Abstract
Introduction
Pancreatic adenocarcinoma (PDAC) has a poor prognosis since often diagnosed too late. Dyslipidemia and hyperglycemia are considered risk factors, but the presence of the tumor itself can determine the onset of these disorders. Therefore, it is not easy to predict which subjects with diabetes or dyslipidemia will develop or have already developed PDAC. Over the past decade, tests based on the use of nanotechnology, alone or coupled with common laboratory tests (e.g., hemoglobin levels), have proven useful in aiding the diagnosis of PDAC. Tests based on magnetic levitation (MagLev) have demonstrated high diagnostic accuracy in compliance with the REASSURED criteria. Here, we aimed to assess the ability of the MagLev test in detecting PDAC when coupled with the blood levels of glycemia, cholesterol, and triglycerides.
Methods
Blood samples from 24 PDAC patients and 22 healthy controls were collected. Human plasma was let to interact with graphene oxide (GO) nanosheets and the emerging coronated systems were put in the MagLev device. Outcomes from Maglev experiments were coupled to glycemia, cholesterol, and triglycerides levels. Linear discriminant analysis (LDA) was carried out to evaluate the classification ability of the test in terms of specificity, sensitivity, and global accuracy. Statistical analysis was performed with Matlab (MathWorks, Natick, MA, USA, Version R2022a) software.
Results
The positions of the levitating bands were measured at the starting point (i.e., as soon as the cuvette containing the sample was subjected to the magnetic field). Significant variations in the starting position of levitating nanosystems in controls and PDACs were detected. The combination of the MagLev outcomes with the blood glycemic levels returned the best value of global accuracy (91%) if compared to the coupling with those of cholesterol and triglycerides (global accuracy of ~ 77% and 84%, respectively).
Conclusion
If confirmed by further studies on larger cohorts, a multiplexed Maglev-based nanotechnology-enabled blood test could be employed as a screening tool for PDAC in populations with hyperglycemia.
Similar content being viewed by others
Introduction
The lack of biomarkers for early detection of pancreatic ductal adenocarcinoma (PDAC) represents a hot topic of research, and their assessment in clinical practice could represent a turning point in the fight against this lethal disease (Jacks, et al. 2022). To date, high costs for screening programs and low reliability of available specific PDAC markers still give a large advantage to the disease that continues to have a poor prognosis as it is too often identified in its advanced stage (Huang et al. 2022). According to what the World Health Organization reported, to be used in clinical practice a marker should satisfy the REASSURED criteria (Land et al. 2019). On this basis, increasing attention is paid to potential easy-to-use markers that can be commonly dosed in all laboratories at low cost. In 2015, based on the results obtained analyzing plasma levels of glucose, total cholesterol, and triglycerides from 7 European cohorts in a very large series composed of 21593 men and 14348 women, Stocks et al. explored the influence of metabolic factors on the risk of developing different cancer types. According to the results of this investigation, in the presence of metabolic alteration, the risk to develop PDAC increase was 53% and women were particularly interested (Stocks et al. 2015). Further investigations focused on the importance that alteration and balance between different metabolic pathways, such as the cholesterogenic and glycolytic ones have on the risk of development and differentiation of PDAC (Karasinska et al. 2020). Moreover, researchers at the University of Pennsylvania showed how changes in total serum cholesterol are predictors of PDAC; on this basis, serum cholesterol has been advocated as a potential biomarker to be used for risk stratification and early detection of pancreatic cancer (Chen et al. 2019). Nevertheless, some authors proposed a mechanism allowing cholesterol metabolism to play a key role in PDAC development (Gabitova-Cornell, et al. 2020). The link between lipid metabolism and the risk of PDAC could be identified in IL-6, a cytokine critically involved in inflammation, a well-known cancer risk condition and serum cholesterol level regulator (Mormile 2020). An association between serum levels of triglycerides and PDAC has been also reported so far (Wang et al. 2021). About the glycolytic pathway, it is known that 1% of new-onset diabetic (NOD) patients will develop pancreatic cancer within 3 years from the diagnosis. Nonetheless, it