Abstract
Background
Autoimmune diseases (ADs) may be complicated by sepsis when intensive care unit (ICU) admission. But repeated sepsis among AD patients has not been studied yet. The aim of this study is to investigate the impact of repeated in-ICU sepsis on the 1-year overall-cause mortality, septic shock and in-ICU death of AD patients.
Methods
Data of AD patients with sepsis retrieved from Medical Information Mart for Intensive Care IV (MIMIC-IV) database were divided into the single group and the repeated group according to the frequency of in-ICU sepsis. Propensity score matching was used to balance inter-group bias. Cox proportional hazard regression and sensitivity analysis were utilized to assess the variables on mortality.
Results
The incidence of repeated in-ICU sepsis in baseline was 19.8%. The repeated in-ICU sepsis was a risk factor for 1-year overall-cause mortality among AD patients (adjusted hazard ratio [HR] = 1.50, 95% CI: 1.16–1.93, P = 0.002), with robust adjusted HRs by the adjustment for confounders in the sensitivity analysis (all P < 0.01). Maximum Sequential Organ Failure Assessment (Max SOFA), Charlson comorbidity index (CCI) and Simplified Acute Physiology Score-II (SAPS-II) were risk factors for 1-year overall-cause mortality among AD with repeated sepsis (Max SOFA: HR = 1.09, P = 0.002; CCI: HR = 1.08, P = 0.039; SAPS-II: HR = 1.03, P < 0.001).
Conclusions
Compared to single hit, repeated in-ICU sepsis was independently related to a higher risk of 1-year overall-cause mortality among AD patients. Assessment tools (Higher SOFA, CCI and SAPS-II scores) were closely linked to poor prognosis of AD with repeated sepsis and helped to reflect ill physical conditions for the patients.
Similar content being viewed by others
Introduction
Autoimmune diseases (ADs) encompass a heterogeneous group of disorders characterized by the self-attack on the tissues or organs of patients themselves. The dysregulation of the immune system in ADs may lead to chronic inflammation, multiple organ damage, and various comorbidities, rendering the patients more susceptible to infections in the long-term periods [1].
Sepsis is another disorder that is attributable to a dysregulated immune response to an infection and successively progress to life-threatening multiorgan failure even deaths [2]. In the USA, severe sepsis is reported to occupy about 10% of all admissions to intensive care units (ICU) and its mortality highly reaches 30% [3]. Moreover, the coexistence between AD and sepsis is common in hospitalization, and the mortality of sepsis overlapping with ADs in ICU is up to 40% [1, 4, 5]. Owing to the high prevalence and high mortality of ADs with sepsis, improving health outcomes among this population has become a notable research focus.
Sepsis is currently found to be a process that starts with inflammation and transitions to a state of immunosuppression in the long period, and the latter phase predisposes individuals to secondary infection or repeated sepsis [6,7,8]. For infection-susceptible AD patients, the occurrence of sepsis may exacerbate the immunity dysregulation and cause recurrent sepsis episodes afterwards. To date, previous studies have demonstrated that recurrent sepsis was associated with higher mid- or long-term mortality for septic patients [9, 10]. However, the finding cannot be extrapolated directly to AD patients in ICU. As for AD population complicated with sepsis, it still remains poorly understood about the impact of single or multiple sepsis episodes on their long-term survival.
Therefore, our study extracted data of AD patients with different episodes of in-ICU sepsis from Medical Information Mart for Intensive Care IV (MIMIC-IV) database, and aimed to investigate the influence of repeated in-ICU sepsis on the prognosis of AD patients, with 1-year overall-cause mortality, septic shock and in-ICU death as outcomes.
Materials and methods
Data source
The data were extracted from MIMIC-IV database version 2.0 from 2008 to 2019 [11]. It is a publicly shared and widely-used database providing critical care information of tens of thousands of ICU patients at the Beth Israel Deaconess Medical Center. The acquisition of the data was permitted by the database and did not require ethical approval.
