Abstract
Background
We investigated the associations between the different doses of tigecycline, its efficacy and safety, and the role of tigecycline therapeutic drug monitoring for patients in the intensive care unit.
Methods
This study was a single-center cohort including patients infected with multidrug-resistant Acinetobacter baumannii (MDR-AB) and multidrug-resistant Klebsiella pneumoniae (MDR-KP) causing pulmonary infections. The steady-state plasma concentration after tigecycline administration was determined by High-Performance Liquid Chromatography (HPLC) in patients admitted to the ICU between October 2020 and December 2021. Multivariate analyses of tigecycline’s clinical efficacy and safety were performed to control confounding factors.
Results
For this study, we included 45 patients and 45 blood samples to determine steady-state trough concentrations of tigecycline. All patients were divided into the High Dose (HD) and Standard Dose (SD) groups. The median trough concentration of tigecycline was 0.56 μg/mL in the HD group, which was higher than in the SD group (0,21 μg/mL), p = 0.000. There was no significant difference between the two groups of patients in terms of bacterial eradication rate, mortality rate, and clinical efficacy. Multiple regression analysis showed that the ICU days were correlated with mortality OR 1.030(1.005–1.056), p = 0.017. APACHE II was significantly associated with clinical efficacy OR 0.870(0.755–1.002), p = 0.045. The level of fibrinogen decline in the HD group was significantly higher than in the SD group (-3.05 ± 1.67 vs -1.75 ± 1.90), p = 0.038. We identified that age and tigecycline treatment duration influenced fibrinogen decline.
Conclusions
Tigecycline plasma concentrations are significantly increased when using a high dose. However, the plasma concentration of tigecycline is not correlated with clinical efficacy and adverse reactions. Fibrinogen decline appears to be related to the patient’s age and days of tigecycline. Large sample data are still needed to confirm the clinical guidance significance of tigecycline TDM.
Similar content being viewed by others
Background
The incidence of pneumonia caused by Gram-negative organisms has significantly increased in the ICU [1]. Multidrug-resistant (MDR) Gram-negative bacteria, especially Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae members, are predominant. Multidrug-resistant A. baumannii((MDR-AB) and Carbapenemase-producing Klebsiella pneumoniae (KPC-KP) infections are significant causes of mortality in intensive care units [2]. Managing these infections is difficult due to their antibiotic resistance, particularly to carbapenem antibiotics. Tigecycline is one of the few broad-spectrum antibiotics highly active in treating pneumonia caused by MDR bacteria,including MDR-AB and KPC-KP [3]. It is off-label used in ventilator-associated pneumonia hospital-acquired pneumonia (HAP) caused by MDR pathogens, especially carbapenem-resistant (CR) bacteria [4]. The recommended initial dose of tigecycline for injection is 100 mg, followed by 50 mg every 12 h. A meta-analysis study indicated that higher doses of tigecycline lead to lower all-cause mortality and higher clinical cure and microbiological eradication rates [5]. Nowadays, the IDSA guidelines recommend high-dose tigecycline (initial dose of 200 mg, followed by 100 mg every 12 h) as an alternative option [6]. However, it will increase the risk of adverse effects of tigecycline for critically ill patients. Gastrointestinal symptoms, including nausea, vomiting, and diarrhea, are the most common adverse reactions in all-case post-marketing surveillance studies [7]. Some studies showed that high-dose tigecycline treatment in ICU patients may decrease plasma fibrinogen levels and activate partial thromboplastin time (APTT) values [8,9,10]. Coagulopathy has been reported as a side effect of high-dose tigecycline in the FDA adverse event reporting systems [10]. A study indicated that age, days of medication, and baseline fibrinogen levels were associated with hypofibrinogenemia [11]. Therefore, preventing or detecting coagulation abnormalities in time is crucial, especially for ICU patients.
