Abstract
Aims
We investigated the antibacterial efficacy of Umonium38 and Virkon® against Burkholderia pseudomallei, Escherichia coli, Pseudomonas aeruginosa and Methicillin-Resistant Staphylococcus aureus (MRSA) up to 14 days following treatment.
Methods and results
Umonium38 was diluted to 0.5%, 1.0%, 1.5%, 2.0%, 2.5% and 3%, tested against the bacterial strains at various contact times (15 min to 24 h), and incubated for up to 14 days. A minimum concentration of 0.5% Umonium38 with a contact time of 15 min effectively killed approximately 108 CFU/ml of all four bacterial species. No growth was observed on agar plates from day 0 until day 14 for all six concentrations. The bacteria were also inactivated by a 30-minute treatment time using Virkon® 1% solution.
Conclusions
Umonium38 effectively inactivates B. pseudomallei, E. coli, P. aeruginosa and MRSA at a concentration of ≥ 0.5% with a contact time of at least 15 min. The antimicrobial effect of Umonium38 remained for 14 days.
Significance
and impact of the study: The study provides evidence supporting the efficacy of Umonium38 as a bactericidal agent against E. coli and P. aeruginosa, specifying the required concentrations and contact time. Our investigation adds to the existing knowledge by demonstrating the bactericidal activity of Umonium38 against B. pseudomallei and MRSA. A limitation of the study is that the results presented have been produced under optimal laboratory conditions, and additional studies are required to determine the effectiveness of Umonium38 and Virkon® under sub-optimal conditions, including high levels of organic matter or variable pH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective disinfection is crucial for infection control as it helps to control potentially hazardous microorganisms, especially in laboratory environments. It is crucial to use validated decontamination protocols to effectively inactivate pathogens as this reduces the likelihood of pathogen exposure, resulting in laboratory-acquired infections and contamination of laboratory and outside environments. The selection of a disinfectant is often based on various factors, including the pathogens to be manipulated, compatibility with laboratory surfaces and efficacy of inactivation [1]. This is particularly important in low-resource settings, where cost, availability, and concentration influence decisions.
Laboratory personnel consistently face significant hazards when exposed to hazardous and potentially lethal pathogens. The routine handling of pathogenic biological agents by laboratory personnel necessitates adherence to stringent safety protocols due to the inherent risk of infection. P. aeruginosa is an opportunistic bacterium classified as risk group 2 [2] that frequently causes nosocomial infections, particularly in patients with burn wounds, cystic fibrosis, acute leukaemias, organ transplantation, and intravenous drug addiction [3]. Inadequate infection control protocols can contribute to its persistence. They can survive under various environmental conditions, such as storage tanks, disinfectant solutions, and urinals in hospital environments [4]. According to the Centers for Disease Control and Prevention (CDC), P. aeruginosa causes 51,000 healthcare-associated infections in US hospitals annually, with 13% of cases exhibiting multidrug resistance, leading to 440 deaths each year [5]. Similarly, Methicillin-Resistant Staphylococcus aureus (MRSA) infection is a significant source of nosocomial and community-associated infections, potentially leading to mortality due to its resistance to conventional beta-lactam antibiotics [6]. The manipulation of S. aureus necessitates adherence to Biosafety Level 2 practices and procedures [7]. Specific populations, including athletes, daycare and school children, military personnel residing in barracks, and individuals undergoing inpatient medical care, surgery, or using medical devices, are more susceptible to MRSA infection [8].
Burkholderia pseudomallei is a gram-negative bacterium classified as risk group 3 and causes melioidosis infection [9, 10]. Patients infected with this bacterium often experience symptoms that can be easily confused with other diseases, such as tuberculosis [11]. B. pseudomallei infection can lead to local infection, bacteremia, pulmonary infection, and disseminated infection, with a mortality rate of approximately 21% [9]. Escherichia coli is mostly harmless to humans; however, some strains, such as enterotoxigenic E. coli O157:H7 [12, 13], can cause serious illness. These pathogenic strains are known to contaminate food and water sources, leading to symptoms such as diarrhoea and poisoning in people who come into contact with them.
