Abstract
Background
Aberrant Wnt5a expression contributes to immunity, inflammation and tissue damage. However, it remains unknown whether Wnt5a is associated with liver injury in chronic hepatitis B virus (HBV) infection. We aimed to explore the potential role of Wnt5a expression in liver injury caused by chronic HBV infection.
Methods
Wnt5a mRNA levels in peripheral blood mononuclear cells (PBMCs) were analyzed in 31 acute-on-chronic hepatitis B liver failure (ACHBLF) patients, 82 chronic hepatitis B (CHB) patients, and 20 healthy controls using quantitative real-time polymerase chain reaction. Intrahepatic Wnt5a protein expression from 32 chronic HBV infection patients and 6 normal controls was evaluated by immunohistochemical staining.
Results
Wnt5a mRNA expression was increased in CHB patients and ACHBLF patients compared to healthy controls and correlated positively with liver injury markers. Additionally, there was a significant correlation between Wnt5a mRNA expression and HBV DNA load in all patients and CHB patients but not in ACHBLF patients. Furthermore, intrahepatic Wnt5a protein expression was elevated in chronic HBV infection patients compared to that in normal controls. Moreover, chronic HBV infection patients with higher hepatic inflammatory grades had increased intrahepatic Wnt5a protein expression compared with lower hepatic inflammatory grades. In addition, the cut-off value of 12.59 for Wnt5a mRNA level was a strong indicator in predicting ACHBLF in CHB patients.
Conclusions
We found that Wnt5a expression was associated with liver injury in chronic HBV infection patients. Wnt5a might be involved in exacerbation of chronic HBV infection.
Similar content being viewed by others
Background
Hepatitis B virus (HBV) infection is a serious public health problem, with approximately 240 million individuals worldwide being chronically infected [1]. Chronic HBV infection can cause immune-mediated inflammation and liver injury, leading to chronic hepatitis B (CHB), and even progression to acute-on chronic liver failure (ACLF) [2]. CHB can be treated with antiviral therapy, but ACLF has a significantly high mortality with few effective treatments [2, 3]. Therefore, monitoring and assessing the severity of liver injury and progression of chronic HBV infection is important in clinical practice. Unfortunately, despite a large number of studies undertaken to further understand the immune response and HBV-associated liver injury [4,5,6], the mechanism remains obscure.
Wnt proteins are a large family of glycoprotein ligands that play important roles in many cellular functions, including proliferation, differentiation, migration, polarization, and apoptosis [7]. Wnt proteins can be grouped into two distinct classes depending on their downstream involvement or absence of β-catenin: canonical and noncanonical [7, 8]. Noncanonical Wnt5a pathways are usually initiated by binding to frizzled (Fzd) receptors, receptor tyrosine kinase-like orphan receptor (Ror) and receptor tyrosine kinase related tyrosine kinase (Ryk), whereas intracellular pathways are mediated by some molecules, such as Ca2+, c-Jun N-terminal kinase (JNK), calmodulin dependent protein kinase II (CaMKII) and protein kinase C (PKC) [7, 9]. Basal expression of Wnt5a has been observed in many immune cells [10, 11]. Wnt5a, which is dependent on Toll-like receptors, can induce phenotypic changes in monocytes, macrophages and dendritic cells, mediating the innate immune response [12,13,14]. Additionally, Wnt5a signaling contributes to CD4 T-cell homeostasis and activation of CD8 T cells, which triggers adaptive immunity [15, 16]. Increasing evidence suggests that Wnt5a signaling may play a crucial role in inflammatory diseases [17, 18]. In highly active antiretroviral therapy (HAART)-associated NeuroAIDS, administration of nucleoside reverse transcriptase inhibitors induced inflammatory cytokine production, including interleukin (IL)-6, IL-1β and TNF (tumor necrosis factor)-α, in various regions of the central nervous system via a Wnt5a signaling-dependent mechanism [19]. Wnt5a signaling mediates the pathogenesis of lung inflammation and airway epithelial injury in benzo(a)pyrene-induced pulmonary dysfunction [20]. In addition, Wnt5a is reported to be increased in experimental and human chronic obstructive pulmonary disease (COPD) and to induce production of inflammatory cytokines such as IL-6 and TNF-α [21]. Box5, a Wnt5a antagonist, is a hexapeptide that antagonizes Wnt5a-mediated signaling via direct frizzled class receptor 5 (FZD5) binding [22]. Many Wnt5a-mediated cellular activities, including migration, invasion, and apoptosis can be antagonized by Box5 [22, 23]. Inhibiting Wnt5a signaling with Box5 decreases the expression of C-C motif chemokine 2 (CCL-2), IL-6 and TNF-α, reduces the percentage of F4/80 + macrophage infiltration in the kidney, and alleviates renal injury in diabetic mice [24]. Furthermore, fine particulate matter (PM2.5) induces upregulation of Wnt5a, which promotes human bronchial epithelial cell proliferation, and production of IL-1β, IL-6 and IL-8, which was demonstrated to be prevented by Box5 [25, 26]. Moreover, limiting Wnt5a activity via Box5 reduces stroke size in rats by middle cerebral artery occlusion, eliciting neurological protection [27].