Therapeutic drug monitoring (TDM) of antibiotics was recommended for critically ill patients to optimize drug effectiveness and adjust the dosage [12]. A study found that trough tigecycline concentrations can predict liver toxicity [13]. However, there is limited clinical experience with tigecycline TDM, and no evidence exists to determine whether tigecycline TDM can predict the risk of hypofibrinogenemia. Therefore, we evaluate the clinical efficacy by monitoring the plasma concentration of tigecycline and provide a reference for early prediction of coagulation abnormalities.
Material and methods
Study design and participants
This retrospective cohort study was conducted in a single health system from December 2020 to October 2021 at a tertiary-care teaching hospital in China. The study included patients over 18, diagnosed with pulmonary infection, and treated with tigecycline. Blood samples were collected from the patients had to fulfill the following mandatory criteria: samples were taken from patients who had received tigecycline therapy for > 72 h (2) adult patients (> 18 years) who needed treatment with tigecycline which bronchoalveolar lavage fluid culture was MDR-AB and KPC-KP. Respiratory tract secretion test was performed daily. Exclusion criteria were pregnancy, (2) liver or kidney failure, (3) non-compliance with the dosing regimen, and (4) infection in other sites. All patients were divided into two groups according to the medical orders by the physician: High Dose (HD) (loading dose 200 mg, maintenance dose 100 mg q12h) group and Standard Dose (SD) (loading dose 100 mg, maintenance dose 50 mg q12h) group, based on their tigecycline dosage. The follow-up time for all patients was transition from the ICU or discharge. Hospital-acquired pneumonia was diagnosed based on IDSA guidelines [14].
Blood sampling and analytical assays
To obtain total tigecycline concentration samples for analysis, 0.5–1.0 mL of peripheral vein blood was collected from patients at trough concentration points or other times after administration. The collected blood was placed in vacutainer tubes without anticoagulant and then centrifuged at 3,000 rpm for 10 min at 10◦C, and the serum was collected and stored at −40℃ until analysis. An HPLC method was used to determine the concentration of tigecycline in the serum, with a calibration curve ranging from 0.10–5.00 μg/mL showing good linearity. The intraday and interday precision RSD for low, medium, and high-dose quality control samples were less than 10%, while the extraction recovery ranged from 99.43% to 116.68% [15].
Data collection
Patient demographic characteristics, including gender, age, Charlson Comorbidity Index, mechanical ventilation, catheter indwelling, and steroid use during treatment, were collected. Data on the usage, dosage, course of treatment, and combination of tigecycline were also collected. Changes in body temperature (T), respiration (R), pulse (P), chest imaging, and laboratory indexes were collected before and after treatment. Laboratory tests, including White blood cell count (WBC), Albumin (ALB), platelet(PLT), procalcitonin (PCT), C-reactive protein (CRP), Albumin, Fibrinogen level(Fib), Prothrombin time (PT), and Activated partial prothrombin time (APTT), were also collected before tigecycline initiation and within 1 week after discontinuation.
Microbiological analysis
All the MDR-KP and MDR-AB were isolated for evaluation with 13 different antibiotics using the VITEK-2 compact system (bioMérieux, France). The minimum inhibitory concentration (MIC) was routinely determined by Clinical and Laboratory Standards Institute (CLSI) guidelines. The results for CR A. baumannii and CRKP were interpreted as susceptible (S), intermediate (I), or resistant (R), based on the breakpoints for Enterobacteriaceae strains released by the US Food and Drug Administration (FDA) (S ≤ 2, I = 4, and R ≥ 8 mg/L for minimum inhibitory concentration (MIC) testing by using the E-test method (BIO-KONT, Wenzhou, China) [16].
Clinical outcomes
All-cause death in the ICU
The patient died during the follow-up period.
Clinical efficacy was divided into effective clinical treatment (improvement of body temperature, symptoms and signs, and improvement or no progress of pulmonary imaging) and ineffective clinical treatment (lack of improvement or aggravation in body temperature and vital signs, progress of pulmonary imaging or death of patients due to infection).