Patient population and variables
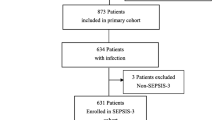
We selected patients over 18 years old who were admitted to the ICU with diagnosis of ADs, which encompassed systemic lupus erythematosus (SLE), rheumatoid arthritis, systemic sclerosis, psoriasis, ankylosing spondylitis, vasculitis, idiopathic inflammatory myopathies, autoimmune hepatic diseases, and inflammatory bowel diseases, namely Crohn’s disease and ulcerative colitis (Supplementary Table S1). Therein, AD patients diagnosed with in-ICU sepsis were retrieved. In-ICU sepsis was defined as a condition existing during the ICU stay and accorded with the Sepsis-3 criteria: an associated suspicion of an infection event and a Sequential Organ Failure Assessment (SOFA) score ≥ 2. We counted the frequency of in-ICU sepsis among these selected AD patients, and those ICU admissions when sepsis did not occur were excluded from the count. Patients with a single record of in-ICU sepsis were grouped into “single group”, before which they each did not experience a sepsis-related hospitalization. Those with records more than one time were into “repeated group”. We selected the last recodes of in-ICU sepsis among the repeated group to compare with the unique episode of the single group, namely the intergroup comparison. Within the repeated group, a within-group comparison of characteristics between the first admissions and the last ones was conducted (Fig. 1).
The variables assessed in the study were as follows: in-ICU sepsis frequency (single and repeated), age at admission (< 65 and ≥ 65 years old), gender, race (white, black, Hispanic, Asian, other and unknown), overlapping syndrome, length of ICU stay, comorbidities, maximum SOFA (Max SOFA), Charlson comorbidity index (CCI), Simplified Acute Physiology Score-II (SAPS-II), maximum of white blood cell (WBC) count, minimum of hemoglobin (Hb), minimum of platelet (PLT) count, use of glucocorticoids, immunosuppressants/biologics and intravenous immunoglobulin (IVIG) (Supplementary Table S2). The comorbidities embodied acute kidney injury, chronic kidney disease, atrial fibrillation, essential hypertension, heart failure, myocardial infarction, respiratory failure, chronic obstructive pulmonary disease (COPD), diabetes mellitus, human immunodeficiency virus (HIV) infection and malignancy. Overlapping syndrome patients were defined as those with the combination of more than one autoimmune diseases, or they would be marked with “no”. Those with unknown or no evidence of comorbidities and drug therapies were also labeled with “no”. SOFA score was a sum of quantified assessment of respiration, coagulation, central nervous system, renal system, cardiovascular system and liver function [12]. CCI was a measurement of the comorbidities based on ICD-9/ICD-10 codes [13] and the SPAS-II is used to assess the illness severity during the first 24 hours of each ICU stay [14]. Individuals with missed data of evaluation tools (Max SOFA, CCI and SAPS-II) or laboratory results were excluded.
Primary and secondary outcomes
In view of the long-term immune dysregulation shared by ADs and sepsis as well as the commonly-used indexes in other previous studies [9, 15], we chose 1-year overall-cause mortality as primary outcome in this study. The occurrence of septic shock (ICD-code 78,552 and R6521) and in-ICU death were identified as the secondary outcomes, as they were commonly adverse events among AD patients in ICU [15,16,17]. Thereinto, the septic shock was the complication during ICU stay. The follow-up of patients started on the date of selected records of ICU admissions and lasted for one year. 1-year overall-cause mortality reflected deaths within a 1-year observation window, including in-hospital deaths and deaths after hospital discharge. Observations of fully one year were deemed as censored events.
Statistical analysis
Considering the small number of missing data, we opted to address missing data by employing a deletion approach (Fig. 1). For the post-deletion data, Chi-square test was implied to assess the categorical variables in the baseline characteristics. If numeric variables of the two groups did not meet normal distribution through Shapiro-Wilk normality test (P < 0.05), they were shown as medians with inter-quartile ranges (IQRs) and their differences were evaluated by Wilcoxon rank sum test. To decrease the potential imbalance between the single group and the repeated group, a 1:1 propensity score matching (PSM) was applied. The algorithm principle of PSM was to match potential confounding variables based on logistic regression and nearest neighbor method with a caliper width of 0.02. All the variables we chose were put into PSM and the balances were acceptable if P > 0.05.