has been demonstrated that every 0.56 mmol/L increase in fasting blood glucose is associated with a 14% increase in PDAC rate (Liao 2015), and long-lasting type 2 diabetes is widely considered a risk factor for the development of PDAC (Keum et al. 2018). On this basis, models to determine the risk of PDAC in these patients have been proposed but they still need to be confirmed on larger prospective randomized trials (Sharma, et al. 2018). Bo Hyung Yoon described type 3 diabetes associated with pancreatic disease as PDAC. However, type 3 diabetes is often confused with type 2 and may lead to a late diagnosis of pancreatic cancer. Even though some differences can be detected between patients affected by type 3 and type 2 diabetes (e.g., lower BMI, older age, and better hemoglobin A1c levels), these signs, symptoms and laboratory data are not specific and do not allow the use of expensive and/or potentially dangerous second level screening tests to be applied on large series identified at higher risk of PDAC (Yoon et al. 2021; Konstantinou et al. 2013; Lankisch et al. 2012). Recently, our group has demonstrated that a proper combination of non-specific laboratory data (e.g., low hemoglobin levels and acute phase protein concentration), with the outcomes of nanoparticle-enabled blood (NEB) tests, can discriminate PDAC patients from healthy controls with high sensitivity, specificity, and diagnostic accuracy. The NEB tests assume that the protein corona that forms around nanoparticles (NPs) upon exposure to human plasma (HP) is personalized and, under certain experimental conditions, contains a signature for PDAC (Caputo et al. 2020; Caputo et al. 2022a). The most recent version of the NEB test is based on the analysis of the magnetic levitation (MagLev) profiles of coronated graphene oxide (GO) nanoflakes in a paramagnetic solution (Digiacomo et al. 2021; Digiacomo et al. 2022; Quagliarini et al. 2022a). We demonstrated that specific features of the profiles such as the starting position after injection in the device, the separation into precipitating and levitating components, and the migration speed of the precipitating component may contain information on the health spectrum of individuals. Here, we aimed to assess the ability of the MagLev test in detecting PDAC when coupled with the blood levels of glycemia, cholesterol, and triglycerides (Fig. 1). The multiplexed strategy was validated using a sample cohort made of 24 PDAC patients and 22 healthy volunteers and its most efficacious version was obtained by coupling the starting position of the coronated materials in the MagLev device with the patients’ glycemia levels in the blood. It exhibited high specificity, sensitivity, and a global classification accuracy of 91%.
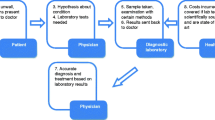
Multiplexed strategy for PDAC diagnosis. Representative scheme of the workflow applied for the classification of non-oncological patients (NOP) and PDAC patients. It consists in a multiplexed strategy that combines the results obtained by a magnetic levitation nanoparticle-enabled blood test with the blood levels of biological markers
Results
A total of 46 subjects, 22 PDACs, and 24 non-oncological patients (NOP), have been studied. Their clinical characteristics are reported in “Materials and Methods”. Preliminary experiments were aimed at characterizing pristine GO nanosheets. The size and zeta potential distributions displayed in Additional file 1: Fig. S1 show that GO nanosheets were homogeneous in size and negatively charged. After incubation with HP, an increase in size and a decrease in zeta potential were observed. Next, GO nanosheets were incubated with HP from N = 24 PDAC patients and N = 22 healthy subjects for 1 h at room temperature, leading to the formation of personalized protein coronas. Each of the 46 GO–HP samples was characterized by the Maglev platform and images of the levitating profiles were acquired and processed (representative images and analysis are reported in Additional file 1: Fig. S2). The positions of the levitating bands were measured at the starting point (i.e., as soon as the cuvette containing the sample was subjected to the magnetic field). As Fig. 2 shows significant variations in the starting position of coronated-GO from NOP (Fig. 2, panel a) and PDAC (Fig. 2, panel b) patients were detected. The one-dimensional intensity profiles (Fig. 2c), derived from the vertical projection of the relative pseudo-color images, NOP and PDAC profiles were distinguishable with starting positions centered around 16 mm and 17 mm for NOP and PDAC, respectively. Starting position distributions for the 22 NOP and 24 PDAC samples are reported in Fig. 2d.