Umonium38 has been reported as a broad-spectrum disinfectant for laboratory purposes for a wide range of bacteria, viruses, and fungi. The active ingredient of Umonium38 is isopropyl-tridecyl-dimethyl-ammonium, a surfactant that breaks the bonds between water molecules and penetrates deeper into micro-asperities, allowing it to dissolve other molecules [14]. Studies have demonstrated that Umonium38 is a highly effective disinfectant against avian influenza virus (AIV) subtype H5N1 and Newcastle disease virus (NDV) [15]. Furthermore, Umonium38, when combined with other active compounds, exhibits anti-mycobacterial and antibacterial properties [16]. Umonium38 offers several advantages, such as its broad antibacterial properties, relative affordability, and user safety since it contains no carcinogenic or endocrine-disrupting components [14]. It is also compatible with several industrial and equipment surfaces, thanks to its neutral pH, non-flammability, and lack of toxic gas emissions [14].
This study aimed to assess the bactericidal efficacy of various concentrations of Umonium38 against four bacterial species: B. pseudomallei, E. coli, MRSA and Pseudomonas aeruginosa. We also examined the bactericidal efficacy of Umonium38 and determined its stability over 14 days post-treatment. Furthermore, we aimed to compare the bactericidal efficacy of Umonium38 with Virkon®, a widely employed and currently available laboratory disinfectant.
Materials and methods
Bacterial strains and disinfectants
The bacterial strains used in the study were all clinical isolates either from the American Type Culture Collection (ATCC) or from clinical studies performed in Thailand: P. aeruginosa (PA) strain Boston 41,501 (ATCC 27,853), E. coli strain Seattle 1946 (ATCC 25,922), MRSA strain S021 (Northeastern Thailand; 2008) and B. pseudomallei (BP) strain 1026b [17, 18] (BEI strain NR-9910, Northeastern Thailand; 1993). Two commercial disinfectants, Umonium38 (Huckert’s International, Belgium) and Virkon® (Antec International Ltd, United Kingdom), were assessed for effectiveness against these four bacterial strains.
Bacterial suspension preparation
Bacteria were retrieved from frozen stocks and sub-cultured using selective Ashdown’s agar for B. pseudomallei and Columbia agar for E. coli, MRSA, and P. aeruginosa. The bacteria were incubated at 37 oC for two days before re-subculturing 3–5 colonies on Columbia agar and incubated at 37 oC overnight. A bacterial suspension was formed by emulsifying pure colonies in 20 mL of normal saline solution (NSS). The bacterial turbidity was adjusted to meet the McFarland standard number 7.0, which resulted in an estimated bacterial concentration ranging from 1.0 to 3.0 × 109 CFU/mL (stock concentration), which was used for in vitro testing purposes.
In vitro bacterial viability testing
Umonium38 was diluted in distilled water at concentrations of 0.5%, 1%, 1.5%, 2%, 2.5%, and 3% (v/v) and stored at room temperature (25–35 °C). Concentrations were selected based on manufacturers’ recommendations and practical considerations, including overnight soaking of contaminated materials. A negative control (NC) of 1% (w/v) Virkon® and a positive control (PC) of distilled water were used for each Umonium38 concentration (refer to Table 1). To achieve a bacterial concentration in the range of 2–6 × 108 CFU/mL in each tube, 1mL of 1–3 × 109 CFU/mL stock concentration of each isolate was dispensed into tubes containing 4 mL (ratio 1:5) of 0.5–3% concentrations of Umonium38 (see Fig. 1). After 15 min, 30 min and 24 h of contact time, 100 µL of each Umonium38 concentration, negative control tubes and positive control tubes was removed and spread onto Columbia agar plates, and another 100 µL was added to 3 mL of enrichment tryptone soya broth (TSB). To determine the bacterial viability result on day 0, plates and broth tubes were incubated at 37 °C for 48 h, and the resulting colonies on cultured plates were counted and calculated to CFU/mL. After 48 h of incubation for broth tubes without shaking, use 100 µL to spread onto Columbia agar plate (perform duplicated plates per broth tube). Plates were incubated at 37 °C for 48 h, and viability testing results were read as growth (+) and no growth (-)(see Fig. 1).