We have previously shown that serum Wnt5a can serve as a predictor for 3-month mortality in liver failure [28]. Furthermore, we demonstrated that Wnt5a expression was enhanced in a mouse model of acute liver failure [29]. Inhibiting Wnt5a signaling with Box5 reduces liver inflammation and injury [29]. In addition, HBV replication was shown to upregulate Wnt5a expression [30]. However, the potential role of Wnt5a as a predictor of liver injury caused by chronic HBV infection remains unknown. In this study, we initially determined mRNA expression of Wnt5a, IL-6, TNF-α and IL-1β in peripheral blood mononuclear cells (PBMCs) from acute-on-chronic hepatitis B liver failure (ACHBLF) patients and CHB patients. Next, we assessed the location and expression of Wnt5a protein in liver tissue. Additionally, we investigated the potential association between Wnt5a expression and the severity of liver injury.
Methods
Study design and subjects
A total of 201 patients, including 140 CHB patients and 61 ACHBLF patients, were retrospectively recruited at the Department of Hepatology, Qilu Hospital (Qingdao) of Shandong University from June 2019 to August 2021. According to inclusion and exclusion criteria, only 82 CHB patients and 31 ACHBLF patients were enrolled in the study. The selection and exclusion of ACHBLF patients and CHB patients is described in Fig. 1. Twenty healthy volunteers were enrolled as healthy controls (HCs). Neither the CHB patients nor the ACHBLF patients displayed evidence of liver cirrhosis. In addition, liver biopsies were obtained from 32 patients with chronic HBV infection, and normal liver tissues were collected from 6 patients with haemangioma who underwent partial hepatectomy and termed normal controls. A pathologist assessed the liver inflammatory grade using the modified histological activity index (HAI). Each subject signed informed consent and was approved by the Ethics Committee in Qilu Hospital (Qingdao) of Shandong University prior to the study.
CHB patients had positive serum hepatitis B surface antigen (HBsAg) for at least six months and exhibited elevation of serum alanine aminotransferase (ALT) (> 40 U/L) [31]. ACHBLF was defined following the guidelines of the Asian Pacific Association for The Study of the Liver (APASL) [32]. Patients meeting any of the following exclusion criteria were excluded: (1) coinfection with hepatitis virus A, C, D, E or G and/or human immunodeficiency virus (HIV); (2) drug hepatitis, autoimmune disease, alcoholic hepatitis, and other liver diseases; (3) any type of cancer and other serious medical illness; (4) receiving any immunotherapy or antiviral treatment at least one year prior to blood collection; and (5) pregnancy.
Peripheral blood mononuclear cell isolation
PBMCs were obtained from ethylenediaminetetraacetic (EDTA)-anticoagulated venous peripheral blood by Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) density gradient centrifugation. The interface mononuclear cells were obtained and washed three times with phosphate-buffered saline.
RNA extraction and quantitative real-time polymerase chain reaction (PCR)
TRIzol reagent (Sparkjade, China) was used to extract total RNA from PBMCs, and the RNA was reverse transcribed into cDNA using RevertAid First Strand cDNA Synthesis Kits (Fermentas, Vilnius, Lithuania). Real-time PCR was conducted using Blaze Taq™ SYBR® Green qPCR Mix 2.0 (GeneCopoeia, Rockville, MD, USA). The primers used are shown in Table 1. GAPDH served as the internal control. All PCR products were determined using the 2−ΔΔCt method.