Outcomes of the optical image processing of GO–HP samples of two representative healthy a and unhealthy b subjects. Corresponding overlapped one-dimensional intensity profiles from non-oncological (NOP) (blue curve) and PDAC (red curve) samples c. Box plot of the starting positions of 22 NOP and 24 PDAC patients d. Asterisks correspond to Student’s t test p values: *p < 0.05; **p < 0.001
Next, we compared the plasma levels of glycemia, total cholesterol, and triglycerides between the two classes of donors (Fig. 3). We found that the blood levels of glycemia (Fig. 3a) and triglycerides (Fig. 3b) were significantly altered in PDAC subjects, while no significant alteration was found in cholesterol levels (Fig. 3c).
Then, we asked whether the combination of the outcomes of the Maglev analysis and the blood levels of glycemia, cholesterol, and triglycerides could be used to develop a version of the NEB test with appreciable sensitivity and specificity. To assess the performance of each possible combination as a PDAC biomarker, we calculated the receiver operating curves (ROC) (Fig. 4a–c) and the corresponding area under the curve (AUC) values (Additional file 1: Table S1). The combination of the MagLev outcomes with the blood glycemic levels returned the highest prediction ability, while lower values were obtained when the MagLev outcomes were coupled to the blood levels of cholesterol and triglycerides.
Receiver operating curves (ROC) for the three possible combinations of the Maglev outcomes with blood levels of glycemia a, cholesterol b, and triglycerides c. ROC curves for three subgroups of PDAC patients calculated using the Maglev outcomes with blood levels of glycemia d, cholesterol e, and triglycerides f. Color code: T1 (gray); T2 + T3 (pink); T4 (purple)
Next, we tried to determine whether the sensibility and specificity of the three couplings could be affected by the tumor stage. According to the TNM staging of cancer, we classified PDAC patients into three subgroups, namely T1, T2 + T3, and T4. Results displayed in Fig. 4d–f and the AUC values reported in Additional file 1: Table S1 show that the performance of the coupling MagLev + levels of glycemia was in the order: T1 < T2 + T3 < T4. On the other hand, no clear correlation was found for the other two combinations.
As a final step, we asked whether disaggregating the data by sex could affect the prediction ability of the technology. Sex disaggregation did not produce appreciable alterations in the distributions of the glycemic levels and the starting positions for NOP and PDAC in women or in men (Additional file 1: Fig. S3). On the other side, the NEB test made with sex-disaggregated data provided substantially better classification accuracy for women (95%) than for men (84%) (Fig. 5).
Sex-disaggregated scatter plots of Maglev starting position and glycemia levels show a larger difference for women a than for men b. Each point indicates a single human subject (red for PDAC and blue for healthy subjects). In both panels, the solid black lines describe the result of the linear discriminant analysis (LDA)
Discussion
Early diagnosis of pancreatic cancer is an extremely challenging topic for the scientific community, and it is critical in clinical practice. When the PDAC patient accesses the clinical consultation, the disease is usually at an advanced stage, so much so that surgery, to date still the most critical therapeutic option for PDAC treatment, is restricted to a small percentage of these patients. This highlights the importance of developing new technologies for the diagnosis of PDAC in its early stages where symptoms are mild and confoundable with those of other diseases (Caputo et al. 2021). Even though CA 19-9, the only clinically approved biomarkers for pancreatic cancer, is gaining importance to predict the advanced stages of PDAC (Coppola, et al. 2021), it is not recommended as an early predictor of this lethal malignancy. Moreover, 10% of the population does not secrete CA 19.9 (Tempero, et al. 2017). In the last five years, a significant contribution towards the development of new diagnostic tools has come from nanotechnology. It has been demonstrated that the protein corona that is absorbed on nanomaterials when they are exposed to patient plasma is personalized. This means that it varies from subject to subject and is influenced by the onset of cancer (Caputo et al. 2020; Caputo et al. 2017; Colapicchioni et al. 2016; Caracciolo et al. 2019; Santo et al. 2020; Santo et al. 2021; Palchetti et al. 2019; Quagliarini et al. 2020) and other human conditions (Hajipour et al. 2015). It is well known that cancer produces alterations in the human proteome from the earliest stages and these changes can significantly influence the composition of the protein corona. Having demonstrated that the protein corona is personalized paved the way for test development of the NEB test for the early diagnosis of PDAC (Quagliarini et al. 2022b; Caputo et al. 2022b) and provided an impetus to serious work in this field.