After PSM, the survival probability among these two groups was shown by Kaplan-Meier survival curve, and the comparison of survival was performed through Log-rank test. Further, univariate and multivariate Cox proportional hazard regressions were used to assess the independent risk factors on survival among AD patients with sepsis. If variables met P ≤ 0.05 in univariate analysis, they would be selected into the multivariate analysis. The proportional hazards assumption was examined by Schoenfeld residuals and was satisfied. To investigate whether the effect of in-ICU sepsis frequency on survival was influenced by other variables, sensitivity analysis was conducted where variables were included through forward selection in multivariate Cox proportional hazard regressions. Multivariate Cox regression analyses were further applied in the subgroup analysis of the single group and the repeated group. Moreover, comparisons of secondary outcomes between the two groups were evaluated by logistic regressions and adjusted by other variables.
All statistical analyses and visualization of results were performed using R version 4.0.5. A two-tailed P ≤ 0.05 was considered statistically significance.
Results
General description among AD patients
Before PSM, a total of 1590 AD patients with sepsis were selected, of whom 315 (19.8%) belonged to the repeated group (Table 1). Supplementary Table S1 provided further details on the distribution of specific AD diagnoses, among whom overlapping syndrome was observed in 90 patients (5.7%) (Table 1). Compared to the single group, the repeated group exhibited fewer white, longer length of ICU stays, higher scores for SOFA, CCI, and SAPS-II, lower levels of Hb and PLT, and a lower incidence of comorbidities including acute kidney injury, chronic kidney disease, essential hypertension, heart failure, respiratory failure and diabetes mellitus (all P < 0.05). However, this imbalance was eliminated after 1:1 PSM and there were 278 patients equally in each matched group (Table 1).
Within the unmatched repeated group (Supplementary Table S3), Max SOFA, CCI and SAPS-II significantly increased in the last admissions than those in the first admissions (all P < 0.05). But insignificant differences were presented in the comparison of comorbidities, laboratory examinations and drug therapies between the last and the first admissions.
In-ICU sepsis on 1-year overall-cause mortality in survival analysis
In survival analysis where in-ICU sepsis frequency acted as a dichotomous variable, 1-year overall-cause mortality significantly ascended among patients in the repeated group than those in the single group (P = 0.028). Since the deaths of each group were fewer than 50%, median survival was unavailable. At the terminal of one year, the survival probability of the repeated group reached 50.7% and the single group reached 60.1% (Fig. 2).
Risk factors for 1-year overall-cause mortality in univariate and multivariate analyses
The results of univariate and multivariate Cox proportional hazard regressions were shown in Table 2. Repeated in-ICU sepsis was a risk factor for the 1-year overall-cause mortality of AD patients with statistical significance (unadjusted hazard ratio [HR] = 1.32, 95% CI: 1.03–1.70, P = 0.028; adjusted HR = 1.50, 95% CI: 1.16–1.93, P = 0.002). Besides, respiratory failure, Max SOFA, CCI and SAPS-II were also risk factors for AD patients with sepsis (all P < 0.05). For three quantified comprehensive tools (Max SOFA, CCI and SAPS-II), an increase of 1 point was associated with a 2-13% increase in the risk of deaths (Max SOFA: adjusted HR = 1.13, 95% CI: 1.08–1.18, P < 0.001; CCI: adjusted HR = 1.09, 95% CI: 1.02–1.17, P = 0.016; SAPS-II: adjusted HR = 1.02, 95% CI: 1.01–1.03, P < 0.001). Length of ICU stay, working as a continuous variable, was associated with a 3% decrease in the risk of death (adjusted HR = 0.97, 95% CI: 0.95-1.00, P = 0.030). The use of immunosuppressants/biologics was a predictor for better prognosis (adjusted HR = 0.55, 95% CI: 0.35–0.87, P = 0.011).
To explore whether in-ICU sepsis frequency was an independent risk factor from other covariates (respiratory failure, Max SOFA, CCI, SAPS-II, length of ICU stay and use of immunosuppressants/biologics), the sensitivity analysis showed relatively robust adjusted HRs of in-ICU sepsis frequency in Model 1–3 (all P < 0.01) (Supplementary Table S4).