Immunohistochemical staining
Liver tissues were fixed, embedded, and sectioned at 4 μm thickness. The sections were deparaffinized with a series of progressive xylenes and ethanol baths. Heat-induced antigen retrieval was performed in citrate buffer, followed by endogenous peroxidase activity blocking with 0.3% H2O2. The sections were incubated with primary antibody against Wnt5a (1:200; Bioss, China) overnight at 4 °C and then with horseradish peroxidase (HRP)-labeled secondary antibody. The Wnt5a protein was stained with diaminobenzideine (DAB). All sections were observed under a light microscope. Immunohistochemical positivity was measured using ImageJ 6.0 software.
Laboratory indices
The laboratory indices of each subject, including serum levels of ALT, aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), hepatitis B e antigen (HBeAg), international normalized ratio (INR), and prothrombin time activity (PTA), were obtained from laboratory reports. HBV DNA load was determined by PCR with a sensitivity of 500 IU/ml.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software. Data are shown as numbers or medians and ranges. Differences between groups were analyzed using the Kruskal–Wallis test. Spearman’s tests were used for correlation analysis. Diagnostic accuracy was evaluated by the area under the receiver operating characteristic curve (AUROC). A P value < 0.05 was considered significant.
Results
The characteristics of the subjects
The general characteristics of the ACHBLF patients, CHB patients and healthy controls are described in Table 2. Patients with ACHBLF were older and showed higher degrees of AST, TBIL and INR, as well as lower levels of ALB and PTA, than healthy controls and CHB patients. Furthermore, CHB patients had higher levels of ALT and AST, than healthy controls. No significant difference in age was found between CHB patients and healthy controls. There were no significant differences in sex distribution among the groups.
mRNA expression of Wnt5a, IL-6, TNF-α and IL-1β in PBMCs from ACHBLF patients, CHB patients, and healthy controls
We initially analyzed the mRNA levels of Wnt5a and its associated inflammatory cytokines in PBMCs from ACHBLF patients, CHB patients and healthy controls. As shown in Fig. 2A, the level of Wnt5a mRNA was higher in CHB patients than in healthy controls (5.01 [1.63, 14.72] vs. 0.96 [0.44, 2.43], P < 0.01) but lower than that in ACHBLF patients (5.01 [1.63, 14.72] vs. 28.43 [15.28, 98.70], P < 0.01]. Similar trends were found in Wnt5a-associated inflammatory cytokine expression, including TNF-α, IL-6 and IL-1β (Fig. 2B, C and D). CHB patients had higher mRNA levels of TNF-α, IL-6 and IL-1β than healthy controls (TNF-α, 3.09 [1.69–6.62] vs. 0.73 [0.59–1.76], P < 0.01; IL-6, 1.70 [0.86–4.94] vs. 0.82 [0.48–1.91], P < 0.05; IL-1β, 4.26 [2.57-1.00] vs. 0.94 [0.59–1.77], P < 0.01). In particular, expression of TNF-α, IL-6 and IL-1β in ACHBLF patients was increased compared with that in CHB patients (TNF-α, 8.51 [5.13–16.10] vs. 3.09 [1.69–6.62], P < 0.01; IL-6, 8.98 [3.90-30.03] vs. 1.70 [0.86–4.94], P < 0.01; IL-1β, 17.52 [3.72–29.16] vs. 4.26 [2.57-1.00], P < 0.05).
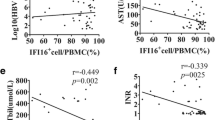
Correlation between Wnt5a mRNA levels and laboratory indices or inflammatory cytokine expression in CHB patients and ACHBLF patients
We applied Spearman rank correlation analysis to analyze the correlation between Wnt5a mRNA levels and laboratory indices or inflammatory cytokine expression in PBMCs from ACHBLF patients and CHB patients (Fig. 3). Our findings indicated that Wnt5a mRNA expression was positively associated with ALT (r = 0.316, P = 0.001, Fig. 3A), AST (r = 0.401, P < 0.001, Fig. 3B), TBIL (r = 0.205, P = 0.029, Fig. 3C), and HBV DNA (r = 0.194, P = 0.039, Fig. 3D) in all patients, including CHB patients and ACHBLF patients. Interestingly, we observed a positive correlation between Wnt5a and AST or HBV DNA in CHB patients (AST, r = 0.316, P = 0.004; HBV DNA, r = 0.231, P = 0.037), but there were no significant correlations in ACHBLF patients (AST, r = 0.272, P = 0.138; HBV DNA load, r = 0.323, P = 0.076; Fig. 3B and D). However, Wnt5a correlated positively with serum levels of TBIL in ACHBLF patients (r = 0.372, P = 0.039) but with no significant correlation in CHB patients (r = 0.198, P = 0.075, Fig. 3C). Furthermore, we assessed correlations between Wnt5a expression and its associated inflammatory cytokines in HBV-associated subjects. Wnt5a exhibited positive correlations with TNF-α (r = 0.204, P = 0.030; Fig. 3E) and IL-6 (r = 0.235, P = 0.012; Fig. 3F) but no significant association with IL-1β.
