Abstract
Objective
Hepatitis C presents a profound global health challenge. The impact of COVID-19 on hepatitis C, however, remain uncertain. This study aimed to ascertain the influence of COVID-19 on the hepatitis C epidemic trend in Henan Province.
Methods
We collated the number of monthly diagnosed cases in Henan Province from January 2013 to September 2022. Upon detailing the overarching epidemiological characteristics, the interrupted time series (ITS) analysis using autoregressive integrated moving average (ARIMA) models was employed to estimate the hepatitis C diagnosis rate pre and post the COVID-19 emergence. In addition, we also discussed the model selection process, test model fitting, and result interpretation.
Results
Between January 2013 and September 2022, a total of 267,968 hepatitis C cases were diagnosed. The yearly average diagnosis rate stood at 2.42/100,000 persons. While 2013 witnessed the peak diagnosis rate at 2.97/100,000 persons, 2020 reported the least at 1.7/100,000 persons. The monthly mean hepatitis C diagnosed numbers culminated in 2291 cases. The optimal ARIMA model chosen was ARIMA (0,1,1) (0,1,1)12 with AIC = 1459.58, AICc = 1460.19, and BIC = 1472.8; having coefficients MA1=-0.62 (t=-8.06, P < 0.001) and SMA1=-0.79 (t=-6.76, P < 0.001). The final model’s projected step change was − 800.0 (95% confidence interval [CI] -1179.9 ~ -420.1, P < 0.05) and pulse change was 463.40 (95% CI 191.7 ~ 735.1, P < 0.05) per month.
Conclusion
The measures undertaken to curtail COVID-19 led to a diminishing trend in the diagnosis rate of hepatitis C. The ARIMA model is a useful tool for evaluating the impact of large-scale interventions, because it can explain potential trends, autocorrelation, and seasonality, and allow for flexible modeling of different types of impacts.
Similar content being viewed by others
Introduction
Hepatitis C is a viral infection caused by the hepatitis C virus (HCV). Symptoms commonly include decreased appetite, nausea, and general weakness, which can progress to liver failure and brain tissue accumulation, posing significant health risks and public health concerns. The World Health Organization (WHO) estimates that HCV infection has become a substantial global public health burden, affecting approximately 3% of the population worldwide [1]. Hepatitis C is the leading cause of chronic liver disease among over 170 million individuals globally [2]. The disease manifests in a wide range of severity, from mild self-limiting cases to cirrhosis and hepatocellular carcinoma [3]. Regrettably, no vaccine is currently available for hepatitis C. Chronic hepatitis C is highly prevalent and associated with an increased risk of progressive liver fibrosis, ultimately leading to cirrhosis and liver failure [4]. However, significant progress has been made with the development of direct-acting antiviral (DAA) medications. Notably, the combination of sofosbuvir and velpatasvir, together with sofosbuvir and ledipasvir, have proven highly successful in clinical trials and are now considered the cornerstone of HCV treatment [5]. Despite these advancements, the global mission to eradicate HCV faces challenges, such as the high cost of DAA medications and the absence of a vaccine. Effective management of hepatitis C remains a complex task due to its widespread prevalence and associated risk factors [5].
The emergence of COVID-19 as a novel coronavirus has placed immense strain on global healthcare systems. Early reports from China highlighted the overwhelming pressure faced by hospital staff during the initial stages of the pandemic[6]. The healthcare infrastructure in many countries has been severely disrupted due to the high demand for COVID-19 care and the reallocation of resources. As a result, the efforts to eliminate hepatitis C, including screening, diagnosis, and treatment, have been significantly reduced or even halted. This poses significant challenges to the WHO’s goal of eradicating hepatitis C, both in terms of screening and treatment. The Chinese CDC has issued guidelines to postpone non-essential procedures and routine outpatient visits in order to alleviate the burden on the healthcare system caused by COVID-19. The pandemic has not only impeded hepatitis eradication plans, but also led to a decrease in routine HCV antibody screening, clinical care, and treatment opportunities in the first half of 2020 [7].
Furthermore, the implementation of social distancing measures, lockdowns, isolation protocols, and the perceived risk of contracting SARS-CoV-2 during hospital visits have resulted in fewer visits for non-COVID-19 related illnesses. This delay in seeking medical care may hinder the early detection and treatment of hepatitis C, thereby increasing the health risks for individuals affected by the disease [8]. The delayed identification and subsequent treatment of hepatitis C-infected individuals may also contribute to a higher likelihood of virus transmission and an increased risk of disease progression in untreated individuals. This can lead to the development of advanced liver disease and potential transmission of the virus [9]. It is estimated that a one-year delay in the diagnosis and treatment of hepatitis could result in 44,800 new cases of liver cancer and 72,300 deaths from hepatitis C worldwide by 2030 [9]. Given the global commitment to eliminate hepatitis by 2030, it is crucial to prioritize hepatitis planning as soon as circumstances permit [10].