Max SOFA, CCI and SAPS-II on 1-year overall-cause mortality in subgroup analysis
We further conducted a subgroup analysis to investigate the effect of Max SOFA, CCI and SAPS-II on the mortality respectively in the single group and the repeated. As depicted in Table 3, the subgroup analysis revealed that Max SOFA, CCI, and SAPS-II were identified as risk factors for 1-year overall-cause mortality within both the repeated group (Max SOFA: HR = 1.09, 95% CI: 1.03–1.16, P = 0.002; CCI: HR = 1.08, 95% CI: 1.00-1.15, P = 0.039; SAPS-II: HR = 1.03, 95% CI: 1.01–1.04, P < 0.001) and the single group (Max SOFA: HR = 1.12, 95% CI: 1.05–1.20, P < 0.001; CCI: HR = 1.13, 95% CI: 1.04–1.21, P = 0.002; SAPS-II: HR = 1.02, 95% CI: 1.00-1.04, P = 0.038).
The effect of risk factors on secondary outcomes
Table 4 and Supplementary Table S5 exhibited the effects of in-ICU sepsis frequency, Max SOFA, CCI and SAPS-II on two secondary outcomes (namely septic shock and in-ICU death). The risk of septic shock was found to be significantly higher in the repeated group compared to the single group (unadjusted odds ratio [OR] = 1.52, 95% CI: 1.05–2.23, P = 0.029; adjusted OR = 1.74, 95% CI: 1.15–2.66, P = 0.009). However, no association was observed between the repeated group and in-ICU death when compared to the single group (P > 0.05 both in univariate and multivariate logistical regression).
For Max SOFA, CCI and SAPS-II among overall AD patients, increased scores of Max SOFA and SAPS-II correlated with septic shock and in-ICU death (all P < 0.05) except for CCI with insignificant differences in multivariate logistical regression (P > 0.05) (Table 4 and Supplementary Table S5). The effects of Max SOFA, CCI and SAPS-II on the secondary outcomes in the subgroups were shown in Supplementary Table S6.
Discussion
To the best of our knowledge, this is the first retrospective cohort study focusing on the impact of repeated in-ICU sepsis on the survival of AD patients using the MIMIC database. We found that repeated in-ICU sepsis acted as a risk factor for 1-year overall-cause mortality and septic shock of AD patients. Furthermore, higher scores in Max SOFA, CCI and SAPS-II were also closely associated with poor prognosis among AD patients with sepsis.
Firstly, repeated in-ICU sepsis episode was significantly associated with higher 1-year overall-cause mortality among AD population, along with robust adjusted HRs in sensitivity analysis by the adjustment of confounders. The findings confirm that repeated in-ICU sepsis served as an independent risk factor for the long-term survival of AD individuals and its effect was less influenced by other covariates. Partly similar to our study, Pandolfi et al. [9] found that hospital readmission due to recurrent sepsis was associated with an increased risk of 1-year mortality. Likewise, some previous studies showed that readmission for recurrent sepsis was indeed a risk factor related to a higher mortality rate [18, 19]. Whereas, these studies on repeated sepsis rarely involved AD population. Other studies on ADs found that sepsis was an important factor influencing the mortality of ADs patients [5, 20], but they did not give a specific definition of whether the sepsis they studied was new-onset or recurrent. These studies provided some clues of the impact of sepsis on ADs, and our findings regarding the long-term prognosis of ADs in relation to recurrent sepsis helps made up for the limitations of these studies.
