transplant recipients. Am J Transpl. 2010;10:1268–75.
Righi E, Ivaldi F, La Rosa A, Carnelutti A, Londero A, Bassetti M. Immunological profiles of HIV-positive recipients of liver transplant. Transpl Immunol. 2019;57:101208.
Albasanz-Puig A, Suanzes P, Esperalba J, Fernández C, Sellarès-Nadal J, Torrella A. Low frequency of cytomegalovirus (CMV) disease despite high prevalence of CMV viraemia in patients with advanced HIV infection: a clinical and immunological 48-week follow-up study. HIV Med. 2021;22:682–9.
Neff GW, Sherman KE, Eghtesad B, Fung J. Review article: current status of liver transplantation in HIV-infected patients. Aliment Pharmacol Ther. 2004;20:993–1000.
Elliot E, Chirwa M, Boffito M. How recent findings on the pharmacokinetics and pharmacodynamics of integrase inhibitors can inform clinical use. Curr Opin Infect Dis. 2017;30:58–73.
Sparkes T, Manitpisitkul W, Masters B, Bartlett ST, Davis C, Husson J, et al. Impact of antiretroviral regimen on renal transplant outcomes in HIV-infected recipients. Transpl Infect Dis. 2018;20:e12992.
Voshavar C. Protease inhibitors for the treatment of HIV/AIDS: recent advances and Future challenges. Curr Top Med Chem. 2019;19:1571–98.
Podany AT, Scarsi KK, Fletcher CV. Comparative clinical pharmacokinetics and pharmacodynamics of HIV-1 integrase strand transfer inhibitors. Clin Pharmacokinet. 2017;56:25–40.
Su S, Fairley CK, Sasadeusz J, He J, Wei X, Zeng H, et al. HBV, HCV, and HBV/HCV co-infection among HIV-positive patients in Hunan province, China: regimen selection, hepatotoxicity, and antiretroviral therapy outcome. J Med Virol. 2018;90:518–25.
Ghare SS, Donde H, Chen WY, Barker DF, Gobejishvilli L, McClain CJ, et al. Acrolein enhances epigenetic modifications, FasL expression and hepatocyte toxicity induced by anti-HIV drug Zidovudine. Toxicol Vitro. 2016;35:66–76.
Ryom L, Lundgren JD, De Wit S, Kovari H, Reiss P, Law M, et al. Use of antiretroviral therapy and risk of end-stage liver disease and hepatocellular carcinoma in HIV-positive persons. Aids. 2016;30:1731–43.
Acknowledgements
Not applicable.
Funding
This study was supported by Shenzhen Third People’s Hospital Scientific Research Project (G2021008, G2022008), Shenzhen Key Medical Discipline Construction Fund (SZXK079), Shenzhen Science and Technology Research and Development Fund (JCYJ20210324131809027, JCYJ20220530163011026).
Author information
Authors and Affiliations
Contributions
Conception and design: Dong Zhao, Jianxin Tang and Ruihui Weng; Administrative support: Dong Zhao; Provision of study materials or patients: Jianxin Tang, Kangjun Zhang and Taishi Fang; Collection and assembly of data: Ruihui Weng, Xu Yan and Xin Jin; Data analysis and interpretation: Ruihui Weng, Linjie Xie; Manuscript writing: Dong Zhao, Jianxin Tang and Ruihui Weng; Final approval of manuscript: All authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol followed the principles of the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Shenzhen Third People’s Hospital (No. 2022-038-02). The study has been registered in Chinese Clinical Trial Registry (ChiCTR2300067631). All patients signed an informed consent form before liver transplantation.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, J., Weng, R., Fang, T. et al. Clinical outcomes of liver transplantation in human immunodeficiency virus/hepatitis B virus coinfected patients in China. BMC Infect Dis 24, 383 (2024). https://doi.org/10.1186/s12879-024-09284-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09284-2