In this study, an interrupted time series (ITS) analysis was employed to assess the influence of COVID-19 on hepatitis C diagnosis rate, contributing a solid scientific foundation for enhancing hepatitis C prevention and control strategies in Henan Province.
Materials and methods
The data used in this study originates from hepatitis C diagnosis cases reported by the Henan Provincial Health Commission spanning from January 2013 to September 2022. The diagnosis data of hepatitis C is defined in accordance with the diagnostic criteria specified by the Henan Provincial Health Commission. Figure 1 illustrates the diagnostic criteria for hepatitis C.
The interrupted time series (ITS) analysis serves to assess the impact of interventions by comparing indicators within the time series both before and after the intervention point. The two primary models commonly utilized for this analysis are the ARIMA and Segmented Regression Model (SRM). In cases where the trend of dependent variables displays non-linearity with strong seasonality and periodicity, as often observed in time series data, a direct application of the SRM could compromise the accuracy of intervention effect evaluation. Instead, ARIMA may be employed for a more appropriate analysis [11].
ARIMA modeling process
The constructed ARIMA model is represented as ARIMA (p, d, q) (P, D, Q) s. Here, p signifies the autoregressive (AR) orders, q represents the moving average (MA) orders, and d stands for the number of differences applied to make the time series stationary [12]. D indicates the degree of seasonal difference, while P and Q denote the AR and MA terms of the seasonal components [13]. The ARIMA modeling process encompasses the following steps: (1) Data smoothing: Initially, the stationarity of the time series data is assessed. For non-stationary time series, iterative differencing is conducted until stationarity is achieved [13]. If the time series has seasonal factors, the seasonal differencing is employed to control or eliminate the impact of seasonal autocorrelation. (2) Model identification: The “auto.arima()” function within the forecast package automatically tests various order combinations and selects the optimal model, aiding in the determination of appropriate orders. (3) Model selection: Based on model selection criteria, the model is evaluated using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) to identify the most suitable one. (4) Model validation: Residuals of the chosen model are examined to determine if they exhibit characteristics of white noise. Autocorrelation testing is carried out by analyzing residual plots and performing the Ljung-Box Q test.
Use ITS-ARIMA model to evaluate interventions
ITS analysis assesses the impact of intervention measures by evaluating their influence on specific outcomes. Three primary types of intervention effects include step change, pulse change, and slope change [13]. Predefining the expected shape of the intervention effect is essential, which is influenced by the intervention’s nature (temporary or continuous) and the evaluation’s outcomes. Combinations of influencing variables are often utilized to express changes, like step change and slope/pulse change or step change and pulse change. It is important to account for potential delays in impact, specifying a reasonable time frame to observe the effects based on prior research to avoid spurious correlations [14]. When necessary, controlling and mitigating the impact’s delay on the time series may involve excluding data during the intervention’s transitional period. By comparing estimated indicator values post-intervention with values assumed under no intervention, the researcher calculates the intervention’s impact at specific time points. These effects can be modeled effectively through step and pulse functions or straight lines with unit slopes [14]. Once the final model is selected, the intervention’s impact can be estimated accurately.
Results
A total of 267,968 cases of hepatitis C were diagnosed between January 2013 and September 2022, resulting in an annual average diagnosis rate of 2.42 per 100,000 people. The highest diagnosis rate occurred in 2013 at 2.97 per 100,000 people, while the lowest was recorded in 2020 at 1.7 per 100,000 people. The average monthly diagnosis cases of hepatitis C was 2291.
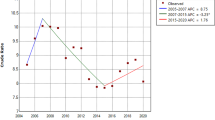
The diagnosis rate for hepatitis C was analyzed using the ARIMA model. Figure 2 displays a time series analysis of hepatitis C diagnosis rate in Henan spanning from January 2013 to September 2022. The data is divided into two segments: pre-COVID-19 (from January 2013 to December 2019) and post-COVID-19 (from January 2020 to September 2022). The insights gained from Fig. 2 highlight a noticeable decrease in hepatitis C diagnosis rate during the COVID-19 outbreak in 2020, followed by a subsequent increase. Across the broader timeframe from 2013 to 2022, the diagnosis series of hepatitis C exhibited cyclic patterns. Through the application of differencing to adjust for this trend and periodicity, the hepatitis C diagnosis series demonstrated enhanced stability.