Stability of disinfection
To determine the stability of Umonium38 over 14 days, the same procedure was followed for preparing the cultures and Umonium38 at the six concentrations (i.e., 0.5%, 1%, 1.5%, 2%, 2.5%, and 3%). Viability testing on days 3, 5, 7, and 14 with different contact times of 15 min, 30 min and 24 h was performed on an agar plate and broth as described on day 0. TSB tubes were inoculated in triplicate and incubated at 37 °C without shaking, and culture attempted after 48 h of incubation by collecting 100 µL from TSB tubes to spread onto Columbia agar plates and plates were incubated at 37 °C for 48 h, with growth (+) or no growth (-) recorded for each organism. In addition to the six Umonium38 concentrations, a 1% Virkon NC and Distilled water PC were also included. A summary of all the tested disinfectants and concentrations is presented in Table 1.
Results
Umonium38 and virkon®
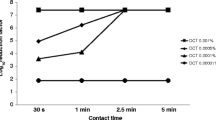
Umonium38 demonstrated potent antimicrobial activity against P. aeruginosa, E. coli, B. pseudomallei, and MRSA. At a concentration of 0.5%, Umonium38 completely inactivated P. aeruginosa, E. coli, and MRSA within 15 min of exposure (Table 2 and Table S1). However, B. pseudomallei required 1% Umonium38 with contact for 15 min or 0.5% concentration with contact for 24 h to achieve a complete kill (Table 2 and Table S1). While also effective against the four bacterial strains, 1% Virkon® required a contact time of 30 min to achieve comparable outcomes (Table S1).
Stability of Umonium38 antibacterial efficiency
Umonium38 consistently demonstrated its efficacy throughout the experiment by inactivating P. aeruginosa, E. coli, B. pseudomallei, and MRSA to the same extent on day 3 as on day 0. No growth of these bacterial strains was observed on day 5. Although B. pseudomallei requires a longer contact time of 30 min to achieve a similar effect, exposure to 0.5% Umonium38 for just 15 min resulted in complete inactivation of all four bacterial strains on days 7 and 14, as shown in Table 2 and Table S1. Additionally, no growth of these organisms was observed after 14 days of incubation with 1% Virkon® (Table S1).
Discussion
This study is the first to determine the most effective Umonium38 concentration and duration of contact for inactivation of P. aeruginosa, E. coli, B. pseudomallei, and MRSA under optimal conditions. 1% Virkon® was also effective against the four bacterial strains following a 30-minute contact time.
The results presented in this study reflected those of the Umonium38 manufacturer (summarised in Table 3) on P. aeruginosa and E. coli using European Standards EN 1276:2019 and EN 1040:2006 [19]. They reported a contact time of > 10 min, and a 0.5% Umonium38 solution resulted in a reduction of over 105 in both bacteria; however, after a 1-minute contact, the reduction reported was < 105 [19]. The report also mentioned that a higher concentration of 2.5% Umonium38 required a minimum contact time of only 1 min to achieve the same bactericidal effect as 0.5% Umonium38 following 10 min of contact [19]. Similar results were achieved for inactivating P. aeruginosa and E. coli using 0.5% and 2.5% Umonium38 following 15 min of contact following the European Standard EN13697:2001 [16].
Our results demonstrated that 0.5% Umonium38 can effectively inactivate MRSA within 15 min, sustained for 14 days, and underscores its potential for safely and effectively disinfecting equipment surfaces and laboratory environments. MRSA inactivation currently focuses on light utilisation, such as far-UVC LEDs with a wavelength below 240 nm [20, 21] and antimicrobial photodynamic therapy with a porphyrinic formulation [22] for antiseptic purposes on the skin. In addition, the efficacy of octenidine hydrochloride has been assessed for the inactivation of MRSA biofilm formation on medical implants and laboratory equipment within hospital settings [23].