Eradication of pathogens
The microbiology efficacy was divided into eradication (negative bacterial culture results), hypothetical eradication (clinically effective but not confirmed due to the inability to obtain respiratory specimens), and replacement (MDR-AB and KPC-KP were clear, while other bacteria were detected) and re-infection (MDR-AB and KPC-KP were still detected in the culture). The microbiology eradication rate = (number of cleared cases + assumed number of cleared cases + number of replacement cases)/total number of cases × 100% [17]. The duplicated cases (multiple isolates in the same patient) were not included.
Safety
Changed of coagulation values
The changes values of platelet (PLT), fibrinogen level (Fib), prothrombin time (PT), and activated partial prothrombin time (APTT) were collected before and after the administration of tigecycline.
The decline of fibrinogen
< 1.0 ~ 0.75 times the lower limit of normal value; If the baseline value is abnormal, it is lower than the baseline < 25% according to the Common Terminology Criteria for Adverse Event (CTCAE) v.5.0 guidelines [18].
Two independent investigators performed confirmation of clinical and safety outcomes.
Statistical analysis
Continuous variables were described using mean and standard deviation or median with interquartile range, while categorical variables were described using frequency or percentage. The Student t-test, or a nonparametric test, was used to compare continuous variables, and the X2 test, or Fisher’s exact test, was used to compare categorical variables. Covariates with p < 0.05 in the univariate analysis were included in the multivariate analysis. IBM SPSS Statistics (version 25.0, IBM, Armonk, NY) was used for statistical analyses, and a two-sided p < 0.05 was considered statistically significant. All graphs were created using GraphPad Prism (version 9.0, GraphPad Software, La Jolla, CA).
Results
Baseline demographics
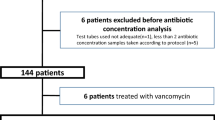
Between October 2020 and December 2021, we identified 139 patients who met the inclusion criteria. After applying the exclusion criteria, 45 patients were included in the study, with 13 in the high-dose group and 32 in the standard-dose group. See the flow chart in the Supplementary Fig. S1. Baseline characteristics of the two groups are presented in Table 1, and all patients were consistent at baseline. The media concentrations of tigecycline differed significantly between the two groups (0.56 μg/mL vs 0.21 μg/mL). The distribution of tigecycline plasma concentration in two groups of patients is presented in the Supplementary Fig. S2. All 45 patients were treated with a combination of tigecycline regimens, with 27 patients being carbapenem-resistant A. baumannii and 18 patients being carbapenem-resistant K. pneumoniae, which were sensitive to tigecycline. However, the two groups had no significant difference in bacterial eradication, mortality, or clinical efficacy.
Antimicrobial susceptibility test
All isolates were resistant to IPM (Imipenem), MEM (meropenem), AMP (ampicillin), CAZ: (ceftazidime), CTX (cefotaxime), CRO (ceftriaxone), PIP (piperacillin), TZP (piperacillin and tazobactam), MNO (minocycline), and ATM (aztreonam) except CT (colistin) and AMK (amikacin). Thirty-three isolates (73.3%) showed MIC = 2 mg/L, 8 (17.8%) MIC = 1 mg/L, 2 (4.4%) MIC ≤ 0.5 mg/L, 2 (4.4%) MIC = 4 mg/L which was intermediate to tigecycline. Please see Table 2. There were no significant clinical outcomes and bacterial clearance rates between the two groups for MIC = 2 mg/L. Please see the results in Supplementary Table 1.
Baseline labs
Before using tigecycline, there were no statistically significant differences in coagulation indicators such as PT, APTT, and Fib between the two groups. Similarly, no statistically significant differences existed in inflammatory response indicators such as CRP and PCT. Please refer to Table 3 for further details.
Logistic regression analysis of mortality in ICU
Multivariate logistic regression analysis was performed to evaluate the mortality in the ICU (Table 4). The results indicated that ICU days were a risk factor after adjusting for these variables (OR 1.030 95% CI 1.005–1.056 p = 0.017).
