Abstract
Background
Community-onset pneumonia (COP) is a combined concept of community acquired pneumonia and the previous classification of healthcare-associated pneumonia. Although ceftriaxone (CRO) is one of the treatment choices for COP, it is unclear whether 1 or 2 g CRO daily has better efficacy. We compared the effectiveness of 1 g with 2 g of CRO for COP treatment. We hypothesized that 1 g CRO would show non-inferiority over 2 g CRO.
Methods
This study was an analysis of prospectively registered data of the patients with COP from four Japanese hospitals (the Adult Pneumonia Study Group-Japan: APSG-J). We included subjects who were initially treated solely with 1 or 2 g of CRO. The propensity score was estimated from the 33 pre-treatment variables, including age, sex, weight, pre-existing comorbidities, prescribed drugs, risk factors for aspiration pneumonia, vital signs, laboratory data, and a finding from chest xrays. The primary endpoint was the cure rate, for which a non-inferiority analysis was performed with a margin of 0.05. In addition, we performed three sensitivity analyses; using data limited to the group in which CRO solely was used until the completion of treatment, using data limited to inpatient cases, and performing a generalized linear mixed-effect logistic regression analysis to assess the primary outcome after adjusting for random hospital effects.
Results
Of the 3817 adult subjects with pneumonia who were registered in the APSG-J study, 290 and 216 were initially treated solely with 1 or 2 g of CRO, respectively. Propensity score matching was used to extract 175 subjects in each group. The cure rate was 94.6 and 93.1% in the 1 and 2 g CRO groups, respectively (risk difference 1.5%; 95% confidence interval − 3.1 to 6.0; p = 0.009 for non-inferiority). The results of the sensitivity analyses were consistent with the primary result.
Conclusions
The propensity score-matched analysis of multicenter cohort data from Japan revealed that the cure rate for COP patients treated with 1 g daily CRO was non-inferior to that of patients treated with 2 g daily CRO.
Similar content being viewed by others
Background
Community-acquired pneumonia (CAP) is one of the most common infectious diseases and leads to morbidity and mortality worldwide [1, 2]. The Infectious Diseases Society of America (IDSA) guidelines recommend that either respiratory quinolone or beta-lactam plus azithromycin to be used as a first line therapy for CAP; Ceftriaxone (CRO) is one of the recommended regimens among beta-lactam antibiotics [2, 3].
The Sanford Guide for Antimicrobial Therapy recommends that the dose of CRO should be 1–2 g daily to treat pneumonia [4]. However, it is unclear whether 1 or 2 g of CRO daily is better. To date there have been a few studies comparing the effectiveness of 1 and 2 g CRO for CAP. In one study CRO was used for pneumonia in addition to other community acquired infections such as urinary tract infections or cellulitis [5]. Another study compared 1 g daily CRO not only with 2 g daily CRO but also with other agents [6]; with such designs it is not possible to determine the optimal dose of CRO for pneumonia. Previous studies have shown that CRO can cause gallstone formation and a study suggests that more than 2 g or 40 mg/kg daily of CRO is one of the risk factors of gallstone formation (odds ratio: 11.9, 95% confidence interval [CI]: 2.6–54.2) [7, 8]. In addition, optimization of antimicrobial dosing is an essential part of antimicrobial stewardship [9]. Conversely, we need to be very cautious about the effectiveness of antibiotics for the treatment of pneumonia, as the proportion of elderly people in populations is increasing dramatically worldwide, and old age is thought to be an independent risk factor for mortality associated with CAP [10, 11].
Community-onset pneumonia (COP) is a combined concept of CAP and the previous classification of healthcare-associated pneumonia (HCAP). The concept of HCAP was removed in the hospital acquired pneumonia (HAP) / ventilator-associated pneumonia (VAP) guidelines in 2016, as the patients with HCAP frequently present from the community and are initially cared in emergency departments [12].
The aim of our study was to compare the effectiveness of 1 and 2 g daily CRO as treatment for COP, using data from a Japanese multicenter registry. We hypothesized that 1 g CRO would show non-inferiority over 2 g CRO for treatment of patients with COP.
