Abstract
Purpose
The significant proportion of asymptomatic patients and the scarcity of genotypic analysis of lymphogranuloma venereum (LGV), mainly among men who have sex with men (MSM), triggers a high incidence of underdiagnosed patients, highlighting the importance of determining the most appropriate strategy for LGV diagnosis, at both clinical and economical levels.
Materials and methods
We conducted L1-L3 serovar detection by molecular biology in stored Chlamydia trachomatis-positive samples from MSM patients with HIV, another STI or belonging to a Pre-exposure prophylaxis program, to make a cost effectiveness study of four diagnostic strategies with a clinical, molecular, or mixed approach.
Results
A total of 85 exudates were analyzed: 35urethral (31 symptomatic/4 positive) and 50 rectal (22 symptomatic/25 positive), 70/85 belonging to MSM with associated risk factors. The average cost per patient was €77.09 and €159.55 for clinical (Strategy I) and molecular (Strategy IV) strategies respectively. For molecular diagnosis by genotyping of all rectal exudate samples previously positive for CT (Strategy II), the cost was €123.84. For molecular diagnosis by genotyping of rectal and/or urethral exudate samples from all symptomatic patients (proctitis or urethritis) with a previous positive result for CT (Strategy III), the cost was €129.39. The effectiveness ratios were 0.80, 0.95, 0.91, and 1.00 for each strategy respectively. The smallest ICER was €311.67 for Strategy II compared to Strategy I.
Conclusions
With 30% asymptomatic patients, the most cost-effective strategy was based on genotyping all rectal exudates. With less restrictive selection criteria, thus increasing the number of patients with negative results, the most sensitive strategies tend to be the most cost-effective, but with a high incremental cost-effectiveness ratio.
Similar content being viewed by others
Introduction
Chlamydia trachomatis (CT) infection is the most frequent sexually transmitted bacterial infection (STI) in developed countries and the one with the highest incidence in Spain among under 25-year-olds [1, 2]. Among other pathologies, CT causes non-gonococcal urethritis, pelvic inflammatory disease, and tubal infertility. Lymphogranuloma venereum (LGV) is predominantly caused by invasive genotypes L1–L3, with molecular biology technologies as the main diagnostic tool [3, 4].
LGV has been considered endemic in Europe since 2003 in men who have sex with men (MSM), with an annual incidence of 1.5 cases per 100,000 population; approximately 70% of those with available serological data are seropositive [5]. CT L1–L3 is among the main pathogens of anorectal sexual transmission, alongside gonococcus, syphilis and HSV [6].
Diagnostic algorithms have traditionally recommended CT genotyping by PCR in CT-positive patients only if LGV-associated symptomatology is present [7]. However, multicenter studies have highlighted the existence of a 27% ratio of asymptomatic patients with LGV, mainly in HIV-positive MSM [8]. Taking these data into account and given the high prevalence of STI among MSM using the pre-exposure prophylaxis program (PrEP) [9], global screening for L1–L3 genotypes could be advisable in these two high-risk groups. However, there is no consensus as to the optimal approach for detecting this pathology or for prior selection of asymptomatic patients, or the risk factors to be considered [6, 10, 11].
CT genotyping results are reported by eleven Autonomous Communities in Spain, of which only seven reported positive cases. A total of 453 positive cases were recorded in 2019, 98% of whom were male [12]. These data show that LGV has a high incidence in Spain, particularly among MSM, and is probably underdiagnosed just as highlights the lack of a unified strategy for detecting genotypes related to lymphogranuloma venereum in patients infected by Chlamydia trachomatis within the country. In view of the above, we carried out a retrospective analysis by CT L1-L3 biovar detection and an economic evaluation to pinpoint the population eligible for these genotypic studies, the real incidence of LGV in our healthcare area, the costs associated with each diagnostic process and the best diagnostic strategy for our setting from a healthcare perspective.
Materials and methods
Patients and samples
L1-L3 biovar detection by molecular biology was carried out retrospectively on all PCR CT-positive urethral and rectal samples of at-risk patients analyzed in the Hospital Clínico Microbiology Service during routine procedures between 2018 and 2023. All samples, CT positive by PCR, had been cryopreserved to -80ºC. Samples from at-risk patients were those corresponding to the following groups: MSM HIV seropositive, MSM PreP-users, or MSM co-infected with Neisseria gonorrhoeae. A control group (18% of the total sample size) without other risk factors was also included.
Analytic methods
We differentiated A–K from L1–L3 CT biovares by multiplex PCR and subsequent hybridization with specific probes in urethral or rectal exudate samples.