As an instance, we have shown that coupling the outcomes of the NEB test with the values of specific clinical parameters can boost the predictive capacity of the test itself (Caputo et al. 2020). The earlier versions of the NEB tests were based on the direct characterization of the protein corona, i.e., after the isolation of plasma proteins from the particle surface. However, several studies demonstrated that protein isolation from NPs is a crucial step that can affect the reproducibility of the data and decrease the sensitivity and specificity of the test (Chetwynd et al. 2019). Therefore, more recently we have explored the chance of distinguishing PDAC patients from healthy subjects by the indirect characterization of the protein corona, i.e., without the protein isolation step. Indirect methods of protein corona characterization look at the protein nanoparticle complex as a single entity. Among these approaches, MagLev has recently shown great promise for the classification of PDAC patients. In a typical test, nanoparticle–protein complexes are injected into a device filled with a liquid paramagnetic where they migrate in a high-intensity magnetic field. Depending on the experimental conditions, typical MagLev outputs such as the initial position in the test tube and the precipitation speed can be identified as fingerprints for PDAC.
Taking advantage of these previous experiences, here we explored the ability of the MagLev technique in detecting PDAC when coupled with the blood levels of glucose, cholesterol and triglycerides. These parameters were chosen considering that the alteration in metabolic pathways, as the glycolytic and cholesterogenic ones, have an impact on the risk of development and differentiation of PDAC (Karasinska et al. 2020).
Furthermore, unlike what happens in the event of the accidental finding of significant alterations of known markers (e.g., Ca 19.9), the choice of using extremely non-specific laboratory parameter alterations (such as glycemia and blood lipid levels) is in line with the main mission of our test: to serve as a first-line test, respecting the REASSURED criteria, to be used before performing the execution of costly and/or invasive second level tests (e.g., MRI, CT-scan, EUS). More in detail, as reported by Feng et al. hypertriglyceridemia represents a risk factor for the development of PDAC via bile acids metabolism (Feng and Chen 2016). Moreover, highlighting how bile acids influence adipose tissue distribution, insulin sensitivity, and triglyceride metabolism, the authors reported how they are associated with risk factors of pancreatic cancer as hypertriglyceridemia, and hyperglycemia. As Chen et al. reported, hyperglycemia boosts the development of precancerous pancreatic lesions by triggering the Wnt/β-catenin signaling pathway (Chen, et al. 2021). Screening for PDAC in patients with hyperglycemia is both an old issue and a current challenge. Indeed, as highlighted in the literature (Illés et al. 2020), the fact that diabetes may improve after resection for pancreatic cancer also suggests that the increase in blood sugar is supported by the tumor. Moreover, the risk of developing PDAC is up to eight times higher in elderly subjects with new-onset diabetes. On the other hand, the widespread diffusion of primary type 2 diabetes in the general population (Liu et al. 2022), which is clinically indistinguishable from diabetes linked to cancer, complicates the matter. The above-mentioned earlier studies in the field of nanotechnology and the pieces of evidence supporting the link between PDAC and metabolic alterations prompted us to explore the combination of the technique's predictions with blood levels of glucose, cholesterol, and triglycerides. Our results show that coupling the MagLev technology with the blood glycemic levels leads to high detection ability, while lower accuracy levels were obtained when the MagLev outcomes were coupled to blood levels of cholesterol and triglycerides. Lastly, the fact that the analysis by gender has shown greater accuracy of the test in women is consistent with what has already been reported in the literature (Stocks et al. 2015). In the presence of metabolic alterations, the risk to develop a pancreatic adenocarcinoma increases more for women. Moreover, while in men, the strongest individual risk factors for global incident cancer are high levels of blood pressure and triglycerides, hyperglycemia represents the strongest risk factor in women.