It remains unclear about the underlying pathogenesis of the increased vulnerability of AD with repeated sepsis to a poorer long-term prognosis; however, immune dysfunction may provide a potential explanation. Sepsis is a disorder as a result of the dysregulated host response to an infection, beginning with inflammation in the early phase and converting into an immunosuppression in the late phase [6, 7]. Previous studies showed that recurrent sepsis or post-sepsis state could induce the exhaustion and impaired response of CD4+ T cell and memory CD8+ T cell [21,22,23], along with the increase of regulatory T cells and myeloid derived suppressor cells that had T cell inhibiting capabilities [22, 24, 25]. Another study also identified HLA-DRlowS100Ahighmonocytes as a cell subtype enriched in the sepsis patients, which was also correlated to the immunosuppression [26]. In addition to the alteration of cell populations, immunosuppressive cytokine secretion and increased expression of negative costimulatory molecules were also found in the long-term immunosuppression status of sepsis [27]. The alteration of immunity might successively cause infection susceptibility and recurrent sepsis episodes. As such, the prolonged immunosuppression status and the recurrence of sepsis formed a bidirectional causality, perpetuating a detrimental loop and eventually causing organ dysfunctions even deaths. For AD populations in our study, the sepsis-induced immunosuppression might be also dysregulated and exacerbated by the ADs; in the meanwhile, the AD-associated multi-organ damage might coexist with the organ dysfunction induced by the sepsis. The underlying pathogenesis on the association between ADs and sepsis episodes needs more studies in the future.
Another important finding was that tools reflecting severe physical dysfunction were closely linked to AD patients with in-ICU sepsis. SOFA, CCI and SAPS-II, three commonly-used quantified assessment tools in ICU, were considered useful to assess organ dysfunction, disease severity, comorbidity status and prognosis of sepsis [12, 13, 28]. In our analysis of 1-year overall-cause mortality, we observed that higher scores in Max SOFA, CCI, and SAPS-II were risk factors for ADs patients. This association remained consistent even when conducting subgroup analyses where the repeated group showed a correlation between high scores of quantified assessment tools and mortality, resembling the findings observed within the single group (all P < 0.05). Moreover, within the repeated group, scores of the tools were higher during the last ICU stays in the comparison between the first admissions and the last ones (all P < 0.05), suggesting that sepsis developing repeatedly were more severely ill than those occurred at the first time. These findings are partly similar to other published literature reporting a correlation between Max SOFA, CCI, and SAPS-II scores and ADs [5, 29,30,31]. To summarize, the higher scores of three quantified assessment tools were long-term mortality-associated risk factors for AD patients whether with recurrent sepsis episodes or with single one. Those subjected to repeated sepsis had a propensity for higher scores after the previous hits of sepsis, indicating more severe physical dysfunction in the recurrence. As such, it is crucial to comprehensively assess the physical conditions and multiorgan involvements among AD patients with sepsis. Given a greater possibility of worse conditions, AD patients with repeated sepsis may require enhanced attention and long-term follow-up following a sepsis episode.
As for secondary outcomes, compared to those with single sepsis episode, individuals with repeated in-ICU sepsis had an increased risk of septic shock, while no significant differences were observed between the repeated and the single group on in-ICU death (or not) (Table 4). Nevertheless, the growth of Max SOFA scores and SAPS-II scores were correlated with both two secondary outcomes. The findings suggested that the septic shock was a vital outcome for AD patients with repeated sepsis. In terms of in-ICU death, one form of mortality in hospital, our patients had an ICU stay duration of one week or less (Table 1), suggesting in-ICU deaths were all within acute phases. During the acute phase, the occurrence of in-ICU death was associated with the severity of diseases (reflecting by SOFA or SAPS-II) instead of the frequency of sepsis episodes. In despite of the irrelevance, we reckon that the underlying mechanisms of in-ICU deaths were different between the single and the repeated group. For AD patients with once-only sepsis episode, early hyperinflammation might manifest in their initial hit, during which organ failure even deaths might happen due to uncontrolled inflammation as well as inadequate medical interventions [32,33,34,35]. On the other hand, for AD survivors with repeated sepsis, the prolonged immunosuppression made them lose the ability to resist the secondary infection and repeated sepsis, rendering them vulnerable to deaths during the acute phase [36, 37]. However, very few is still known about the association between in-ICU deaths and speiss episodes among AD population.