Methods
Setting and study population
This propensity score-matching study was a sub-study of the Adult Pneumonia Study Group-Japan (APSG-J) study; this study aimed to compare the effectiveness of 1 g CRO treatment to 2 g of COP treatment for adult patients. The APSG-J study was initiated after obtaining approval by the Institutional Review Boards (IRBs) of all five study hospitals. Written consents from participants were waived by all IRBs because of the study’s observational nature, without any deviation from the current medical practice. The study was conducted on all of the four main islands of Japan from September 2011 through August 2014.
The APSG-J study collected data from adult patients with pneumonia prospectively to elucidate trends in COP and its etiologies in the aging society [13]. Eligible patients were enrolled in the APSG-J study if they fulfilled all of the following criteria: patients 1) ≥ 15 years; 2) exhibited symptoms compatible with pneumonia (e.g., fever, cough, sputum, pleuritic chest pain, or dyspnea); and 3) displayed new pulmonary infiltrates on chest X-ray images (CXR) or computed tomography scans that were consistent with pneumonia. Patients were enrolled from both inpatient and outpatient services. In our study, subjects who were initially treated solely with 1 or 2 g daily CRO were enrolled.
Assessment of outcomes
The primary outcome was the cure rate, which was defined based on the state on discharge in the patient’s record; this was assessed using the frequency of cured patients in each group. The states in the record included cure, stable condition, exacerbation, death, hospital transfer, and others. Secondary outcomes included in-hospital mortality, the duration of antibiotics, and length of hospital stay between the two groups.
Data preparation and sample size estimation
All statistical analyses were performed with the R 3.2.3 software for statistical computing (https://www.r-project.org/); the add-on packages “mice” for multiple imputation and “matching” for propensity score matching were used [14, 15]. These analyses were conducted according to the methods used in a previous propensity-score matching study [16]. The primary analysis of the cure rate was conducted using a non-inferiority analysis with a one-sided alpha level of 0.05. The non-inferiority margin was set at an absolute value of 5.0%, based on the Food and Drug Administration non-inferiority clinical trial guidance to determine the margin for pneumonia, and two previous clinical trials [5, 6, 17]. We chose the Farrington and Manning test because propensity-score matching would only give us the same sample size for the two groups, and the Farrington and Manning test requires that the same sample size be enrolled in the two groups. The sample size for the primary outcome was calculated based on the previous randomized control trials, which suggested that the cure rate in the 1 and 2 g CRO groups would be 92 and 87%, respectively [5, 6]. We calculated that a sample of 161 patients per group would give the study 90% power to detect non-inferiority for 1 g CRO treatment.
Apart from the primary outcome of the main analysis, we also used a two-sided alpha level of 0.05, and differences were considered significant if p-values were ≤ 0.05. The survival of patients was shown using a Kaplan-Meier survival curve, and adjusted hazard ratios (aHRs) were calculated using multivariable Cox proportional hazards regression analyses. As there were several missing values observed (Additional file 1: Table S1), we used multiple imputation by employing chained equations to complement all missing values for each study variable. Thereby we generated 25 datasets with 20 iterations.
Propensity score matching
Logistic regression analyses were used to estimate the propensity scores, which were then utilized to predict the efficacy of the use of 1 g over 2 g of CRO. This prediction incorporated 33 pre-treatment covariates, including age, sex, weight, pre-existing comorbidities, if the medical histories were consistent with CAP or not, prescribed drugs prior to admission (specifically prednisolone, anti-acid drug, and sleeping drug), risk factors for aspiration pneumonia, vital signs (respiratory rate, systolic blood pressure, heart rate, and body temperature), laboratory data (hematocrit and blood urea nitrogen, sodium, glucose, and albumin), and a finding from CXRs (pleural effusion). Propensity score matching was conducted for the selected subjects on a pairwise basis after all propensity scores across the imputed datasets had been averaged and logit-transformed. The match caliper was set to 0.2. We used absolute standardized mean differences (ASMDs) for all variables included in the propensity score estimation to assess the match balance; an ASMD of < 0.1 was defined as an appropriate match balance.