Logistic regression analysis of clinical efficacy
Multivariate logistic regression analysis was performed to evaluate the clinical efficacy of tigecycline (Table 5). The results showed that APACHE II was significantly associated with clinical efficacy after adjusting for potential confounding variables (OR 0.870 95% CI 0.755–1.002 p = 0.045).
Safety
Changes in coagulation
This study revealed that the decrease in fibrinogen levels was significantly higher among high-dose patients compared to the standard-dose group, with statistical significance (p = 0.038). Please refer to Table 6 for further details.
Logistic regression analysis of fibrinogen decline
After conducting a multivariate regression analysis on the decline of fibrinogen, the study revealed that age (OR 0.231, 95% CI 0.086–0.616, p = 0.003) and the duration of tigecycline treatment (OR 1.115, 95% CI 1.006–1.235, p = 0.038) were the primary influencing factors. However, no significant correlation was found between the trough concentration of tigecycline in the blood and the decline of fibrinogen, as demonstrated in Table 7.
Discussion
In this study, we used an established HPLC method to evaluate the concentration of tigecycline in ICU patients. All patients had consistent baselines; no significant difference was observed between the two groups. All K. pneumoniae isolates were resistant to ß-lactam antibiotics and carbapenems. The prevalent resistance to carbapenems was detected in all 52 isolates, whereas the presence of metallo-beta-lactamase was not detected. Meropenem-vaborbactam or ceftazidime–avibactam is not available in our hospital formulary. However, according to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for patients with severe CRKP infections [19], tigecycline is still an option.
Our study found that patient mortality and clinical efficacy were associated with APACHE II sore and ICU days, respectively. However, the blood concentration of tigecycline was not correlated with clinical effectiveness and mortality. Regarding safety, the fibrinogen decline was not related to the blood concentration of tigecycline, except for the patient’s age and days of tigecycline. It suggests that close monitoring of the days of treatment is necessary for ICU patients.
Studies have shown that the clinical efficacy of tigecycline for MDR Gram-negative bacteria was 57.57%-71.43%, and the bacterial eradication rate was 25%-56.5% [20]. Our study also demonstrated a clinical treatment efficacy of 71%, consistent with the literature. However, the bacterial eradication rate observed in our study was 11%, which was lower than reported in previous studies.
The ICU mortality rate of 24.4% was comparable to a meta-analysis study with a mortality rate of 31.4% [21]. There is a tendency for higher mortality in the HD group with decreased fibrinogen, but it is not statistically significant. These results were also consistent with Zhang’s study, which found that hospital mortality was higher in patients with hypofibrinogenemia than those with normal fibrinogen. Nevertheless, the patients in this study were not critically ill [22].
Critically ill patients can experience a range of pathophysiological changes that complicate antibiotic dosing. Therefore, knowledge of the pharmacokinetic and pharmacodynamic properties of the antibiotics used to manage critically ill patients is essential for selecting antibiotic dosing regimens. AUC/MIC was the main pharmacodynamic factor for tigecycline [23]. However, calculating AUC usually requires multiple consecutive samples, which is difficult to achieve in clinical ICU patients. Hence, therapeutic drug monitoring (TDM) is necessary to evaluate the clinical efficacy of tigecycline. In ICU patients, steady-state blood drug concentration monitoring plays a crucial role due to the possibility of one-time sampling. TDM studies of tigecycline use a standard dose, and there are no studies on high doses [24]. Our study found that the drug concentration of tigecycline in HD group patients was significantly higher than in the SD group. It has been the first time reported that the blood concentration of high-dose tigecycline has significantly increased. However, there was no significant association between the plasma concentration of tigecycline and clinical efficacy in our study. These results were also consistent with another study [13].
Recent studies have shown that a combination of the Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥ 24 and AUC0–12 h × V/MIC ≥ 100 (where V is the apparent distribution volume of the central compartment) is also closely related to the clinical efficacy of tigecycline [25]. Our research found that APACHE II also affected drug efficacy, consistent with previous studies’ results.