Receiver operating characteristic (ROC) analysis of Wnt5a mRNA expression to discriminate ACHBLF from CHB
We performed ROC analysis to evaluate the ability of Wnt5a mRNA expression in PBMCs to distinguish ACHBLF from CHB. As depicted in Fig. 4, the area under the receiver operating characteristic (AUROC) curve of relative Wnt5a mRNA in predicting the occurrence of ACHBLF in CHB patients was 0.831 (95% CI: 0.749–0.895, P < 0.001). The optimal cut-off value was 12.25 for the relative Wnt5a mRNA level with a sensitivity of 83.87% and a specificity of 73.17%.
Association between intrahepatic Wnt5a expression and inflammation in chronic HBV Infection patients
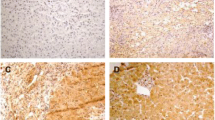
We further analyzed intrahepatic Wnt5a protein expression in 6 normal controls and 32 chronic HBV infection patients. The characteristics of these participants are shown in Tables 3 and 4. The Wnt5a protein primarily localized to the cytoplasm, as depicted in the representative immunohistochemistry images shown in Fig. 5A, B, and C. Quantitative analysis revealed that intrahepatic Wnt5a protein expression was increased in chronic HBV infection patients compared to normal controls (P < 0.001; Fig. 5D). Furthermore, intrahepatic Wnt5a expression in patients with higher liver inflammatory grade scores (G3-G4) was significantly elevated compared to that in patients with lower liver inflammatory grade scores (G0-G2) (P < 0.05; Fig. 5D). Interestingly, we found no significant difference in serum levels of ALT or AST between patients with higher liver inflammatory grade scores and those with lower scores (ALT, P > 0.05; AST, P > 0.05; Table 5).
Intrahepatic Wnt5a protein expression was determined in patients with chronic HBV infection and normal controls by immunohistochemical staining. Representative immunohistochemical photographs for intrahepatic Wnt5a protein in normal controls (A) and chronic HBV infection patients with hepatic inflammatory grade scores G1 (B) and G3 (C) (magnification, × 400). D. Quantitative analysis of Wnt5a immunohistochemistry staining. * P < 0.05. AOD, average optical density
Discussion
Aberrant Wnt5a expression has been observed in infection, inflammation, and immunity [17, 33, 34]. However, the role of Wnt5a in progression of chronic HBV infection has not been well investigated. The findings of the present study indicate that peripheral and intrahepatic Wnt5a expression is increased and positively associated with liver injury in patients with chronic HBV infection.
Initially, we found that Wnt5a mRNA expression was increased in PBMCs from CHB patients compared to those from HCs. In addition, intrahepatic Wnt5a protein expression was increased in patients with chronic HBV infection compared to that in normal controls. Previous studies have suggested that mutations in the X protein of HBV regulate Wnt5a expression in hepatocellular carcinoma (HCC) tissues and an HCC cell line [35]. Intrahepatic Wnt5a protein was increased in HBV-infected patients as liver inflammation and fibrosis improved, and it was upregulated by HBV replication in Hep AD38 cells [30]. Consistent with these studies, we observed that Wnt5a expression in PBMCs from CHB patients correlated positively with HBV DNA load. In our study, we also observed significantly higher mRNA levels of TNF-α, IL-6, and IL-1β in CHB patients than in HCs. A number of studies have demonstrated that Wnt5a can activate the nuclear factor-kappa B (NF-κB) and JNK pathways, leading to production of proinflammatory cytokines, including TNF-α, IL-6 and IL-1β [36, 37]. In line with these findings, we previously showed that exogenous Wnt5a stimulation dependent on JNK signaling resulted in production of TNF-α and IL-6 in THP-1 cells [29]. Furthermore, our results suggest that Wnt5a correlates positively with IL-6 and TNF-α. Therefore, our findings indicate that Wnt5a might play a potential role in the pathogenesis and progression of chronic HBV infection.