Respiratory failure, with acute ones occupying the majority (90.3% of overall respiratory failure before PSM), also emerged as another risk factor for long-term mortality, which was in line with previous study finding that respiratory failure was associated with the mortality of rheumatoid arthritis with sepsis [38]. On the other hand, the factors for a good prognosis in our study included longer length of ICU stay and the use of immunosuppressants. In the published literature, there were conflicting findings about the association between the length of ICU stay and mortality of AD patients. One study reported no significant relationship between the length of ICU stay and mortality of SLE patients [39], but it was restricted by small size of patient sample. Another study with a relatively large sample found that an extended ICU stay (2 ~ 14 days) was a risk factor for 3-year mortality for rheumatoid arthritis with sepsis [38], which was inconsistent with our results. But differently, the maximum ICU stay did not exceed 1 week in the baseline of our AD patients. The decreased risk of mortality observed among our AD patients who had a relatively longer ICU stay might be due to the supporting critical care and sufficient anti-sepsis therapy in an appropriate length of ICU stay [40]. As for immunosuppressants or biologics, a current study showed that the occurrence of sepsis among AD was not relevant to the use of immunosuppressants in the ICU [41]. but another small-scale study found biologics or corticosteroids could improve the 30-day survival of SLE patients with sepsis [39]. The contradiction of the results on ICU staying and drugs for ADs with sepsis needed more studies to resolve.
Our study had some strengths and limitations. A major strength was that our study was conducted based on a large patient sample and its design of a retrospective cohort study can better indicate the association between repeated sepsis and ADs. Also, variables of interest were adjusted by other confounders through post-PSM multivariate analysis and sensitivity analysis so as to make the results as reliable as possible. However, our study is subject to limitations imposed by the MIMIC database. Detailed causes of death were not available, which hindered further analysis of specific causes of mortality. In the meanwhile, due to privacy protection principles followed by the MIMIC database, the maximum follow-up period for each patient is limited to one year after the last discharge. Consequently, we were only able to focus on the 1-year overall-cause mortality as one of the outcomes. Secondly, we found it difficult to exclusively evaluate the disease activity and immunity of ADs due to the lack of some AD-specific measurements from the database, including autoantibody titer, cytokines, complements, the count of lymphocyte subtypes, etc. We could only utilize SOFA, CCI and SAPS-II, some quantified assessment tools commonly used in ICU, to assess the conditions of disease severity. As shown in our results, the tools indeed worked. Future studies should consider incorporating additional disease activity-related indices to further investigate the relationship between ADs and sepsis. Thirdly, this study utilized an open-access database derived from in-hospital database systems that were collected from routine clinical practice. It is possible that inaccuracies or non-standardization may exist in the documentations.
Conclusion
This study demonstrated that repeated in-ICU sepsis was as a risk factor for 1-year overall-cause mortality among AD patients. Increased scores of Max SOFA, CCI and SAPS-II, which reflected severely ill physical conditions, were closely linked to unfavorable prognosis for AD patients experiencing repeated episodes. Thus, when AD patients encounter sepsis, physicians need more vigilance and pay more attention on their follow-up, especially among cases with multiple episodes. Future research endeavors and prospective trials are needed to unravel the underlying mechanisms.
Data availability
The datasets supporting the conclusions of this article are available in the Physionet (https://physionet.org/content/mimiciv/2.0/) [11].
Abbreviations
- AD:
-
Autoimmune disease
- CCI:
-
Charlson comorbidity index
- COPD:
-
Chronic obstructive pulmonary disease
- Hb:
-
Hemoglobin
- HIV:
-
Human immunodeficiency virus
- HR:
-
Hazard ratio
- ICU:
-
Intensive care unit
- IQR:
-
Inter-quartile range
- IVIG:
-
Intravenous immunoglobulin
- MIMIC-IV:
-
Medical Information Mart for Intensive Care IV
- OR:
-
Odds ratio
- PLT:
-
Platelet
- PSM:
-
Propensity score matching
- SAPS-II:
-
Simplified Acute Physiology Score-II
- SLE:
-
Systemic lupus erythematosus
- SOFA:
-
Sequential Organ Failure Assessment
- WBC:
-
White blood cell
References
Singh JA, Cleveland JD. Hospitalized infections in Lupus: a nationwide study of types of infections, Time Trends, Health Care utilization, and In-Hospital mortality. Arthritis & Rheumatology. 2021;73(4):617–30.
van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54(11):2450–64.