The Box-Cox method was employed, along with the “auto.arima” function in the R software, to fit the hepatitis C diagnosis series in Henan Province from January 2013 to September 2022. ACF and PACF plots are depicted in Supplementary Fig.S1. In this illustration, bars above or below the dotted line represent statistically significant autocorrelation (P < 0.05). Both ACF and PACF plots (Supplementary Fig.S1a) reveal undifferentiated autocorrelation and partial autocorrelation patterns, with noticeable significant autocorrelation. Supplementary Fig.S1b presents autocorrelation and partial autocorrelation post-differencing. In comparison to Supplementary Fig.S1a, differencing effectively removed much of the autocorrelation.
The ARIMA (0,1,1) (0,1,1)12 model yielded the lowest AIC (1459.58), AICc (1460.19), and BIC (1472.8). Consequently, this model structure was selected as the optimal. Diagnostic results indicated that for the ARIMA (0,1,1) (0,1,1)12 model, MA1=-0.62 (t=-8.06, P < 0.001) and SMA1=-0.79 (t=-6.76, P < 0.001); ACF and PACF plots of residuals indicated most correlation coefficients were within the confidence interval (Supplementary Fig.S2). The Ljung-Box Q test results demonstrated no statistically significant difference among residuals for different lag periods (P = 0.408), affirming that model residuals constituted white noise. These results validate the ARIMA (0,1,1) (0,1,1)12 model.
Over time, the time series plot displayed relatively constant variance. The histogram of the time series showed normally distributed prediction errors, with the mean adhering to normal distribution as well. Residuals showcased no discernible pattern or significant autocorrelation, and they followed a normal distribution. The Ljung-Box Q test yielded a P-value of 0.408, confirming the chosen model’s good fit.
The final model estimated a step change of -800.0 (95% CI -1179.9 ~ -420.1, P < 0.05) and a pulse change of 463.40 (95% CI 191.7 ~ 735.1, P < 0.05) per month. Figure 3; Table 1 depict the comparison between predicted and observed values of the ARIMA model without intervention (counterfactual).
From January 2013 to September 2022, an analysis was conducted on the fitting and observed values of hepatitis C diagnosis cases both before and after the onset of COVID-19. The findings suggested a decrease in hepatitis C diagnosis cases in January 2020, with an anticipation of the impact being transient. Since January 2020, the influence of COVID-19 on hepatitis C diagnosis rate was modeled using a pulse function. Following the emergence of COVID-19, a decline in hepatitis C diagnosis cases was observed. A step function was utilized to fit potential long-term fluctuations in the number of hepatitis C diagnosis cases. The final model revealed a sudden reduction in diagnosis cases post the onset of COVID-19, followed by a gradual increase back to pre-COVID-19 levels. Given the nature of the intervention, we assumed an immediate decrease in diagnosis cases post-intervention (step change) and an accompanying pulse change. Hence, variables representing both types of impacts were incorporated into the model.
Moreover, when comparing the ITS-ARIMA model with the BSTS model (Fig. 3), results indicated that the mean absolute percentage error (MAPE = 19.95%) of the ITS-ARIMA model’s predictions was lower than that of the BSTS model (MAPE = 25.7%). This implies that the prediction performance of the ITS-ARIMA model surpassed that of the BSTS model, demonstrating the former’s superior predictive capabilities.
Discussion
Throughout the period from 2013 to 2020, the diagnosis rate of hepatitis C displayed a declining trend following the onset of the COVID-19 pandemic, particularly evident in early 2020. As COVID-19 emerged, Henan Province implemented emergency response measures to curb its spread, which subsequently affected hepatitis C diagnosis rate.