In this present experience, the diagnostic accuracy of the test was increased, although without statistically significant differences, when the technology was matched to disease stage (i.e., T parameter) and bilirubin levels. In our opinion, these results are consistent with what has been previously reported for tests based on the use of nanoparticles and which are potentially able to distinguish PDAC in different stages (Caputo et al. 2018) and are in line both with the fact that jaundice per se represents a high-risk feature of PDAC (Schmidt-Hansen et al. 2016) and that it is likely that the larger the pancreatic tumor, the more likely it can cause biliary obstruction resulting in jaundice.
Conclusions
We have developed a multiplexed NEB test that combines the outcomes of MagLev analysis carried out on graphene oxide–protein complexes with the blood levels of glucose. This strategy correctly distinguished PDAC patients from healthy subjects with a global accuracy of 91%. If confirmed by further studies on larger cohorts, this in vitro diagnostic test could be employed as a screening tool for the population at high risk for developing PDAC as are those affected by hyperglycemia.
Materials and methods
Patients’ enrollment and inclusion criteria
The case series included 24 PDAC patients and 22 non-oncological patients (NOP). The inclusion criteria for the study were age > 18 years, adequate renal function (creatinine < 1.5 mg/dL, blood urea nitrogen < 1.5 times the upper limit), previous personal medical history negative for malignancy, renal, liver, or blood disorders, no previous chemotherapy or radiotherapy, absence of uncontrolled infections, and obtained written informed consent. All patients in the oncology group had a cyto-histological diagnosis of PDCA. Healthy controls have been identified among patients admitted to the Surgery Center of the Campus Bio-Medico University Hospital Foundation of Rome for benign surgical diseases. Data regarding medical history and clinical-instrumental workup has been assessed for each patient; in detail, triglyceride blood levels, cholesterol blood levels, and blood glucose were obtained. Among NOPs, 13 (54%) patients had undergone laparoscopic cholecystectomy for cholelithiasis, 7 patients (29%) had undergone inguinal hernia repair, one (4%) to left hemicolectomy for diverticular disease and one (4%) has undergone upper limb lipoma excision. Among the healthy donors, the median age was 64.5 years (Interquartile Range: 46–74); 13 subjects (60%) were female and 9 (40%) were male. Of them, 7 subjects (31%) were smokers. Pancreatic cancer patients had a median age of 73 years (Interquartile Range: 64–79). Thirteen (54%) patients were female and 11 (46%) were male. In the healthy group, the median value of triglyceride blood was 102 mg/dl (Interquartile Range: 72–167 mg/dl); the median value of cholesterol blood levels was 178.5 mg/dl (Interquartile Range: 117.75–265.75); the median value of blood glucose was 89 mg/dl (Interquartile Range: 71–109 mg/dl). In the PDCA group, the median value of triglyceride blood levels was 116 mg/dl (Interquartile Range: 91–210); the median value of cholesterol blood levels was 176 mg/dl (Interquartile Range: 129–216); the median value of blood glucose was 98 mg/dl (Interquartile Range: 87–159). Demographic and clinic characteristics of the PDAC patients and NOP have been reported in Additional file 1: Table S2.
Human plasma collection
Blood and plasma samples have been collected and stored according to the procedure described in Caputo et al. (2017).
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University Campus Bio-Medico di Roma (Prot. 10/12 ComEt CBM and further amendments).
Preparation of graphene oxide
Graphene oxide (GO) water solution has been purchased by Graphenea (San Sebastian, Spain). GO solution was diluted with distilled water to reach a final concentration of 0.25 mg/ml and subjected to sonication for 2 min with Vibra cell sonicator VC505 (Sonics and Materials, Newton, CT, USA) to obtain a homogenous solution as described elsewhere (Santo et al. 2020).
Preparation of graphene oxide–protein corona complexes
GO–protein corona complexes were prepared by a bulk mixing incubation between 50 µl GO solution and 20 µl of human plasma (HP) derived from 24 non-oncological patients (NOP) and 22 PDAC-affected subjects. For each sample, 30 µl of distilled water was added to reach a final volume of 100 µl, necessary for the following experiments. The GO–HP mixtures were incubated for 1 h at 37 °C to produce coronated-GO complexes.