Gene extraction and purification was performed using the QIASymphony platform (QIAGEN, Venlo, The Netherlands) with the DSP DNA Pathogen kit. CT DNA detection was performed using the Allplex™ II STI-7 Detection kit (Seegene Inc, South Korea) on the AriaMX Real-time PCR platform, and the subsequent genotypic characterization using the STD Direct Flow Chip amplification assay (Vitro, Granada, Spain), in all cases following the manufacturer’s instructions.
Economic evaluation
We carried out an economic evaluation using the decision tree approach to calculate the incremental cost-effectiveness ratio (ICER) of four hypothetical diagnostic strategies following the JBI critical appraisal checklist for economic evaluation recommendations [13]. These strategies were as follows: 1.- Clinical diagnosis of LGV based on the macroscopic rectal lesions characteristic of the pathology. 2.- Molecular diagnosis by genotyping of all rectal exudate samples previously positive for CT. 3.- Molecular diagnosis by genotyping of rectal and/or urethral exudate samples from all symptomatic patients (proctitis or urethritis) with a previous positive result for CT. 4.- Molecular diagnosis by genotyping of rectal and/or urethral exudate samples from all patients with a positive result for CT regardless of symptomatology. The proportion of true positives, false positives, true negatives, and false negatives for each strategy evaluated was obtained by comparing the hypothetical result we had obtained with each strategy and the real value obtained by the molecular procedure, considering the latter as the reference standard. Clinical data were obtained by clinical chart review.
To select the most cost-effective diagnostic strategy, the different strategies were ranked in order of increasing cost. Strategies dominated by their competitors were excluded due to their higher cost and lower efficacy. The ICER was then calculated using the following formula, and the strategy with the lowest cost-effectiveness ratio (C/E) was selected as optimal [14].
The cost attributable to each branch of the decision tree was calculated by adding the costs associated with the clinical consultation, PCR and genotyping, follow up visits and the time taken for diagnostic testing, supervision and validation by both the specialist technician and the attending physician; this was multiplied in each case by the probability associated with each branch. The cost of additional visits by the specialist in charge were imputed in the case of false negatives. The costs attributable to healthcare employee hours invested and the cost associated with the medical consultation were obtained from official data published by the Conselleria de Sanitat [15] and are described in Table 1.
Sensitivity analysis
We used the deterministic threshold value method for sensitivity analysis: this involves arbitrary substitution of the probabilities defining the model within logical ranges to evaluate the influence of these changes on the decision taken, and thus find the critical value that would change the direction of the decision [16, 17]. To do this, we first increased the proportion of symptomatic patients gradually from 60 to 99%, then in a second analysis we increased the number of asymptomatic patients from 20 to 50%.
Results
Of the 136 patients who met the inclusion criteria, samples were available in only 85, of which 14 corresponded to MSM co-infected with Neisseria gonorrhoeae, 25 to seropositive patients, 31 to patients included in PreP and 15 to MSM infected with CT but without another associated risk factors. The samples (one per patient) were stratified as follows: 35 urethral exudates, 31 from symptomatic patients, of which only 4 (13%) belonged to genogroups L1–L3 (all symptomatic). The remaining 50 samples were rectal exudates, 21 of which corresponded to symptomatic patients. In total, 25 of the 50 exudates belonged to the L1–L3 genogroup (50%), a significantly higher proportion (p < 0.001) than was observed in urethral exudates, and 17 (68%) of these 25 were from symptomatic patients. No cases of LGV were detected in the patient subgroup without associated risk factors (Table 2).
Economic evaluation
The number of true positives (VP), false positives (FP), true negatives (VN) and false negatives (FN) for each of the four strategies is shown in Table 3.
In the clinical arms, the cost associated with TP and FP was 100 euros, corresponding to diagnostic and follow-up visits. The TN cost 50 euros (diagnostic visit only), and the FN totaled 150 euros (added appointment due to diagnostic error). In the arms with genotypic characterization, the TP corresponded to 192.5 euros (150 euros for medical visits, 17.5 euros for laboratory personnel hours and 25 euros for genotypic characterization reagents). The TN amounted to 142.5 euros for the two visits, laboratory time and reagents used for genotypic characterization. Finally, the FN cost 242.5 euros, resulting from the clinical visits, laboratory personnel costs, genotypic reagents and additional follow-up visit associated with diagnostic error (Table 1).