In addition to its involvement in inflammation, recent studies have highlighted the significant role of Wnt5a in tissue damage, such as lung, kidney, and myocardial injuries [36, 38, 39]. Nevertheless, the association between Wnt5a and liver injury in chronic HBV infection remains unknown. In our study, we initially observed higher levels of Wnt5a mRNA in PBMCs from ACHBLF patients than in those from CHB patients. Additionally, ACHBLF patients exhibited higher mRNA expression of IL-6, IL-1β and TNF-α than CHB patients. ACHBLF serves as an acute and severe deterioration in liver function leading to liver failure in CHB [32]. In other words, ACHBLF patients exhibited significantly more severe hepatic injury than CHB patients. Therefore, our findings suggest that an increase in Wnt5a expression is associated with deterioration of liver function in CHB patients. Furthermore, we observed that Wnt5a was positively associated with serum ALT and AST levels, which are commonly utilized as serum markers for liver injury, in CHB patients. In contrast, in ACHBLF patients, Wnt5a expression exhibited a positive correlation with TBIL levels, a crucial indicator of severe liver damage. Our findings also suggest that intrahepatic Wnt5a protein levels are increased in chronic HBV infection patients with high liver inflammatory grades (G3-G4) compared to those with low liver inflammatory grades (G0-G2) and HCs. Importantly, we made an intriguing observation that despite having normal serum ALT/AST levels, several patients with higher hepatic inflammation (G3-G4) displayed increased protein expression of hepatic Wnt5a. Consistent with our findings, previous clinical studies have suggested that CHB patients can exhibit persistently normal ALT levels despite significant inflammation in liver tissues [40, 41]. In this case, a strong correlation between Wnt5a and liver inflammation still existed. However, a larger sample is needed for validation. In summary, our results suggest that Wnt5a might be associated with liver injury during chronic HBV infection.
Moreover, we investigated the role of Wnt5a as a predictor in diagnosis of ACHBLF in CHB patients by ROC curve analysis. Our findings indicated that a cut-off value of 12.25 for relative Wnt5a mRNA expression had substantial discriminatory ability in distinguishing ACHBLF from CHB. ACHBLF is associated with considerable morbidity and mortality. Liver transplantation is the definitive and effective treatment for ACHBLF; however, its feasibility is limited by the availability of compatible donor livers [42]. Early diagnosis and timely intervention are generally managed to improve prognosis of ACHBLF [43]. Unfortunately, reliable predictors are lacking to identify early which patients with previously stable chronic HBV infection may experience progression to liver failure [44]. Our findings may offer a novel diagnostic tool for early detection of ACHBLF in CHB patients. However, the diagnostic value of Wnt5a should be validated through a larger, multicenter validation cohort.
There are some limitations in our present study. First, the sample size of subjects enrolled from our single institution was relatively small. We collected only 32 liver tissues from chronic HBV infection patients, and no liver tissues were obtained from ACHBLF patients due to their poor health condition and the limited acceptance of liver biopsy. Second, to explore the extensive role of Wnt5a in progression of chronic HBV infection, we should consider other HBV-infected diseases, including cirrhosis and HCC. A series of studies should be conducted on this issue. Recently, we investigated the prognostic role of serum Wnt5a in ACHBLF patients [28] and explored the underlying mechanism of Wnt5a contributing to acute liver failure with a mouse model [29]. Third, the precise mechanism underlying the contribution of Wnt5a to liver injury in chronic HBV infection remains obscure. Future studies should utilize Wnt5a knockout and overexpression cell lines as well as HBV transgenic mice to gain further insight.
Conclusion
Our findings indicate a significant increase in peripheral and intrahepatic Wnt5a expression in patients with chronic HBV infection. Wnt5a expression was observed to be associated with liver injury in chronic HBV infection. Moreover, Wnt5a mRNA expression in PBMCs may serve as a predictive marker for distinguishing ACHBLF from CHB.
Data Availability
All data generated or analyzed during the study are available from the corresponding author upon reasonable request.