Maglev experiments
The Maglev system can levitate a diamagnetic object in a paramagnetic solution according to its density. Maglev device consists of two N42-grade neodymium coaxial square permanent magnets of 2.5 cm length and width and 5.0 cm height (Magnet4less). The N poles of the two magnets face each other with a distance of 2.8 cm, to generate a highly intense magnetic field of 0.5 T. The sample container is a plastic cuvette of 4 ml capacity and 2.5 cm height. A paramagnetic aqueous solution of Dysprosium (III) nitrate hydrate (Sigma-Aldrich, Inc. Merk KGaA, Darmstadt, Germany) was used with a concentration of 50 mg/mL, as applied elsewhere (Digiacomo et al. 2021). After GO–HP incubation, coronated-GO samples were injected with a syringe at the bottom of the cuvette until the complete dissolution of the 100 µl sample. When the whole sample volume was distributed homogenously on the surface, the cuvette was inserted between the magnets. The diamagnetic object (e.g., plasma proteins) in a paramagnetic solution levitates when the magnetic and gravitational forces cancel each other out:
where \(\overrightarrow{F}\) mag depends on the magnetic susceptibility of the paramagnetic medium (Xm), the magnetic susceptibility (Xs) and the volume (V) of the diamagnetic object, the magnetic field (\(\overrightarrow{B}\)), and the magnetic permeability of free space (0) are as follows:
and Fg is the buoyancy-corrected gravitational force, i.e.,
where the sample density and medium density are, respectively represented by s and m, and the gravity acceleration by g. When the final equilibrium (Eq. (1)) is reached, the diamagnetic sample reaches a steady height (h) that depends on the density of the object in the paramagnetic solution:
In Eq. (4), the information related to the expression of the magnetic force (Eq. (2)), the gravitational force (Eq. (3)), and the geometry of the magnetic setup are collected. Finally, the Nikon D5600 camera (time-lapse mode, 1 frame per 20 s) was used to acquire image series of MagLev patterns (at a controlled temperature of 25 °C) of coronated-GO sample and Matlab (Mathworks, Portola Valley, CA, USA) scripts were applied for image processing. Briefly, for each frame, the vertical intensity profile was computed by averaging the recorded intensity over a region of interest containing the inner part of the cuvette. Then, after background subtraction, profiles were normalized to the maximum detected intensity over a reference window to avoid undesired effects due to exposure variations.
Statistical data analysis
A multivariate analysis was performed to classify healthy and PDAC-affected subjects. Briefly, the outcome from Maglev experiments, i.e., the height reached by the coronated-GO sample as soon as injected in the Maglev device (i.e., the ‘starting position’), was coupled to the circulating level of nutritional metabolites including glycemia, cholesterol, and triglycerides. ROC analysis was carried out to evaluate the classification ability of the test. ROC curves were obtained from the Linear Discriminant Analysis (LDA) performed for each coupling and by plotting the false-positive ratio (1 − specificity) vs the true-positive ratio (sensitivity). Statistical data analysis was performed with Matlab (MathWorks, Natick, MA, USA, Version R2022a) software.
Availability of data and materials
The content of the manuscript has not been published or submitted for publication elsewhere.