In terms of mortality, ICU days are an influential factor. A meta-analysis suggests that high-dose use of tigecycline can reduce the mortality rate of HAP [5]. This effect contrasts with our studies, possibly due to drug combination, patient age, etc. In our research, most patients received combination treatment. In a meta-analysis study, combination treatment significantly lowered the mortality risk in treating hospital-acquired pneumonia (HAP) [26]. Combination with meropenem and tigecycline showed significantly lower in-hospital mortality [27]. Combined with polymyxins, it has a good synergistic effect in vitro [28]. However, a propensity score-matched study showed that the combination therapy was not associated with clinical outcomes, mortality, and microbiological cure [29]. A recent study indicated that the ceftazidime–avibactam-based regimen was superior to the TGC-based regimen [30]. Ceftazidime–avibactam was limited in some hospitals in China, so tigecycline was still a treatment option.
One study indicated that MIC ≤ 2 mg/L was associated with tigecycline’s clinical efficacy in treating hospital-acquired pneumonia caused by MDR A. baumannii [31]. Our study showed no significant difference in clinical efficacy between the two groups in patients with MIC = 2 mg/l. This is not consistent with Zhou’s study. This may be why patients with MIC ≤ 2 mg/l were the main population in this study.
The resistance phenotype of CRE can be classified into two main sub-groups: non-carbapenemase-producing and carbapenemase-producing [32]. The application of whole genome sequencing in identifying antimicrobial-resistant genes has demonstrated guiding treatment decisions [33]. A recent study indicated that antibiotic resistance genes (aac3iia) and virulence genes (AREO-iutA, Capsule-wzc) were independent mortality risk factors for patients with carbapenemase-producing K. pneumoniae infections [34].
Studies have shown that albumin also affects the clinical efficacy of tigecycline. For every 10.3 g/L increase in the albumin, the odds of microbiological success increased 8–21 times compared to the albumin ≤ 26.00 g/L [35]. This observation may be due to the high protein-binding rate of tigecycline, which may increase the free plasma concentration and be further associated with clinical efficacy. Therefore, during tigecycline treatment, if the albumin value is below the normal limit, the albumin can be supplemented appropriately to obtain a more significant curative effect. Due to the small number of patients in our study and most patients with an albumin value of ≥ 26.00 g/L, the correlation between the albumin and PK parameters is not analyzed.
Literature research found that the adverse reactions of tigecycline were diarrhea, coagulation abnormalities, and thrombocytopenia in a real-world study [36]. Whether TDM monitoring can prevent adverse reactions is still unclear. A study reported that blood tigecycline concentration was related to hepatotoxicity [13]. Our study found no significant difference in ALT, AST, ALP, and Total bilirubin before or after tigecycline treatment in both groups. However, there was a significant decrease in fibrinogen levels in the HD group but no significant changes in APTT and PT. A retrospective study of 55 cases found that albumin level and weight-adjusted tigecycline dosage affected PT and APTT prolongation [37]. However, this study did not indicate whether patients used large doses of tigecycline. Retrospective studies suggested that large doses of tigecycline lead to fibrinogen decline [38]. Besides age, our study indicated that tigecycline treatment duration may also lead to decreased fibrinogen levels, which has not been reported in other studies. Lastly, our study found that the trough concentration of tigecycline in the blood did not predict the level of fibrinogen decline. Literature reported that Fibrinogen falls to critically low levels (< 1.0 g/L) during major hemorrhage was clinically significant [39]. However, early detection of fibrinogen decline can reduce the risk of bleeding in the ICU, and a large sample size is still needed to confirm it.