Our study demonstrated that B. pseudomallei required 1% Umonium38 with contact for 15 min for effective inactivation. Other chemical treatments, heat exposure, autoclaving, and radiation are also effective for B. pseudomallei inactivation. Chemical agents, including chlorine dioxide solution [24], pH-adjusted bleach, ethanol solution (70%), quaternary ammonium compounds, and PineSol® [25] have been proven effective. B. pseudomallei can be effectively inactivated by heat treatment at 80oC for 1 h [26] or 121 oC for 15 min [27]. Exposure to sunlight with wavelengths ranging from 295 to 305 nm can inactivate B. pseudomallei concentrations from 104 to 106 CFU/ml in 60 to 180 min [28]. Furthermore, ultraviolet (UV) light with a wavelength of 365 nm, emitting a radiant flux of 90,000 mWs/cm2 at a flow rate of 5 L/min, resulted in the inactivation of B. pseudomallei 106 CFU/mL [29].
A limitation of the results presented in this study is that it has been performed under optimal conditions, and the results should be interpreted as such. The effectiveness of chemical disinfectants under different background conditions, including pH, and in the presence of significant biological matrices, including culture media or organic contamination, may affect the efficacy of the inactivation of Umonium38. Raffo et al. [16] investigated the impact of “clean” and “dirty” conditions on the effectiveness of 0.5% and 2.5% Umonium38 for inactivating P. aeruginosa and E. coli. The “clean” condition involved 0.3 g of bovine serum albumin per liter of water, while the “dirty” condition involved the combination of 3.3 g of bovine serum albumin with 3.0 ml of red blood cells per liter of water. The study found that for both P. aeruginosa and E. coli. under “clean” conditions, 0.5% Umonium38 treatment gave a 4-log10 reduction in infectivity after 15 min; however, 60 min was required under “dirty” conditions [16]. Interestingly, 2.5% Umonium38 achieved a 4-log10 reduction in infectivity of both pathogens after 15-minute exposure for both “clean” and “dirty” conditions [16]. Therefore, additional studies are required to determine the optimal concentrations for the inactivation of B. pseudomallei and MRSA under sub-optimal conditions, including organic loads or varied pH.
The results presented here demonstrate the effectiveness of Umonium38 and Virkon® for a selected group of bacteria under optimal conditions. When used correctly, Umonium38 offers laboratory staff an alternative for effective disinfection and provides an affordable and practical method for routine disinfection. Further work is required to determine the effectiveness of this and other disinfectants under the practical circumstances of everyday use.
Data availability
All data generated or analysed during this study are included in this published article.
References
World Health Organization. Laboratory biosafety manual, 4th edition: Decontamination and waste management. Available at: https://www.who.int/publications/i/item/9789240011359.
Public Health Agency of Canada. Pathogen Safety Data Sheets: Infectious Substances– Pseudomonas spp. Available at: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/pseudomonas.html.
Bodey GP, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983;5(2):279–313.
Bush LM, Vazquez-Pertejo MT. Pseudomonas and Related Infections. MSD Manual. 2022.
Weiner LM, Webb AK, Limbago B, et al. Infect Control Hosp Epidemiol. 2016;37(11):1288–301. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014.
Siddiqui AH, Koirala J, Methicillin-Resistant. Staphylococcus Aureus. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482221/.
Vitko NP, Richardson AR. Laboratory maintenance of methicillin-resistant Staphylococcus aureus (MRSA). Curr Protoc Microbiol 2013; Chap. 9: Unit 9 C.2.
Centres for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA). Available at: https://www.cdc.gov/mrsa/community/index.html#:~:text=Athletes%2C%20daycare%20and%20school%20students,higher%20risk%20of%20MRSA%20infection.
Perumal Samy R, Stiles BG, Sethi G, Lim LHK. Melioidosis: clinical impact and public health threat in the tropics. PLoS Negl Trop Dis. 2017;11(5):e0004738.
Public Health Agency of Canada. Burkholderia pseudomallei: Infectious substances Pathogen Safety Data Sheet. Available at: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/burkholderia-pseudomallei.html.
Centers for Disease Control and Prevention. Signs and Symptoms. Available at: https://www.cdc.gov/melioidosis/symptoms/index.html.
Escherichia coli (eColi 0157 H7). Available at: https://www.statpearls.com/physician/cme/activity/87849/?specialty=specialty°=MD.
Zhang Y, Tan P, Zhao Y, Ma X. Enterotoxigenic Escherichia coli: intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes. 2022;14(1):2055943.
Laboratoire Huckert’s International. Umonium38. Master in disinfection. Available at: https://huckerts.net/en/umonium38/.