References
Caputo D et al (2017) A protein corona-enabled blood test for early cancer detection. Nanoscale 9(1):349–354
Caputo D et al (2018) Improving the accuracy of pancreatic cancer clinical staging by exploitation of nanoparticle-blood interactions: a pilot study. Pancreatology 18(6):661–665
Caputo D et al (2020) Synergistic analysis of protein corona and haemoglobin levels detects pancreatic cancer. Cancers 13(1):93
Caputo D et al (2021) Nanotechnology and pancreatic cancer management: State of the art and further perspectives. World J Gastrointest Oncol 13(4):231
Caputo D et al (2022a) Multiplexed detection of pancreatic cancer by combining a nanoparticle-enabled blood test and plasma levels of acute-phase proteins. Cancers 14(19):4658
Caputo D et al (2022b) Nanotechnology meets oncology: a perspective on the role of the personalized nanoparticle-protein corona in the development of technologies for pancreatic cancer detection. Int J Mol Sci 23(18):10591
Caracciolo G et al (2019) Disease-specific protein corona sensor arrays may have disease detection capacity. Nanoscale Horizons 4(5):1063–1076
Chen WC-Y et al (2019) Total serum cholesterol and pancreatic cancer: a nested case-control studytotal serum cholesterol and pancreatic cancer. Cancer Epidemiol Biomark Prev 28(2):363–369
Chen H et al (2021) Hyperglycemia promotes pancreatic cancer initiation and progression by activating the Wnt/β-catenin signaling pathway. Anti Cancer Agents Med Chem 21(18):2592–2602
Chetwynd AJ, Wheeler KE, Lynch I (2019) Best practice in reporting corona studies: minimum information about nanomaterial biocorona experiments (MINBE). Nano Today 28:100758
Colapicchioni V et al (2016) Personalized liposome–protein corona in the blood of breast, gastric and pancreatic cancer patients. Int J Biochem Cell Biol 75:180–187
Coppola A et al (2021) CA19. 9 serum level predicts lymph-nodes status in resectable pancreatic ductal adenocarcinoma: a retrospective single-center analysis. Front Oncol 11:690580
Di Santo R et al (2020) Personalized graphene oxide-protein corona in the human plasma of pancreatic cancer patients. Front Bioeng Biotechnol 8:491
Di Santo R et al (2021) Protein corona profile of graphene oxide allows detection of glioblastoma multiforme using a simple one-dimensional gel electrophoresis technique: a proof-of-concept study. Biomater Sci 9(13):4671–4678
Digiacomo L et al (2021) Detection of pancreatic ductal adenocarcinoma by ex vivo magnetic levitation of plasma protein-coated nanoparticles. Cancers 13(20):5155
Digiacomo L et al (2022) Magnetic levitation patterns of microfluidic-generated nanoparticle-protein complexes. Nanomaterials 12(14):2376
Feng H-Y, Chen Y-C (2016) Role of bile acids in carcinogenesis of pancreatic cancer: an old topic with new perspective. World J Gastroenterol 22(33):7463
Gabitova-Cornell L et al (2020) Cholesterol pathway inhibition induces TGF-β signaling to promote basal differentiation in pancreatic cancer. Cancer Cell 38(4):567-583 e11
Hajipour MJ et al (2015) Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale 7(19):8978–8994
Huang B et al (2022) Artificial intelligence in pancreatic cancer. Theranostics 12(16):6931
Illés D et al (2020) New onset of diabetes in association with pancreatic ductal adenocarcinoma (NODES Trial): protocol of a prospective, multicentre observational trial. BMJ Open 10(11):e037267
Jacks BE et al (2022) Molecular markers of pancreatic cancer: a 10-year retrospective review of molecular advances. Cureus. https://doi.org/10.7759/cureus.29485
Karasinska JM et al (2020) Altered gene expression along the glycolysis-cholesterol synthesis axis is associated with outcome in pancreatic cancermetabolic gene expression heterogeneity and survival in PDAC. Clin Cancer Res 26(1):135–146
Keum N et al (2018) Long-term patterns of fasting blood glucose levels and pancreatic cancer incidence. Cancer Causes Control 29(1):135–142
Konstantinou F, Syrigos KN, Saif MW (2013) Pancreatic cancer: what about screening and detection? JOP J Pancreas 14(4):312–315
Land KJ et al (2019) REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol 4(1):46–54
Lankisch PG et al (2012) Pancreatic pseudocysts: prognostic factors for their development and their spontaneous resolution in the setting of acute pancreatitis. Pancreatology 12(2):85–90
Liao W (2015) Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ 350:g7371