The exact mechanism by which tigecycline causes a decline in fibrinogen levels is not fully understood. It is believed that tigecycline is supposed to inhibit interleukin six (IL-6) expression, which usually stimulates gene expression and increases fibrinogen levels [40]. Recently, a study found that inhibiting mitochondrial DNA translation was a potential molecular mechanism [41]. A study showed that MDR A. baumannii strains can bind fibrinogen through two domain tip adhesins, Abp1D and Abp2D [42]. Plasminogen bound to CipA, which mediates complement resistance of A. baumannii, could be activated into plasmin to degrade fibrinogen [43]. This may explain why there were no differences between APTT and PT. Several methods have been proposed for preventing or minimizing fibrinogen decline. These include monitoring fibrinogen levels before and during treatment with tigecycline, adjusting doses based on patient characteristics such as age and underlying medical conditions, and avoiding concomitant use of other medications known to affect clotting. Additionally, it has been suggested that prophylactic administration of vitamin K may benefit some patients receiving long-term tigecycline therapy due to its role in clotting factor synthesis.
We found some limitations in our study due to i) the lack of pharmacokinetic/pharmacodynamic (PK/PD) data on tigecycline, which could lead to suboptimal dosing regimens or adverse drug effects; ii) the blood concentration of tigecycline was not the concentration of free drug. A further analysis of albumin’s effect on clinical effectiveness is not conducted.
Conclusion
Our study found that patients receiving high-dose tigecycline exhibited significantly higher blood concentrations than those receiving low doses. The two groups had no significant differences in mortality, clinical efficacy, and bacterial eradication rates. High amounts of tigecycline were found to reduce the level of fibrinogen. Nevertheless, additional research is needed to understand the mechanism behind this reduction better and identify potential preventive measures for patients experiencing this complication during tigecycline therapy. Furthermore, further investigation is required to evaluate the efficacy of prophylactic administration of vitamin K in preventing this complication during tigecycline treatment. Based on our findings, we recommend monitoring fibrinogen levels in patients who receive high doses of tigecycline.
Availability of data and materials
The datasets are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
References
Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–54.
Jernigan JA, Hatfield KM, Wolford H, et al. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012–2017. N Engl J Med. 2020;382:1309–19. https://doi.org/10.1056/NEJMoa1914433.
Stein GE, Craig WA. Tigecycline: a critical analysis. Clin Infect Dis. 2006;43:518–24.
Frampton JE, Curran MP. Tigecycline. Drugs. 2005;65:2623–35 discussion 2636–7.
Zha L, Pan L, Guo J, et al. Effectiveness and safety of high dose tigecycline for the treatment of severe infections: a systematic review and meta-analysis. Adv Ther. 2020;37:1049–64.
Tamma PD, Aitken SL, Bonomo RA, et al. Infectious Diseases Society of America 2022 guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2022;75:187–212.
Ohashi T, Sugiyama N, Watanabe T, et al. Drug use investigation on the safety and efficacy of tigecycline in Japan (all-case post-marketing surveillance). J Infect Chemother. 2022;28:866–74.
Pieringer H, Schmekal B, Biesenbach G, et al. Severe coagulation disorder with hypofibrinogenemia associated with the use of tigecycline. Ann Hematol. 2010;89:1063–4.
Routsi C, Kokkoris S, Douka E, et al. High-dose tigecycline-associated alterations in coagulation parameters in critically ill patients with severe infections. Int J Antimicrob Agents. 2015;45:90–3.
Guo M, Liang J, Li D, et al. Coagulation dysfunction events associated with tigecycline: a real-world study from FDA adverse event reporting system (FAERS) database. Thromb J. 2022;20:12.
Liu J, Yan Y, Zhang F. Risk factors for tigecycline-associated hypofibrinogenemia. Ther Clin Risk Manag. 2021;17:325–32. https://doi.org/10.2147/TCRM.S302850.
Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper(). Intensive Care Med. 2020;46:1127–53.
Fan G, Jin L, Bai H, et al. Safety and efficacy of tigecycline in intensive care unit patients based on therapeutic drug monitoring. Ther Drug Monit. 2020;42:835–40.
Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-61e111.
Wen-chao LYW, Qian SSS, Xiao-ling LXB. Establishment and application of a HPLC method for determination of tigecycline in human serum. Chin J Drug Appl Monit. 2022;19:16–20.