Regarding the decision tree (Fig. 1), the average cost per patient of the different strategies was 77.09, 123.84, 129.39 and 159.55 euros for strategies I, II, III and IV respectively, with effectiveness of 0.80, 0.95, 0.91 and 1.00. This gave an ICER of 311.67 for strategy II compared to strategy I, 412.3 for strategy IV compared to strategy I, and 714.2 for strategy IV compared to strategy II. Strategy III was dominated by its comparators (Supplementary Fig. 1).
Sensitivity analysis
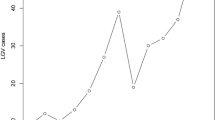
As shown in Fig. 2, the gradual increase in the proportion of symptomatic patients led to progressively higher effectiveness for strategies I and III, while the efficacy of strategy IV was maintained and that of strategy II decreased. The threshold ratio of symptomatic patients was 80%, from which point strategy number III ceased to be dominated, with ICER values of 312.23 € and 479.71€ for strategy II and strategy III respectively, compared to strategy I (Supplementary Table 1).
Studying the influence of asymptomatic patients on optimal strategy selection (Supplementary Fig. 2), the effectiveness of strategy II rose in parallel with an increased proportion of these patients. This was in contrast with other strategies: strategy I and III saw slightly reduced efficacy and strategy IV remained constant. Diagnostic costs decreased in strategies I and III compared to strategy II and IV, the former increasing slightly and the later remaining without changes.
Taking strategy I as a reference once more, the strategy with the lowest ICER at 20% asymptomatic patients was strategy II (312.46 euros). Thereafter, strategy II and IV become progressively closer, although without reaching a threshold value that would modify the meaning of our analysis (Suppementary Table 2).
Discussion
LGV is an infectious pathology transmitted by the CT genogroups L1–L3, which had previously been associated with tropical and subtropical regions. Nonetheless, an outbreak of LGV detected in the Netherlands at the beginning of the century revealed the presence of a large number of asymptomatic patients, mainly in MSM groups [18]. In a previous work carried out in Portugal, Neves et al. [6] found the prevalence of L1–L3 among CT-positive patients to be close to 10%, but nearly 90% of these were seropositive MSM. Another study, carried out in France, observed in anorectal specimens that 20% of patients were L1–L3-positive [19]. In our study we found that 86.2% L1-L3 genotype CT belonged to anorectal exudates and 50% of all anorectal samples tested. The difference found with the abovementioned papers could be caused by the ratio of preselected patients. Taking into account only high risk VIH patients, our ratio of LGV-positive CT was 70%. This discrepancy also highlights the variability in prevalence of this pathology between population groups and underlines the importance of correct pre-analytical selection of patients to optimize resources [6].
Previous studies such as Vries [20] show that urogenital infections due to LGV are less frequent than rectal ones, with an estimated ratio of 1:15 for urogenital vs. rectal infections. In our case, the proportion observed was higher (1/6.25). This difference could be associated with the size of the population, given our limited sample size; however, an alternative form of transmission such as the oral-rectal route also stands out in this context. According to the previously mentioned study [8] as well as the European Guideline on the management of proctitis, proctocolitis and enteritis caused by sexually transmitted pathogens, the proportion of asymptomatic patients infected by LGV reaches between 25 and 30% of all cases of infection [20]. In our work, this value is very similar, reaching 27.5%, which further supports the use of strategies based on molecular biology such as II and IV compared to clinical strategies such as I or III.
Regarding the economic evaluation of the four diagnostic strategies, strategy II was the most cost-effective strategy in our cohort. Australian STI management guidelines [10] recommend testing for all MSM coinfected with another STI pathogen with related symptomatology, and MSM living with a seropositive partner even if asymptomatic provided they have a positive rectal exudate CT result [10]; in contrast, American guidelines support their diagnostic strategy on clinical suspicion and epidemiological information provided there is a positive CT result in a compatible anatomical site [11]. The 2021 European guidelines [6] recommend performing genotyping of all CT-positive anorectal samples from MSM patients and considering genotyping in patients with signs of proctitis/proctocolitis, femoral or inguinal lymphadenopathy or bubo, and/or history of genital ulcers, with priority given to patients with associated risk factors such as PrEP or HIV positive [4].