Abbreviations
- HBV:
-
Hepatitis B virus
- CHB:
-
Chronic hepatitis B
- ACLF:
-
Acute-on chronic liver failure
- Fzd:
-
Frizzled
- Ror:
-
Receptor tyrosine kinase-like orphan receptor
- Ryk:
-
Receptor tyrosine kinase related tyrosine kinase
- JNK:
-
c-Jun N-terminal Kinases
- CaMKII:
-
Calmodulin dependent protein kinase II
- PKC:
-
Protein kinase C
- HAART:
-
Highly active antiretroviral therapy
- IL-6:
-
Interleukin-6
- IL-1β:
-
Interleukin-1β
- IL-8:
-
Interleukin-1β
- TNF-α:
-
Tumor necrosis factor-α
- COPD:
-
Chronic obstructive pulmonary disease
- FZD5:
-
Frizzled class receptor 5
- CCL2:
-
C-C motif chemokine 2
- PM2.5:
-
Fine particulate matter
- PBMCs:
-
Peripheral blood mononuclear cells
- ACHBLF:
-
Acute-on-chronic hepatitis B liver failure
- HCs:
-
Healthy controls
- HAI:
-
The modified histological activity index
- HBsAg:
-
Hepatitis B surface antigen
- ALT:
-
Alanine aminotransferase
- APASL:
-
The Asian Pacific Association for The Study of the Liver
- HIV:
-
Human immunodeficiency virus
- EDTA:
-
Ethylenediaminetetraacetic
- HRP:
-
Horseradish peroxidase
- DAB:
-
Diaminobenzideine
- AST:
-
Aspartate aminotransferase
- TBIL:
-
Total bilirubin
- ALB:
-
Albumin
- HBeAg:
-
Hepatitis B e antigen
- INR:
-
International normalized ratio
- PTA:
-
Prothrombin time activity
- PCR:
-
Quantitative real-time polymerase chain reaction
- AUROC:
-
The receiver operating characteristic curve
- HCC:
-
Hepatocellular carcinoma
- NF-κB:
-
Nuclear factor-kappa B
References
EASL. 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of hepatology. 2017;67(2):370 – 98.
Chen EQ, Zeng F, Zhou LY, Tang H. Early warning and clinical outcome prediction of acute-on-chronic Hepatitis B Liver Failure. World J Gastroenterol. 2015;21(42):11964–73.
Li J, Liang X, Jiang J, Yang L, Xin J, Shi D, et al. PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF. Gut. 2022;71(1):163–75.
Zhang GL, Xie DY, Ye YN, Lin CS, Zhang XH, Zheng YB, et al. High level of IL-27 positively correlated with Th17 cells may indicate liver injury in patients infected with HBV. Liver International: Official Journal of the International Association for the Study of the Liver. 2014;34(2):266–73.
Guan Z, Ding Y, Liu Y, Zhang Y, Zhao J, Li C, et al. Extracellular gp96 is a crucial mediator for driving immune hyperactivation and liver damage. Sci Rep. 2020;10(1):12596.
Yu X, Chen Y, Cui L, Yang K, Wang X, Lei L, et al. CXCL8, CXCL9, CXCL10, and CXCL11 as biomarkers of liver injury caused by chronic Hepatitis B. Front Microbiol. 2022;13:1052917.
Zou G, Park JI. Wnt signaling in liver regeneration, Disease, and cancer. Clin Mol Hepatol. 2023;29(1):33–50.
Zhu Y, Li X. Advances of wnt signalling pathway in Colorectal Cancer. Cells. 2023;12(3).
Akoumianakis I, Polkinghorne M, Antoniades C. Non-canonical WNT signalling in Cardiovascular Disease: mechanisms and therapeutic implications. Nat Reviews Cardiol. 2022;19(12):783–97.
Shao Y, Zheng Q, Wang W, Xin N, Song X, Zhao C. Biological functions of macrophage-derived Wnt5a, and its roles in human Diseases. Oncotarget. 2016;7(41):67674–84.
He W, Zhu H, Liu C. Profiles of inflammation factors and inflammatory pathways around the peri-miniscrew implant. Histol Histopathol. 2021;36(9):899–906.
Zhang Q, Liu J, Ma L, Bai N, Xu H. Wnt5a is involved in LOX-1 and TLR4 induced host inflammatory response in peri-implantitis. J Periodontal Res. 2020;55(2):199–208.
Valencia J, Martínez VG, Hidalgo L, Hernández-López C, Canseco NM, Vicente A, et al. Wnt5a signaling increases IL-12 secretion by human dendritic cells and enhances IFN-γ production by CD4 + T cells. Immunol Lett. 2014;162(1 Pt A):188–99.