Yin D, Guo Y, Li M, et al. Performance of VITEK 2, E-test, Kirby-Bauer disk diffusion, and modified Kirby-Bauer disk diffusion compared to reference broth microdilution for testing tigecycline susceptibility of carbapenem-resistant K. pneumoniae and A. baumannii in a multicenter study in China. Eur J Clin Microbiol Infect Dis. 2021;40:1149–54.
Kwon SH, Ahn HL, Han OY, et al. Efficacy and safety profile comparison of colistin and tigecycline on the extensively drug resistant Acinetobacter baumannii. Biol Pharm Bull. 2014;37:340–6.
Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90–2.
Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28(4):521–47.
Wang J, Pan Y, Shen J, et al. The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2017;16:24.
Ni W, Han Y, Liu J, et al. Tigecycline treatment for carbapenem-resistant Enterobacteriaceae infections: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e3126.
Zhang Q, Wang J, Liu H, et al. Risk factors for tigecycline-induced hypofibrinogenaemia. J Clin Pharm Ther. 2020;45:1434–41.
Singh R, Mukker JK, Drescher SK, et al. A need to revisit clinical breakpoints of tigecycline: effect of atypical non-linear plasma protein binding. Int J Antimicrob Agents. 2017;49:449–55.
Shao R, Li X, Hu Y, et al. Determination of tigecycline in human plasma by LC-MS/MS and its application to population pharmacokinetics study in Chinese patients with hospital-acquired pneumonia. Biomed Chromatogr. 2018;32. https://doi.org/10.1002/bmc.4045.
Zhou Y, Xu P, Li H, et al. Population pharmacokinetics and exposure-response analysis of tigecycline in patients with hospital-acquired pneumonia. Br J Clin Pharmacol. 2021;87:2838–46.
Bai XR, Liu JM, Jiang DC, et al. Efficacy and safety of tigecycline monotherapy versus combination therapy for the treatment of hospital-acquired pneumonia (HAP): a meta-analysis of cohort studies. J Chemother. 2018;30(3):172–8.
Park JM, Yang KS, Chung YS, et al. Clinical outcomes and safety of meropenem-colistin versus meropenem-tigecycline in patients with carbapenem-resistant Acinetobacter baumannii pneumonia. Antibiotics (Basel, Switzerland). 2021;10(8):903.
Cai Y, Lim TP, Teo J, Sasikala S, et al. In Vitro activity of polymyxin B in combination with various antibiotics against extensively drug-resistant Enterobacter cloacae with decreased susceptibility to polymyxin B. Antimicrob Agents Chemother. 2016;60(9):5238–46.
Zha L, Zhang X, Cheng Y, et al. Intravenous polymyxin B as adjunctive therapy to high-dose tigecycline for the treatment of nosocomial pneumonia due to carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae: a propensity score-matched cohort study. Antibiotics (Basel, Switzerland). 2023;12(2):273.
Shi Y, Hu J, Liu P, et al. Ceftazidime-avibactam-based versus tigecycline-based regimen for the treatment of carbapenem-resistant Klebsiella pneumoniae-induced pneumonia in critically ill patients. Infect Dis Ther. 2021;10(4):2721–34.
Zhou Y, Chen X, Xu P, et al. Clinical experience with tigecycline in the treatment of hospital-acquired pneumonia caused by multidrug resistant Acinetobacter baumannii. BMC Pharmacol Toxicol. 2019;20(1):19.
Teng J, Imani S, Zhou A, et al. Combatting resistance: understanding multidrug resistant pathogens in intensive care units. Biomed Pharmacother. 2023;167:115564.
Yang J, Zhang K, Ding C, et al. Exploring multidrug-resistant Klebsiella pneumoniae antimicrobial resistance mechanisms through whole genome sequencing analysis. BMC Microbiol. 2023;23(1):245.