Angus DC, van der Poll T. Severe Sepsis and septic shock. New Engl J Med 2013, 369(9):840–51.
Jinno S, Lu N, Jafarzadeh SR, Dubreuil M. Trends in Hospitalizations for Serious infections in patients with rheumatoid arthritis in the US between 1993 and 2013. Arthritis Care Res. 2018;70(4):652–8.
Krasselt M, Baerwald C, Petros S, Seifert O. Sepsis mortality is high in patients with connective tissue diseases admitted to the Intensive Care Unit (ICU). J Intensive Care Med. 2022;37(3):401–7.
Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15(5):496–7.
van Vught LA, Wiewel MA, Hoogendijk AJ, Frencken JF, Scicluna BP, Klouwenberg PMCK, Zwinderman AH, Lutter R, Horn J, Schultz MJ, et al. The Host Response in patients with Sepsis developing Intensive Care unit–acquired secondary infections. Am J Respir Crit Care Med. 2017;196(4):458–70.
Cui Z, Wang L, Li H, Feng M. Study on immune status alterations in patients with sepsis. Int Immunopharmacol. 2023;118:110048.
Pandolfi F, Brun-Buisson C, Guillemot D, Watier L. One-year hospital readmission for recurrent sepsis: associated risk factors and impact on 1-year mortality-a French nationwide study. Crit Care. 2022;26(1):371–1.
DeMerle KM, Royer SC, Mikkelsen ME, Prescott HC. Readmissions for recurrent Sepsis: New or relapsed infection?*. Crit Care Med 2017, 45(10).
Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–220.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10.
Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson Comorbidity Index: a critical review of Clinimetric Properties. Psychother Psychosom. 2022;91(1):8–35.
Le Gall J-R, Lemeshow S, Saulnier F. A New Simplified Acute Physiology score (SAPS II) based on a European/North American Multicenter Study. JAMA. 1993;270(24):2957–63.
Mageau A, Sacré K, Perozziello A, Ruckly S, Dupuis C, Bouadma L, Papo T, Timsit JF. Septic shock among patients with systemic lupus erythematosus: short and long-term outcome. Analysis of a French nationwide database. J Infect. 2019;78(6):432–8.
Bernal-Macías S, Reyes-Beltrán B, Molano-González N, Augusto Vega D, Bichernall C, Díaz LA, Rojas-Villarraga A, Anaya JM. Outcome of patients with autoimmune diseases in the intensive care unit: a mixed cluster analysis. Lupus Sci Med. 2015;2(1):e000122–2.
Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11.
Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med. 2015;43(4):738–46.
Fry CH, Fluck D, Han TS. Frequent identical admission-readmission episodes are associated with increased mortality. Clin Med (Lond). 2021;21(4):e351–6.
Oud L. Epidemiology and outcomes of sepsis among hospitalizations with systemic lupus erythematosus admitted to the ICU: a population-based cohort study. J Intensive Care. 2020;8:3–3.
Ammer-Herrmenau C, Kulkarni U, Andreas N, Ungelenk M, Ravens S, Hübner C, Kather A, Kurth I, Bauer M, Kamradt T. Sepsis induces long-lasting impairments in CD4 + T-cell responses despite rapid numerical recovery of T-lymphocyte populations. PLoS ONE. 2019;14(2):e0211716.
He W, Xiao K, Xu J, Guan W, Xie S, Wang K, Yan P, Fang M, Xie L. Recurrent Sepsis exacerbates CD4(+) T cell exhaustion and decreases antiviral Immune responses. Front Immunol. 2021;12:627435.
Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J Immunol (Baltimore Md: 1950). 2014;192(8):3618–25.
Cavassani KA, Carson WFt, Moreira AP, Wen H, Schaller MA, Ishii M, Lindell DM, Dou Y, Lukacs NW, Keshamouni VG, et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115(22):4403–11.
Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL et al. Human myeloid-derived suppressor cells are Associated with Chronic Immune suppression after severe Sepsis/Septic shock. Annals of Surgery 2017, 265(4):827–34.