To our knowledge, no economic evaluation has been published which elucidates the most cost-effective strategy from among different possible alternatives. In Europe, where LGV prevalence among heterosexual patients is very low [4], initial diagnosis via patient screening is fundamental from an efficiency point of view. On the other hand, given the substantial proportion of asymptomatic patients, anorectal symptomatology is not in itself an adequate screening criterion. With the incidence detected in our healthcare area, therefore, the strategy that analyzes all CT positive rectal samples in MSM is the most cost-effective one and therefore we have adopted in our heathcare area. As the sensitivity study shows, an increased proportion of symptomatic patients improves the effectiveness of the test and therefore decreases the ICER ratio of strategies based on clinical diagnosis, or mixed strategies such as strategy III. However, there is a large body of evidence pointing to a high proportion of asymptomatic patients. In fact, this number is likely to increase as the proportion of the population undiagnosed for this pathology is reduced. Finally, the choice of less restrictive criteria, with the consequent reduction of the pretest effect, tends to select the strategy with highest sensitivity as the most profitable one, although we were unable to establish a threshold value that would select strategy IV, based entirely on molecular biology.
Our work has several limitations, the first of which is the small sample size, although this weakness was overcome by the sensitivity analysis. Second, in the cohort of patients using PreP, although all patients included in the program were screened in different anatomical locations, difficulties in control and follow-up during their stay in the program contributed to a lack of sufficient samples from this group. Third, no other studies reporting economic data associated with LGV diagnosis were found for comparison with our results. Fourth, the costs attributed to each branch were estimates, since a micro-costing strategy, which would reflect the expenditure specific to each strategy more accurately, was beyond the scope of this study. Likewise, without access to antibiotic prescription data we were unable to include treatment-associated costs in the economic study. Finally, both costs and efficacy were obtained with in-house experimental data in a specific cohort. This could affect the external validity of the results.
Conclusions
Adequate patient selection enables us to optimize diagnostic strategies. The most cost-effective strategy in our setting was based on genotyping all rectal exudates of at-risk patients with a positive result for CT by PCR (Strategy II).
Less restrictive selection criteria for patient prescreening enables the most sensitive diagnostic approach (genotyping of all rectal samples or genotyping all CT-positive samples) to be the most cost-effective one.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
López-de-Munain J, del Mar Cámara-Pérez M, Imaz-Pérez M, Pereda-Berroeta J, López-Azcarreta I, Muñoz-Sánchez J, Cisterna-Cáncer R, Ferrero-Beneitez OL, Ibarra-Ugarte S, Zubero-Sulibarria Z, Artetxe JM. Chlamydia trachomatis re-infection in Spain: a STI clinic-based cohort study. Enfermedades Infecciosas y microbiología clínica. 2017;35(3):165–73. https://doi.org/10.1016/J.EIMC.2016.08.011. B.-E.
De Munain JL. Epidemiology and current control of sexually transmitted infections. The role of STI clinics. Enfermedades Infecciosas Y Microbiologia Clinica (English Ed.), 2019;37(1):45–9. https://doi.org/10.1016/J.EIMC.2018.10.015 De Munain 2019.
Heijer CDJ, den, Hoebe CJPA, Driessen JHM, Wolffs P, Broek IVF, van den, Hoenderboom BM, Williams R, de Vries F, Dukers-Muijrers NH T. M. Chlamydia trachomatis and the risk of pelvic inflammatory disease, ectopic pregnancy, and female infertility: a Retrospective Cohort Study among Primary Care patients. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2019;69(9):1517–25. https://doi.org/10.1093/CID/CIZ429.
De Vries HJC, de Barbeyrac B, de Vrieze NHN, Viset JD, White JA, Vall-Mayans M, Unemo M. 2019 European guideline on the management of lymphogranuloma venereum. J Eur Acad Dermatology Venereology: JEADV. 2019a;33(10):1821–8. https://doi.org/10.1111/JDV.15729.
ECDC. (2018). Lymphogranuloma venereum - Annual Epidemiological Report for 2016.
De Vries HJC, Nori Av, Larsen HK, Kreuter A, Padovese V, Pallawela S, Vall-Mayans M, Ross J. 2021 European Guideline on the management of proctitis, proctocolitis and enteritis caused by sexually transmissible pathogens. J Eur Acad Dermatology Venereology: JEADV. 2021;35(7):1434–43. https://doi.org/10.1111/JDV.17269.
Ward H, Alexander S, Carder C, Dean G, French P, Ivens D, Ling C, Paul J, Tong W, White J, Ison CA. The prevalence of lymphogranuloma venereum infection in men who have sex with men: results of a multicentre case finding study. Sex Transm Infect. 2009;85(3):173–5. https://doi.org/10.1136/STI.2008.035311.