Bai XR, Cao JR, Wang ZZ, et al. Clinical efficacy, antibiotic resistance genes, virulence factors and outcome of hospital-acquired pneumonia induced by Klebsiella pneumoniae carbapenemase 2-producing with tigecycline treatment in the ICU. Infect Drug Resist. 2022;15:5545–55.
Bhavnani SM, Rubino CM, Hammel JP, et al. Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with tigecycline. Antimicrob Agents Chemother. 2012;56:1065–72.
Shi X, Zuo C, Yu L, et al. Real-world data of tigecycline-associated drug-induced liver injury among patients in China: a 3-year retrospective study as assessed by the updated RUCAM. Front Pharmacol. 2021;12:761167.
Wang D, Lin C, Gu C, et al. Tigecycline-associated coagulopathy: a single-center retrospective analysis. Pharmacology. 2022;107:524–36.
Campany-Herrero D, Larrosa-Garcia M, Lalueza-Broto P, et al. Tigecycline-associated hypofibrinogenemia in a real-world setting. Int J Clin Pharm. 2020;42:1184–9.
Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion. 2014;54:1389–405; quiz 1388.
Vasse M, Paysant I, Soria J, et al. Down-regulation of fibrinogen biosynthesis by IL-4, IL-10 and IL-13. Br J Haematol. 1996;93:955–61.
Vandecasteele SJ, Seneca S, Smet J, et al. Tigecycline-induced inhibition of mitochondrial DNA translation may cause lethal mitochondrial dysfunction in humans. Clin Microbiol Infect. 2018;24(431):e1-431.e3.
Tamadonfar KO, Di Venanzio G, Pinkner JS, et al. Structure-function correlates of fibrinogen binding by Acinetobacter adhesins critical in catheter-associated urinary tract infections. Proc Natl Acad Sci U S A. 2023;120:e2212694120.
Ries JI, Heß M, Nouri N, et al. CipA mediates complement resistance of Acinetobacter baumannii by formation of a factor I-dependent quadripartite assemblage. Front Immunol. 2022;13:942482.
Acknowledgements
The authors would like to thank all participants for giving data analyzing data and the hospital administration for granted access to all patients.
Funding
This study was funded by the National Key R&D Program of China (2020YFC2008305), Beijing Municipal Science and Technology Commission Special Funding Project (D181100000218002), National Clinical Research Center for Geriatric Disorders, Beijing, China. Beijing Municipal Commission of Health and Family Planning, No. PXM2018_026283_000002; Beijing Municipal Administration of Hospitals Incubating Program (PX2020038).
Author information
Authors and Affiliations
Contributions
Xiang-rong Bai: Conceptualization, Resources, Data Curation, Writing-Original Draft, Writing-Reviewing and Editing. Zhi-zhou Wang: Data collection. Wen-chao Li: Data collection. Yan-gai Wang: Resources, Data Curation. Ran Lou: Resources, Data Curation. Xin Qu: Resources, Data Curation. Fan linlin: Resources, Data Curation. Zhang Wei: Resources, Data Curation. Yan-chuan Wu: Visualization, Project Administration. Su-ying Yan: Supervision, Project Administration. Lan Zhang: Resources, Supervision, Project Administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was carried out by the Declaration of Helsinki criteria and received approval from the Ethics Committee of Xuanwu Hospital Capital Medical University (IRB, protocol number: 2020038 6/20/2020). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all study participants prior to the start of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure S1.
Flowchart of patient enrollment. Supplementary Figure S2. Violin plot of the blood concentration distribution of tigecycline in the two groups. Supplementary Table 1. Clinical outcomes and bacterial clearance rates between the two groups for MIC = 2 mg/L.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bai, Xr., Wang, Zz., Li, Wc. et al. Clinical efficacy and safety of tigecycline based on therapeutic drug monitoring for carbapenem-resistant Gram-negative bacterium pneumonia in intensive care units. BMC Infect Dis 23, 830 (2023). https://doi.org/10.1186/s12879-023-08815-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08815-7