Yao RQ, Zhao PY, Li ZX, Liu YY, Zheng LY, Duan Y, Wang L, Yang RL, Kang HJ, Hao JW, et al. Single-cell transcriptome profiling of sepsis identifies HLA-DR(low)S100A(high) monocytes with immunosuppressive function. Military Med Res. 2023;10(1):27.
Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, Li L, Cao J, Xu F, Zhou Y, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Military Med Res. 2022;9(1):56.
He Y, Xu J, Shang X, Fang X, Gao C, Sun D, Yao L, Zhou T, Pan S, Zou X et al. Clinical characteristics and risk factors associated with ICU-acquired infections in sepsis: a retrospective cohort study. Front Cell Infect Microbiol 2022, 12.
Quintero OL, Rojas-Villarraga A, Mantilla RD, Anaya JM. Autoimmune diseases in the intensive care unit. An update. Autoimmun Rev. 2013;12(3):380–95.
Kim S-K, Choe J-Y, Lee S-S. Charlson Comorbidity Index is related to organ damage in systemic Lupus Erythematosus: data from KORean lupus Network (KORNET) Registry. J Rheumatol 2017, 44(4):452–2.
Krasselt M, Baerwald C, Petros S, Seifert O. Mortality of Sepsis in patients with rheumatoid arthritis: a single-center retrospective analysis and comparison with a Control Group. J Intensive Care Med. 2021;36(7):766–74.
Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock (Augusta Ga). 2015;43(1):68–73.
Chandra J, de la Armengol MA, Lee G, Lee A, Thoral P, Elbers P, Lee HC, Munger JS, Celi LA, Kaufman DA. A novel vascular Leak Index identifies sepsis patients with a higher risk for in-hospital death and fluid accumulation. Crit Care. 2022;26(1):103.
Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–8.
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96.
Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet (London England). 2018;392(10141):75–87.
van der Slikke EC, An AY, Hancock REW, Bouma HR. Exploring the pathophysiology of post-sepsis syndrome to identify therapeutic opportunities. EBioMedicine. 2020;61:103044.
Barrett O, Abramovich E, Dreiher J, Novack V, Abu-Shakra M. Short- and long-term mortality due to sepsis in patients with rheumatoid arthritis. Rheumatol Int. 2017;37(6):1021–6.
Abramovich E, Barrett O, Dreiher J, Novack V, Abu-Shakra M. Incidence and variables associated with short and long-term mortality in patients with systemic lupus erythematosus and sepsis admitted in intensive care units. Lupus. 2018;27(12):1936–43.
Dupuis C, Bouadma L, Ruckly S, Perozziello A, Van-Gysel D, Mageau A, Mourvillier B, de Montmollin E, Bailly S, Papin G, et al. Sepsis and septic shock in France: incidences, outcomes and costs of care. Ann Intensiv Care. 2020;10(1):145.
Assan F, Bay P, Mathian A, Hekimian G, Bréchot N, Quentric P, Moyon Q, Schmidt M, Cohen-Aubart F, Haroche J, et al. In-ICU-acquired infections in flare-up systemic rheumatic disease patients receiving immunosuppressant. Clin Rheumatol. 2022;41(9):2845–54.
Acknowledgements
We thank the research staff from MIMIC database for providing data.
Funding
This research was funded by grant from the National Natural Science Foundation of China (No. 81871285).
Author information
Authors and Affiliations
Contributions
JY and JC contributed equally to this work. JY: Conception, study design, data collection, data analysis, result interpretation, figure design, draft writing, article–review and editing; JC: study design, result interpretation, figure design, draft writing and article–review. MZ: Data collection and data clearness; QZ: Data analysis; BY: Funding acquisition, result interpretation, project administration and supervision. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The MIMIC-IV resource was approved by the Beth Israel Deaconess Medical Center, and consent was obtained for the original data collection. We have got a permission to use the database.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, J., Chen, J., Zhang, M. et al. Prognostic impacts of repeated sepsis in intensive care unit on autoimmune disease patients: a retrospective cohort study. BMC Infect Dis 24, 197 (2024). https://doi.org/10.1186/s12879-024-09072-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09072-y