Liu Y et al (2022) Exploring facilitators and barriers to self-management engagement of Chinese people with type 2 diabetes mellitus and poor blood glucose control: a descriptive qualitative study. BMC Endocr Disord 22(1):1–13
Mormile R (2020) Total serum cholesterol and pancreatic cancer risk: what is the link? Pathol Oncol Res 26(2):1361–1361
Palchetti S et al (2019) Protein corona fingerprints of liposomes: New opportunities for targeted drug delivery and early detection in pancreatic cancer. Pharmaceutics 11(1):31
Quagliarini E et al (2020) Protein corona-enabled serological tests for early stage cancer detection. Sens Int 1:100025
Quagliarini E et al (2022a) Magnetic levitation of personalized nanoparticle-protein corona as an effective tool for cancer detection. Nanomaterials 12(9):1397
Quagliarini E et al (2022b) A decade of the liposome-protein corona: lessons learned and future breakthroughs in theranostics. Nano Today 47:101657
Schmidt-Hansen M, Berendse S, Hamilton W (2016) Symptoms of pancreatic cancer in primary care: a systematic review. Pancreas 45(6):814–818
Sharma A et al (2018) Model to determine risk of pancreatic cancer in patients with new-onset diabetes running title: END-PAC risk stratification score. Gastroenterology. https://doi.org/10.1053/j.gastro.2018.05.023
Stocks T et al (2015) Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol 44(4):1353–1363
Tempero MA et al (2017) Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Comprehens Cancer Network 15(8):1028–1061
Wang F et al (2021) Dyslipidemia in Chinese pancreatic cancer patients: a two-center retrospective study. J Cancer 12(17):5338
Yoon BH et al (2021) Pancreatic cancer-associated diabetes is clinically distinguishable from conventional diabetes. J Surg Res 261:215–225
Acknowledgements
Morteza Mahmoudi is gratefully acknowledged for providing the NanoDelivery Lab with the MagLev device. Luca Digiacomo is acknowledged for the revision of the statistical analysis.
Funding
The research leading to this work has received funding from the AIRC Foundation under IG 2020-ID. 24521 project—P.I. Pozzi Daniela.
Author information
Authors and Affiliations
Contributions
Conceptualization, DC, GC, and DP; methodology, EQ and RC; software, EQ; formal analysis, EQ; investigation, EQ and RC; resources, DC, GC and DP; data curation, EQ and RC; writing—original draft preparation, EQ, DC, GC, and DP; writing—review and editing, DC and DP, visualization, EQ; supervision, DC and DP; project administration, DC and DP; funding acquisition, DP All the authors have read and agreed to the published version of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethical Committee of the University Campus Bio-Medico di Roma approved this study (Prot. 10/12 ComEt CBM).
Consent for publication
All the authors have approved the manuscript for submission.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Size (a) and zeta potential (b) distributions of Graphene Oxide (GO) nanosheets before (black distribution) and after (orange distribution) exposure to human plasma (HP). Figure S2. Representative outcome of the image processing. (a) Optical image of a MagLev pattern. (b) Corresponding pseudo-color image and derived 3D profile of the detected band. (c) Evaluation of the intensity profile and determination of locations of the detected peak. Figure S3. Distributions of the blood glycemic levels and starting position for women (panels a and b, respectively) and men (panels c and d, respectively). Asterisks correspond to the Student’s t test p values: * p < 0.05; ** p < 0.001, lack of asterisks means no significance. Figure S4. Scatter plot of the MagLev pattern’s starting position with bilirubin level for a set of 22 NOP (blue dots) and 24 PDAC samples (red dots). The black line is the output of linear discriminant analysis (LDA). (b) Receiver operating curves (ROC) for the entire PDAC cohort. (c) ROC analysis for subgroups of PDAC patients: T1, T2 + T3, and T4. Table S1. Area under the curve (AUC) values obtained from the ROC curves reported in Fig.4. Table S2. Demographic and clinic characteristics of the PDAC and control groups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Quagliarini, E., Caputo, D., Cammarata, R. et al. Coupling magnetic levitation of graphene oxide–protein complexes with blood levels of glucose for early detection of pancreatic adenocarcinoma. Cancer Nano 14, 16 (2023). https://doi.org/10.1186/s12645-023-00170-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12645-023-00170-1