Table 1 provides a comparison of predicted and actual values under a scenario without the COVID-19 pandemic (counterfactual). At the beginning of 2020, the actual diagnosis rate was considerably lower than the predicted value. Findings suggest that hepatitis C diagnosis rate in Henan Province experienced a decrease due to COVID-19 prevention measures, consistent with European research outcomes [8]. The decline in hepatitis C diagnosis cases can be attributed to two primary factors [15].First, long-standing effective measures for hepatitis C contributed significantly, including continuous efforts by the Chinese government like robust monitoring systems, increased budgets, and efficient control and treatment. Second, the impact of COVID-19 played a role. There may be several reasons for the decline of hepatitis C diagnosis rate caused by the COVID-19 [16,17,18,19,20]. First, the COVID-19 crisis caused most of the medical resources to be invested in the treatment of COVID-19 patients. Second, the impact of COVID-19 on the elimination of HCV included the redistribution of medical resources and the interruption of treatment. Third, the unwillingness of Hepatitis C patients to seek medical treatment for fear of COVID-19 infection. Fourth, the follow-up plan of Hepatitis C patients could not be implemented owing to the suspension of the medical center, which led to the reduction of the diagnosis rate and treatment rate of Hepatitis C. Fifth, to reduce hospital congestion, it is not recommended for patients with chronic diseases or symptoms to seek medical assistance. Sixth, the opening hours of health facilities are shortened, and the ability of the elderly or those with limited mobility to pay for care or transportation costs is reduced. Individual hepatitis C patients find it difficult to access medical services. Seventh, people with HCV infection may be reluctant to have a medical check-up due to the strict prevention and control measures and the mandatory requirement for the negative results of nucleic acid testing. Eighth, during the lockdown period, under-reporting or delayed reporting may be inevitable for a passive monitoring system.
In general, from 2013 to 2020, the HCV diagnosis rate seems to be declining. As can be seen from Fig. 3, the COVID-19 pandemic will begin to decline sharply in 2020, and then the HCV diagnosis rate will return to the previous level. This trend can be attributed to the relaxation of COVID-19 policy. The redistribution of medical resources and changes in medical demand may affect the diagnosis rate, making it return to the level before COVID-19 [21]. These findings align with Qin Zhou’s research utilizing ITS analysis to evaluate COVID-19’s impact on infectious diseases, including hepatitis C [21]. Through sensitivity analysis, the ITS-ARIMA model demonstrated superior predictive performance compared to the BSTS model, confirming the former’s reliability.
It is essential to educate hepatitis C patients, especially those with cirrhosis or advanced liver disease, about COVID-19 risks and preventive measures, given the potential severity of COVID-19 in such cases [22]. The global aim of eliminating hepatitis C by 2030 has been hindered by the COVID-19 pandemic [10]. Although Henan Province implemented preventive measures against COVID-19 early on, the pandemic negatively impacted chronic disease patient care. The postponement of non-emergency healthcare services strained the healthcare system. Delayed identification of HCV-infected individuals increases transmission risks [23]. Telemedicine offers a solution for patient follow-ups, safeguarding patients and medical staff from COVID-19 transmission [24]. However, its expanded use also introduces security concerns, necessitating ongoing improvements.
This study’s strength lies in its utilization of Henan Province’s notifiable infectious disease data to analyze COVID-19’s impact on hepatitis C diagnosis rate, controlling for seasonal and cyclical effects. However, the post-COVID-19 data collection cycle is relatively short, potentially leading to delays, inaccuracies, and data omissions. Besides, the data in the study is diagnosis cases for hepatitis C that has been legally reported, and is not the actual incidence data for hepatitis C. Rapid policy changes further affect the study’s scope, warranting future research.
Conclusion
Under the influence of COVID-19 prevention measures, hepatitis C diagnosis rate in Henan Province experienced a downward trend, possibly linked to these preventative efforts. The disease exhibited cyclical and seasonal traits. The ARIMA model effectively explained trends, autocorrelation, and seasonality, offering flexibility in understanding intervention impacts. Balancing COVID-19 prevention with hepatitis C management is crucial.
Data Availability
Hepatitis C data obtained from Henan Provincial Health Commission (https://wsjkw.henan.gov.cn/zfxxgk/yqxx/index.html).
References
Sung PS, Hong SH, Chung JH, Kim S, Park SH, Kim HM, et al. IFN-λ4 potently blocks IFN-α signalling by ISG15 and USP18 in hepatitis C virus infection. Sci Rep. 2017;7(1):3821.
Global prevalence. And genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76.
Navaneethan U, Kemmer N, Neff GW. Predicting the probable outcome of treatment in HCV patients. Th Adv Gastroenterol. 2009;2(5):287–302.
Takemoto R, Nakamuta M, Aoyagi Y, Fujino T, Yasutake K, Koga K, et al. Validity of FibroScan values for predicting hepatic fibrosis stage in patients with chronic HCV infection. J Dig Dis. 2009;10(2):145–8.
Bernal LA, Soti V, Hepatitis C, Virus. Insights into its history, treatment, Challenges, and future directions. Cureus. 2023;15(8):e43924.
Liu Q, Luo D, Haase JE, Guo Q, Wang XQ, Liu S, et al. The experiences of health-care providers during the COVID-19 crisis in China: a qualitative study. Lancet Glob Health. 2020;8(6):e790–e8.