Sensitivity analysis
We performed three sensitivity analyses as follows. All the outcomes were reassessed using data limited to the group in which CRO solely was used until the completion of treatment. We evaluated the primary outcome exclusively for inpatient cases, given that the treatment environment (i.e., inpatient vs. outpatient) might influence mortality. Finally, we included a generalized linear mixed-effect logistic regression analysis to assess the primary outcome after adjusting for random hospital effects, since antibiotic selection preferences might differ between the hospitals.
Results
Baseline characteristics of the participants before and after propensity score matching
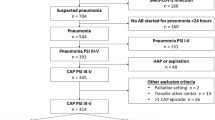
Of the 3817 adult subjects with pneumonia who were registered in the APSG-J study, 290 and 216 were initially treated solely with 1 or 2 g of CRO, respectively. Propensity score matching was employed to finally extract 175 subjects in each group (Fig. 1, Table 1, Additional file 2: Figure S1).
Primary outcome for patients after propensity score matching
Overall, the cure rate was 94.6% in the 1 g CRO group and 93.1% in the 2 g CRO group (risk difference 1.5%; 95% CI − 6.6 to 3.6; p = 0.009 for non-inferiority; p = 0.572 for superiority) (Table 2).
Secondary outcomes for patients after propensity score matching
When considering all of the propensity score-matched subjects, the length of hospital stay in the 1 g CRO group (17 days [95% CI: 14–21 days]) was significantly shorter than that in the 2 g CRO group (26 days [95% CI: 22–30 days]; p < 0.001). Duration of antibiotic treatment in the 1 g CRO group was also significantly shorter (8 days [95% CI: 8 − 9 days]) than that in the 2 g CRO group (10 days [95% CI: 9–10 days]; p = 0.002). The in-hospital mortality rate did not significantly differ between the 1 (4.7% [95% CI: 1.5–8.0%]) and 2 g CRO groups (4.0% [95% CI: 1.1–6.9%]; p = 0.740) (Table 2). Survival analysis of the propensity score-matched subjects revealed a similar survival time in the two groups. Specifically, the aHR for mortality in the 1 g CRO group vs. the 2 g CRO group was 1.58 (95% CI: 0.56–4.43) (p = 0.385) (Fig. 2).
Results of the sensitivity analyses
For the patients in which treatment was solely CRO for the duration of treatment, we could not ascertain an appropriate match using the same variables for the main analysis; thus, we decreased the number of variables used, limiting the sample size to 94 subjects in each group. The cure rate was 88.9% (95% CI: 82.3–95.4%) in the 1 g CRO group and 91.5% (95% CI: 85.7–97.2%) in the 2 g CRO group (p = 0.549 for superiority). The difference in the length of hospital stay was significantly shorter in the 1 g CRO group (18 days [95% CI: 14 − 22 days]) than that in the 2 g CRO group (26 days [95% CI: 21–32 days]; p = 0.007). The duration of antibiotics treatment and the in-hospital mortality rate did not significantly differ between the two groups (Table 3). The analysis using only inpatient cases produced similar results (1 g CRO group: 95.4% [95% CI: 91.8–99.0%] vs. 2 g CRO group: 90.7% [95% CI: 85.8–95.6%]; p = 0.127 for superiority). Finally, the analysis using the random hospital effects as a sensitivity measure also supported the above finding (odds ratio, 0.77 [95% CI: 0.32–1.90], p = 0.576).
Discussion
In this study, the cure rate for COP patients treated with 1 g daily CRO was non-inferior to those treated with 2 g daily CRO. The aim of this study was to show that the smaller antibiotic dose was non-inferior to the higher dose. Although the optimization of antibiotics dose is one of the major aims in the era of antimicrobial stewardship [9] in order to make resistant pathogens less likely to occur, many clinicians and researchers alike may consider the difference between treatment with 1 g and that with 2 g CRO as subtle. To our knowledge, high-quality evidence regarding the difference in efficacy between 1 and 2 g of CRO is lacking. Although a few studies recently evaluated the effectiveness of 1 and 2 g CRO and some other agents for CAP [17, 18], the numbers of patients enrolled were small, leading to a lack of power [5, 6]. Our study will further enhance the results of the previous clinical trials, given that our study was performed with adequate power.