Mehmeti M, Bergenfelz C, Källberg E, Millrud CR, Björk P, Ivars F, et al. Wnt5a is a TLR2/4-ligand that induces t olerance in human myeloid cells. Commun Biology. 2019;2:176.
Sengupta S, Jati S, Maity S, Sen M. Wnt5A Signaling regulates gut bacterial survival and T cell homeostasis. mSphere. 2022;7(6):e0050722.
Sarraf TR, Sen M. Wnt5A signaling supports antigen processing and CD8 T cell activation. Front Immunol. 2022;13:960060.
Tian F, Mauro TM, Li Z. The pathological role of Wnt5a in psoriasis and psoriatic arthritis. J Cell Mol Med. 2019;23(9):5876–83.
Nienhüser H, Kim W, Malagola E, Ruan T, Valenti G, Middelhoff M, et al. Mist1 + gastric isthmus stem cells are regulated by Wnt5a and expand in response to injury and inflammation in mice. Gut. 2021;70(4):654–65.
Wu T, Zhang J, Geng M, Tang SJ, Zhang W, Shu J. Nucleoside reverse transcriptase inhibitors (NRTIs) induce proinflammatory cytokines in the CNS via Wnt5a signaling. Sci Rep. 2017;7(1):4117.
Fan L, Li W, Ma J, Cheng M, Xie L, Ye Z, et al. Benzo(a)pyrene induces airway epithelial injury through Wnt5a-mediated non-canonical Wnt-YAP/TAZ signaling. Sci Total Environ. 2022;815:151965.
Zhang X, Wang Y, He X, Sun Z, Shi X. Diagnosis of Chronic Obstructive Pulmonary Disease and Regulatory mechanism of mir-149-3p on alveolar inflammatory factors and expression of surfactant proteins a (SP-A) and D (SP-D) on lung surface mediated by Wnt Pathway. Comput Intell Neurosci. 2022;2022:7205016.
Hwang J, Park JS, Ramalingam M, Kim BC, Jeong HS, Jang S. Neuroprotective effects of a wnt antagonist in Quinolinic Acid-Induced Excitotoxicity in N18D3 cells. Cellular and molecular biology. France). 2022;68(8):167–72. (Noisy-le-Grand.
Prgomet Z, Axelsson L, Lindberg P, Andersson T. Migration and invasion of oral squamous carcinoma cells is promoted by WNT5A, a regulator of cancer progression. J oral Pathol Medicine: Official Publication Int Association Oral Pathologists Am Acad Oral Pathol. 2015;44(10):776–84.
Li X, Wen J, Dong Y, Zhang Q, Guan J, Liu F, et al. Wnt5a promotes renal tubular inflammation in diabetic Nephropathy by binding to CD146 through noncanonical wnt signaling. Cell Death Dis. 2021;12(1):92.
Zou W, Wang X, Hong W, He F, Hu J, Sheng Q, et al. PM2.5 induces the expression of inflammatory cytokines via the Wnt5a/Ror2 pathway in human bronchial epithelial cells. Int J Chronic Obstr Pulm Dis. 2020;15:2653–62.
Zou W, Wang X, Sun R, Hu J, Ye D, Bai G, et al. PM2.5 induces Airway Remodeling in Chronic Obstructive Pulmonary Diseases via the Wnt5a/β-Catenin pathway. Int J Chronic Obstr Pulm Dis. 2021;16:3285–95.
Calandria JM, Do KV, Kala-Bhattacharjee S, Obenaus A, Belayev L, Bazan NG. cRel and Wnt5a/Frizzled 5 receptor-mediated inflammatory regulation reveal Novel Neuroprotectin D1 targets for Neuroprotection. Cell Mol Neurobiol. 2023;43(3):1077–96.
Ji XF, Li XY, Fan YC, Zhao ZH, Gao S, Sun FK et al. Serum wnt5a is a predictor for the prognosis of acute on chronic Hepatitis B Liver Failure. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2015;20(1):26–34.
Ji XF, Fan YC, Sun F, Wang JW, Wang K. Noncanonical Wnt5a/JNK signaling contributes to the development of D-Gal/LPS-Induced Acute Liver Failure. Inflammation. 2022;45(3):1362–73.
Li W, Yu X, Zhu C, Wang Z, Zhao Z, Li Y, et al. Notum attenuates HBV-related liver fibrosis through inhibiting wnt 5a mediated non-canonical pathways. Biol Res. 2019;52(1):10.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-pacific clinical practice guidelines on the management of Hepatitis B: a 2015 update. Hep Intl. 2016;10(1):1–98.
Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, et al. Acute-on-chronic Liver Failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hep Intl. 2019;13(4):353–90.
Padwal M, Liu L, Margetts PJ. The role of WNT5A and Ror2 in peritoneal membrane injury. J Cell Mol Med. 2020;24(6):3481–91.
Jati S, Sengupta S, Sen M. Wnt5A-Mediated Actin Organization Regulates Host Response to bacterial pathogens and non-pathogens. Front Immunol. 2020;11:628191.
Liu X, Wang L, Zhang S, Lin J, Zhang S, Feitelson MA, et al. Mutations in the C-terminus of the X protein of Hepatitis B virus regulate Wnt-5a expression in hepatoma Huh7 cells: cDNA microarray and proteomic analyses. Carcinogenesis. 2008;29(6):1207–14.
Li G, Wei W, Suo L, Zhang C, Yu H, Liu H, et al. Low-dose aspirin prevents kidney damage in LPS-Induced Preeclampsia by inhibiting the WNT5A and NF-κB signaling pathways. Front Endocrinol. 2021;12:639592.
Xie C, Jiang J, Liu J, Yuan G, Zhao Z. Ginkgolide B attenuates collagen-induced rheumatoid arthritis and regulates fibroblast-like synoviocytes-mediated apoptosis and inflammation. Annals of Translational Medicine. 2020;8(22):1497.
Zhang J, Pan Z, Zhou J, Zhang L, Tang J, Gong S, et al. The myosin II inhibitor, blebbistatin, ameliorates pulmonary endothelial barrier dysfunction in acute lung injury induced by LPS via NMMHC IIA/Wnt5a/β-catenin pathway. Toxicol Appl Pharmcol. 2022;450:116132.
Ding N, Zheng C. Secreted frizzled-related protein 5 promotes angiogenesis of human umbilical vein endothelial cells and alleviates myocardial injury in diabetic mice with Myocardial Infarction by inhibiting Wnt5a/JNK signaling. Bioengineered. 2022;13(5):11656–67.
Sheng Q, Wang N, Zhang C, Fan Y, Li Y, Han C, et al. HBeAg-negative patients with chronic Hepatitis B Virus Infection and normal alanine aminotransferase: wait or treat? J Clin Translational Hepatol. 2022;10(5):972–8.
Liu J, Wang J, Yan X, Xue R, Zhan J, Jiang S, et al. Presence of Liver inflammation in Asian patients with chronic Hepatitis B with Normal ALT and detectable HBV DNA in absence of liver fibrosis. Hepatol Commun. 2022;6(4):855–66.
Karvellas CJ, Francoz C, Weiss E. Liver transplantation in Acute-on-chronic Liver Failure. Transplantation. 2021;105(7):1471–81.
Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic Liver Failure. Lancet (London England). 2015;386(10003):1576–87.
Wu ZB, Zheng YB, Wang K, Mo ZS, Zhen X, Yan Y, et al. Plasma Interleukin-6 level: a potential Prognostic Indicator of Emergent HBV-Associated ACLF. Can J Gastroenterol Hepatol. 2021;2021:5545181.
Acknowledgements
We would like to gratefully acknowledge all of the funding sources and sincerely thank all study participants.
Funding
This work was supported by National Natural Science Foundation of China (82272313), National Key Research and Development Program of China (2021YFC2301801), Natural Science Foundation of Shandong Province (ZR2022MH006) and Scientific Research Foundation of Qilu Hospital (Qingdao) of Shandong University (QDKY2017QN13).
Author information
Authors and Affiliations
Contributions
Author contributionsXiang-Fen Ji designed the study, conducted the experiments and wrote the first draft of the manuscript. Qi Zhou collected clinical data and performed some experiments. Fei Sun and Jing-Wei Wang analyzed some data. Shuai Gao analyzed some data and involved in designing the study. Kai Wang revised it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics committee approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee in Qilu Hospital (Qingdao) of Shandong University (KYLL-2017033). All subjects provided informed consent, and the study design was approved by an ethics review board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ji, XF., Zhou, Q., Wang, JW. et al. Associations of Wnt5a expression with liver injury in chronic hepatitis B virus infection. BMC Infect Dis 23, 860 (2023). https://doi.org/10.1186/s12879-023-08865-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08865-x