Saxon C, Hughes G, Ison C. Asymptomatic Lymphogranuloma Venereum in men who have sex with men, United Kingdom. Emerg Infect Dis. 2016;22(1):112–6. https://doi.org/10.3201/EID2201.141867.
Jansen K, Steffen G, Potthoff A, Schuppe AK, Beer D, Jessen H, Scholten S, Spornraft-Ragaller P, Bremer V, Tiemann C, Knechten H, Panstruga P, Beer D, Cordes C, Lieb C, Rodriguez E, Hillenbrand H, Jessen H, Krznaric I, Trein A. STI in times of PrEP: high prevalence of chlamydia, gonorrhea, and mycoplasma at different anatomic sites in men who have sex with men in Germany. BMC Infect Dis. 2020;20(1). https://doi.org/10.1186/S12879-020-4831-4.
Australian STI. Management Guidelines For Use In Primary Care [https://www.ashm.org.au/resources/australian-sti-management-guidelines/] visitada 15/09/2022.
Hazra A, Collison MW, Davis AM. CDC sexually transmitted infections Treatment guidelines, 2021. JAMA. 2022;327(9):870–1. https://doi.org/10.1001/jama.2022.1246.
Unidad de vigilancia de VIH, ITS y hepatitis B y C. Vigilancia epidemiológica de las infecciones de transmisión sexual., 2019. Centro Nacional de Epidemiología, Instituto de Salud Carlos III/Plan Nacional sobre el Sida, Dirección General de Salud Pública; 2021.
Gomersall JS, Jadotte YT, Xue Y, Lockwood S, Riddle D, Preda A. (2015) Conducting systematic reviews of economic evaluations. Int J Evid Based Healthc. 2015;13(3):170–178.
Drummond MF. (dir.). (2007). Methods for the Economic Evaluation of Health Care Programmes (3e éd.). Oxford: Oxford University Press.
Diari oficial de la Generalitat Valenciana. (2018). Acuerdo por el cual se actualizan las retribuciones del personal al servicio del sector público valenciano y se publican las tablas retributivas de la Administración de la Generalitat y de sus organismos autónomos. Número 8350.
Deterministic Sensitivity Analysis. [https://yhec.co.uk/glossary/deterministic-sensitivity-analysis/]. (2016). York; York Health Economics Consortium; 2016. https://yhec.co.uk/glossary/deterministic-sensitivity-analysis/.
Andronis L, Barton P, Bryan S. Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making. Health Technol Assess (Winchester Eng). 2009;13(29):iii–61. https://doi.org/10.3310/hta13290.
Viccarese G, Drago F, Rebora A, Parodi A. Updates on lymphogranuloma venereum. J Eur Acad Dermatology Venereology: JEADV. 2021;35(8):1606–7. https://doi.org/10.1111/jdv.17468.
Peuchant O, Touati A, Laurier-Nadalié C et al. Sex Transm Infect Epub ahead of print: [please include Day Month Year]. https://doi.org/10.1136/sextrans-2019-054346.
de Vries HJC. The Enigma of Lymphogranuloma Venereum Spread in men who have sex with men: does ano-oral transmission plays a role? Sex Transm Dis. 2016;43(7):420–2. https://doi.org/10.1097/OLQ.0000000000000466.
Acknowledgements
Estela Giménez (Juan Rodés Contract, JR18/00053), Ignacio Torres (Río Hortega Contract; CM20/00090) and Eliseo Albert (Juan Rodés Contract; JR20/00011) hold contracts funded by the Carlos III Health Institute (co-financed by the European Regional Development Fund, ERDF/FEDER).
Funding
The current work received not public or private funds.
Author information
Authors and Affiliations
Contributions
D.S., J.F., I.T. and D.C.: Methodology and data validation. E.G., MJ.A. and MJ.C. Verified the analytical methods. D.N. and E.A.: Conceptualization, supervision and writing the original draft. All authors reviewed the original draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current study was approved by the research and ethics committee of the University Clinical Hospital of Valencia, Spain (reference, 2022/210). The need for Informed Consent was waived by the same committee, the Research Ethics Committee (CEI) of the University Clinical Hospital of Valencia, due to the retrospective nature of the study. Chlamydia genotyping was performed by medical indication as part of the clinical diagnosis of the patients in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sánchez, D., Ferrer, J., Giménez, E. et al. Genotypic study of Chlamydia trachomatis for lymphogranuloma venereum diagnosis in rectal specimens from men who have sex with men: a cost-effectiveness analysis. BMC Infect Dis 24, 298 (2024). https://doi.org/10.1186/s12879-024-09185-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09185-4