We were aware that determining the non-inferiority margin should be prudent and cautious, and it is important that the margin is set appropriately based on the findings from previous studies [19, 20]. We set 5% as the non-inferiority margin for the cure rate based on the findings from previous clinical trials [5, 6]. There is a trade-off relationship between the non-inferiority margin and the potential benefits, which in our study included promoting the antimicrobial stewardship and potential fewer side effects (e.g., gallstone formation). We believe that the 5% non-inferiority margin was a reasonable value from a clinical standpoint.
The length of hospital stay and duration of antibiotics were significantly shorter in the 1 g CRO group based on the main analysis, and the sub analysis showed that the length of hospital stay was also significantly shorter in the 1 g CRO group, in which the treatment was completed exclusively using 1 g CRO. As these results were unexpected, we conducted a generalized linear mixed-effect logistic regression model analysis, and confirmed that the length of the hospital stay and duration of antibiotics were not influenced by random hospital effects. These results might be explained by the fact that a lower dose of antibiotics might lead to fewer side effects, because a variety of adverse events related to CRO were reported [21]. We are, however, uncertain whether all of those adverse events, with the exception of gallstones, were dose-dependent [8, 22]. In this study, we did not have information regarding the side effects caused by antibiotic treatment reported in the various hospitals. Another possible explanation for our findings is that patients’ socioeconomic status (SES) in the 1 g CRO group could have been higher compared to those in the 2 g CRO group. The higher the SES and the fewer problems encountered by the family in preparing a well-ordered environment or nursing home for the patient, the greater the chances might be that the patient would be discharged earlier. These factors suggest that our results should be interpreted prudently.
This study was subject to several limitations. First, this study was designed as non-inferiority trial, and the margin was set retrospectively. However, we discussed the appropriateness of the non-inferiority margin carefully. Second, this was an observational study, and the information was not collected regarding the factors related to SES. Third, we were unable to make comparisons between the two groups based on the sputum cultures. Our results may be associated with differences in bacterial etiology, so their interpretation requires some caution. However, we could not include culture results as pre-treatment variables, as these results were obtained after treatment was initiated, i.e., we usually prescribed antibiotics without any significant sputum culture results since approximately 3 days are required to identify pathogens. The frequency of pneumonia related bacteria in the APSG-J study population was previously reported [13]. Fourth, the overall in-hospital mortality rate in our study was lower (4.3%) than that of a COP mortality (11.5%) in the APSG-J study [13]. This might reflect the situation, in which our participants might be healthier than typical COP patients.
Our study had several strengths despite these limitations. To our knowledge, this was the first study to consider the body weight of the patient to investigate the optimal dose of CRO. The mechanism in which CRO is distributed throughout the body depends on the patient's body weight, so we would not be able to investigate the effectiveness of CRO without also taking body weight into account. Second, we used prospectively collected multicenter registry data; multiple imputation and propensity score matching were conducted to increase the robustness of the analysis. Additionally, many covariates were analyzed to increase the consistency of the results. We chose a variety of covariates not only based on major criteria such as the CURB-65 or Pneumonia Severity Index [23, 24], but we also included factors associated with pneumonia mortality [25,26,27]. Finally, our study had sufficient power as discussed above, while one of the major criticisms of the previous studies was the insufficient power. This study, therefore, provided additional meaningful insights regarding the optimal dose of CRO for the treatment of COP.
Conclusions
In this propensity score-matched analysis of multicenter cohort data, the cure rate of COP patients treated with 1 g daily CRO was non-inferior to those treated with 2 g daily CRO. Our study offers useful insights regarding the optimal dose of CRO for patients with COP. Further studies, for example randomized control studies with adequate power are needed to strengthen the evidence regarding this treatment alternative.
Availability of data and materials
The datasets used and/or analyzed during the current study are not publicly available due to other ongoing research projects using the datasets, but could be available from the corresponding author on reasonable request.