Patnayak DP, Prasad AM, Malik YS, Ramakrishnan MA, Goyal SM. Efficacy of disinfectants and hand sanitizers against avian respiratory viruses. Avian Dis. 2008;52(2):199–202.
Raffo P, Salliez AC, Collignon C, Clementi M. Antimicrobial activity of a formulation for the low temperature disinfection of critical and semi-critical medical equipment and surfaces. New Microbiol. 2007;30(4):463–9.
Massey S, Yeager LA, Blumentritt CA, et al. Comparative Burkholderia pseudomallei natural history virulence studies using an aerosol murine model of infection. Sci Rep. 2014;4:4305.
Sahl JW, Allender CJ, Colman RE, et al. Genomic characterization of Burkholderia pseudomallei isolates selected for medical countermeasures testing: comparative genomics associated with differential virulence. PLoS ONE. 2015;10(3):e0121052.
Laboratoire Huckert’s International. UMONIUM38® Innovation in cold disinfection. Available at: https://henrotech.be/sites/default/files/product/manual/16pUK_light_8.pdf.
Glaab J, Lobo-Ploch N, Cho HK, et al. Skin tolerant inactivation of multiresistant pathogens using far-UVC LEDs. Sci Rep. 2021;11(1):14647.
Zwicker P, Schleusener J, Lohan SB, et al. Application of 233 nm far-UVC LEDs for eradication of MRSA and MSSA and risk assessment on skin models. Sci Rep. 2022;12(1):2587.
Braz M, Salvador D, Gomes ATPC, et al. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus on skin using a porphyrinic formulation. Photodiagn Photodyn Ther. 2020;30:101754.
Amalaradjou MA, Venkitanarayanan K. Antibiofilm Effect of Octenidine Hydrochloride on Staphylococcus aureus, MRSA and VRSA. Pathogens. 2014;3(2):404–16.
Shams AM, O’Connell H, Arduino MJ, Rose LJ. Chlorine dioxide inactivation of bacterial threat agents. Lett Appl Microbiol. 2011;53(2):225–30.
Calfee MW, Wendling M. Inactivation of Burkholderia pseudomallei on environmental surfaces using spray-applied, common liquid disinfectants. Lett Appl Microbiol. 2015;61(5):418–22.
Suttisunhakul V, Wuthiekanun V, Brett PJ, et al. Development of Rapid enzyme-linked immunosorbent assays for detection of antibodies to Burkholderia pseudomallei. J Clin Microbiol. 2016;54(5):1259–68.
Gilmore G, Barnes J, Ketheesan N, Norton R. Use of antigens derived from Burkholderia pseudomallei, B. thailandensis, and B. cepacia in the indirect hemagglutination assay for melioidosis. Clin Vaccine Immunol. 2007; 14(11): 1529-31.
Sagripanti JL, Levy A, Robertson J, Merritt A, Inglis TJ. Inactivation of virulent Burkholderia pseudomallei by sunlight. Photochem Photobiol. 2009;85(4):978–86.
Howard K, Inglis TJ. Disinfection of Burkholderia pseudomallei in potable water. Water Res. 2005;39(6):1085–92.
Acknowledgements
Not applicable.
Funding
This research was funded in whole, or in part, by the Wellcome Trust [220211]. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
“All authors have read and approved the manuscript. Each author has contributed significantly to the development of the manuscript. SB, KKL, SR, PA and VW wrote the main manuscript text. PA, SR, SL and PM performed the laboratory activities. SB provided the funding.”
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12879_2024_9102_MOESM1_ESM.docx
Supplementary Material 1 - Growth of tested pathogens in various concentrations and contact times of Umonium38, 1% Virkon (NC) and distilled water (PC) observed at days 0, 3, 5, 7, and 14.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ruanchaiman, S., Amornchai, P., Wuthiekanun, V. et al. Effectiveness of Umonium38 against Burkholderia pseudomallei, Escherichia coli, Pseudomonas aeruginosa and Methicillin-Resistant Staphylococcus aureus (MRSA). BMC Infect Dis 24, 212 (2024). https://doi.org/10.1186/s12879-024-09102-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09102-9