Kaufman HW, Bull-Otterson L, Meyer WA 3rd, Huang X, Doshani M, Thompson WW, et al. Decreases in Hepatitis C Testing and Treatment during the COVID-19 pandemic. Am J Prev Med. 2021;61(3):369–76.
Kondili LA, Buti M, Riveiro-Barciela M, Maticic M, Negro F, Berg T, et al. Impact of the COVID-19 pandemic on hepatitis B and C elimination: an EASL survey. JHEP Rep. 2022;4(9):100531.
Buti M, Domínguez-Hernández R, Casado MA. Impact of the COVID-19 pandemic on HCV elimination in Spain. J Hepatol. 2021;74(5):1246–8.
Blach S, Kondili LA, Aghemo A, Cai Z, Dugan E, Estes C, et al. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74(1):31–6.
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309.
Jian Y, Zhu D, Zhou D, Li N, Du H, Dong X, et al. ARIMA model for predicting chronic kidney disease and estimating its economic burden in China. BMC Public Health. 2022;22(1):2456.
Schaffer AL, Dobbins TA, Pearson SA. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med Res Methodol. 2021;21(1):58.
Gilmour S, Degenhardt L, Hall W, Day C. Using intervention time series analyses to assess the effects of imperfectly identifiable natural events: a general method and example. BMC Med Res Methodol. 2006;6:16.
Ding W, Li Y, Bai Y, Li Y, Wang L, Wang Y. Estimating the Effects of the COVID-19 outbreak on the reductions in tuberculosis cases and the epidemiological Trends in China: a causal impact analysis. Infect Drug Resist. 2021;14:4641–55.
Karimi-Sari H, Rezaee-Zavareh MS. COVID-19 and viral hepatitis elimination programs: are we stepping backward? Liver Int. 2020;40(8):2042.
Teslya A, Pham TM, Godijk NG, Kretzschmar ME, Bootsma MCJ, Rozhnova G. Impact of self-imposed prevention measures and short-term government-imposed social distancing on mitigating and delaying a COVID-19 epidemic: a modelling study. PLoS Med. 2020;17(7):e1003166.
Mutyambizi C, Wilkinson L, Rees K, Moosa S, Boyles T. Outcomes of a model integrating tuberculosis testing into COVID-19 services in South Africa. Afr J Prim Health Care Fam Med. 2022;14(1):e1–e4.
Min J, Ko Y, Kim HW, Koo HK, Oh JY, Jeong YJ, et al. Increased Healthcare Delays in Tuberculosis Patients during the First Wave of COVID-19 pandemic in Korea: a nationwide cross-sectional study. J Korean Med Sci. 2022;37(3):e20.
McQuaid CF, Vassall A, Cohen T, Fiekert K, White RG. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis. 2021;25(6):436–46.
Zhou Q, Hu J, Hu W, Li H, Lin GZ. Interrupted time series analysis using the ARIMA model of the impact of COVID-19 on the incidence rate of notifiable communicable diseases in China. BMC Infect Dis. 2023;23(1):375.
Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, et al. Clinical best practice advice for Hepatology and Liver Transplant Providers during the COVID-19 pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72(1):287–304.
Barutçu S, Yıldırım Ç, Yıldırım AE, Konduk BT, Sayiner ZA, Gülşen MT. Changes in Hepatitis C awareness in different disciplines during COVID-19. Turk J Gastroenterol. 2022;33(10):838–43.
Sperring H, Ruiz-Mercado G, Schechter-Perkins EM. Impact of the 2020 COVID-19 Pandemic on Ambulatory Hepatitis C Testing. J Prim Care Community Health. 2020;11:2150132720969554.
Acknowledgements
We appreciate Henan Health Commission for providing the monthly number of HCV cases. We also thank all the people who participated in the gathering of HCV cases.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Yanyan Li and Xin-xiao Li conceived, initiated, and performed this work. Xianxiang Lan, Chen-Lu, Xue, Bing-Jie Zhang, Yanyan Li, and Xin-xiao Li collected and analyzed, and interpreted the data for this study. Yong-bin Wang edited and improved this original manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was approved by the study institutional review board of the Xinxiang Medical University (No: XYLL-2019072). We collect data anonymously. This data is second-hand and publicly available, so it does not need ethics. This study does not involve the human body/tissues/organs, therefore there is no need for informed consent from patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Li, X., Lan, X. et al. Impact of COVID-19 on epidemic trend of hepatitis C in Henan Province assessed by interrupted time series analysis. BMC Infect Dis 23, 691 (2023). https://doi.org/10.1186/s12879-023-08635-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08635-9