Abbreviations
- aHRs:
-
Adjusted hazard ratios
- APSG-J:
-
Adult Pneumonia Study Group-Japan
- ASMDs:
-
Absolute standardized mean differences
- CAP:
-
Community-acquired pneumonia
- COP:
-
Community-onset pneumonia
- CRO:
-
Ceftriaxone
- CXR:
-
Chest xray
- HAP:
-
Hospital-acquired pneumonia
- HCAP:
-
Healthcare-associated pneumonia
- IDSA:
-
Infectious Diseases Society of America
- IRBs:
-
Institutional Review Boards
- SES:
-
Socioeconomic status
- VAP:
-
Ventilator-associated pneumonia
References
Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619–28.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372:1312–23.
David N, George M, Henry F, et al. The Sanford Guide to Antimicrobial Therapy 2018. Virginia: Antimicrobial Therapy Inc; 2018.
Segev S, Raz R, Rubinstein E, et al. Double-blind randomized study of 1 g versus 2 g intravenous ceftriaxone daily in the therapy of community-acquired infections. Eur J Clin Microbiol Infect Dis. 1995;14:851–5.
de Klerk GJ, van Steijn JH, Lobatto S, et al. A randomised, multicentre study of ceftriaxone versus standard therapy in the treatment of lower respiratory tract infections. Int J Antimicrob Agents. 1999;12:121–7.
Park HZ, Lee SP, Schy AL. Ceftriaxone-associated gallbladder sludge: Identification of calcium-ceftriaxone salt as a major component of gallbladder precipitate. Gastroenterology. 1991;100(6):1665-1670.
Ettestad PJ, Campbell GL, Welbel SF, et al. Biliary complications in the treatment of unsubstantiated Lyme disease. J Infect Dis. 1995;171:356–61.
Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77.
Kothe H, Bauer T, Marre R, et al. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J. 2008;32:139–46.
World Health Organization. Ageing and life-course. World report on ageing and health. http://www.who.int/ageing/publications/world-report-2015/en. Accessed 2 June 2019.
Kalil AC, Metersky ML, Klompas M, et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111.
Morimoto K, Suzuki M, Ishifuji T, et al. The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS One. 2015;10:e0122247.
Van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations. J Stat Softw. 2011;45:1–67.
Sekhon J. Multivariate and propensity score matching. J Stat Softw. 2011;42:52.
Shiraishi A, Kushimoto S, Otomo Y, et al. Effectiveness of early administration of tranexamic acid in patients with severe trauma. Br J Surg. 2017;104:710–7.
Zhong NS, Sun T, Zhuo C, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of Asian patients with community-acquired pneumonia: a randomised, controlled, double-blind, phase 3, non-inferiority with nested superiority trial. Lancet Infect Dis. 2015;15:161–71.
Lodise TP, Anzueto AR, Weber DJ, et al. Assessment of time to clinical response, a proxy for discharge readiness, among hospitalized patients with community-acquired pneumonia who received either ceftaroline fosamil or ceftriaxone in two phase III FOCUS trials. Antimicrob Agents Chemother. 2015;59:1119–26.
Opatowski L, Mandel J, Varon E, et al. Antibiotic dose impact on resistance selection in the community: a mathematical model of beta-lactams and Streptococcus pneumoniae dynamics. Antimicrob Agents Chemother. 2010;54:2330–7.
Althunian TA, de Boer A, Klungel OH, et al. Methods of defining the non-inferiority margin in randomized, double-blind controlled trials: a systematic review. Trials. 2017;18:107.
Shalviri G, Yousefian S, Gholami K. Adverse events induced by ceftriaxone: a 10-year review of reported cases to Iranian Pharmacovigilance Centre. J Clin Pharm Ther. 2012;37:448–51.
Lopez AJ, O'Keefe P, Morrissey M, et al. Ceftriaxone-induced cholelithiasis. Ann Intern Med. 1991;115:712–4.
Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–82.
Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50.
Adamuz J, Viasus D, Jiménez-Martínez E, et al. Incidence, timing and risk factors associated with 1-year mortality after hospitalization for community-acquired pneumonia. J Inf Secur. 2014;68:534–41.
Torres A, Peetermans WE, Viegi G, et al. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68:1057–65.
Shindo Y, Ito R, Kobayashi D, et al. Risk factors for 30-day mortality in patients with pneumonia who receive appropriate initial antibiotics: an observational cohort study. Lancet Infect Dis. 2015;15:1055–65.
Acknowledgments
The Adult Pneumonia Study Group-Japan (APSG-J) comprises Masahiko Abe 1, Takao Wakabayashi 1, Masahiro Aoshima 2, Naoto Hosokawa 3, Norihiro Kaneko 2, Naoko Katsurada 2, Kei Nakashima 2, Yoshihito Otsuka 4, Eiichiro Sando 5, Kaori Shibui 5, Daisuke Suzuki 3, Kenzo Tanaka 6, Kentaro Tochitani 3, Makito Yaegashi 5, Masayuki Chikamori 7, Naohisa Hamashige 7, Masayuki Ishida 7, Hiroshi Nakaoka 7, Norichika Aso 8, Hiroyuki Ito 8, Kei Matsuki 8, Yoshiko Tsuchihashi 8, Koya Ariyoshi 9, Bhim G. Dhoubhadel 9, Akitsugu Furumoto 9, Sugihiro Hamaguchi 1, 9, Tomoko Ishifuji 9, Shungo Katoh 1,9, Satoshi Kakiuchi 9, Emi Kitashoji 9, Takaharu Shimazaki 9, Motoi Suzuki 9, Masahiro Takaki 9, Konosuke Morimoto 9, Kiwao Watanabe 9, and Lay-Myint Yoshida 10.
1 Department of General Internal Medicine, Ebetsu City Hospital, Hokkaido, Japan.
2 Department of Pulmonology, Kameda Medical Center, Chiba, Japan.
3 Department of Infectious Diseases, Kameda Medical Center, Chiba, Japan.
4 Department of Laboratory Medicine, Kameda Medical Center, Chiba, Japan.
5 Department of General Internal Medicine, Kameda Medical Center, Chiba, Japan.
6 Emergency and Trauma Center, Kameda Medical Center, Chiba, Japan.
7 Department of Internal Medicine, Chikamori Hospital, Kochi, Japan.
8 Department of Internal Medicine, Juzenkai Hospital, Nagasaki, Japan.
9 Department of Clinical Medicine, Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan.
10 Department of Pediatric Infectious Diseases, Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan.
Funding
The APSG-J study was partially supported by Pfizer in the design of the study and collection.
Author information
Authors and Affiliations
Consortia
Contributions
Literature search: SH Data collection: KM Study design: SH, RS, TM. Analysis of data: SH, TM. Manuscript preparation: SH, RS, TM. Review of manuscript critically for important intellectual content: SH, RS, MY, KM, TM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The APSG-J study adhered to the Guidelines for Ethical Aspects in Epidemiological Study (MHLW, 2008). Ethics approval was obtained by the Institutional Review Boards at all of the five study hospitals, namely the Institute of Tropical Medicine at Nagasaki University, Ebetsu City Hospital, Kameda Medical Center, Chikamori Hospital, and Juzenkai Hospital.
This study was of noninterventional nature and did not include primary data collection (i.e., was based on published secondary data only). Therefore, ethic committee or institutional review board approval was not required. Data used were taken from published cohort trials, which were conducted according to the principles of the Declaration of Helsinki and with informed consent from participants. The APSG-J study was approved by the Institutional Review Boards in the Tropical Medicine at Nagasaki University, and the committee’s reference number was 11063070.
Consent for publication
Not applicable.
Competing interests
KM reports and Grants from Pfizer, speaking fee from MSD and Pfizer. TM’s joint appointment as an associate professor at the University of Tsukuba was sponsored by JMDC Inc. in the 2018 financial year (i.e., April 2018 to March 2019), and by SMS CO., LTD. in the 2019 financial year (i.e., April 2019 to the present). JMDC Inc. or SMS CO., LTD had no role in conducting this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
The number of missing values of pretreatment variables in patients with aspiration-associated pneumonia.
Additional file 2: Figure S1.
The distributions of the propensity scores before and after matching.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hasegawa, S., Sada, R., Yaegashi, M. et al. 1g versus 2 g daily intravenous ceftriaxone in the treatment of community onset pneumonia – a propensity score analysis of data from a Japanese multicenter registry. BMC Infect Dis 19, 1079 (2019). https://doi.org/10.1186/s12879-019-4552-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4552-8