Abstract
Objectives
This study aimed to investigate the spectrum of ocular characteristics and viral presence in the conjunctival swab of patients with COVID-19.
Methods
In this cross-sectional study, fifty-three patients were recruited from two COVID-19 referral hospitals in Jakarta (Cipto Mangunkusumo Hospital and Persahabatan Hospital) from July 2020 to March 2021. The inclusion criteria were patients who were suspected of or confirmed cases of COVID-19 with or without ocular symptoms. Demographic data, history of COVID-19 exposure, underlying medical condition, systemic symptoms, ocular symptoms, supporting laboratory results, reverse-transcriptase polymerase chain reaction (RT-PCR) of naso-oropharyngeal (NOP) swab and conjunctival swab were collected.
Results
Fifty-three patients who were suspected, probable or confirmed cases of Covid-19 were included. Forty-six out of 53 patients (86.79%) tested positive for either Covid-19 antibody rapid test or naso-oropharyngeal (NOP) swab. Forty-two patients tested positive for NOP swab. Fourteen out of 42 patients (33.33%) experienced symptoms of ocular infection including red eye, epiphora, itchy eyes, and eye discharge. None of these patients were tested positive for conjunctival swab. Two out of 42 patients (4.76%), who were tested positive for conjunctival swab, did not experience any ocular symptoms.
Conclusions
Establishing the relationship between Covid-19 infection, ocular symptoms, and presence of SARS-CoV-2 virus on the ocular surface proves to be challenging. In Covid-19 patients, ocular symptoms did not warrant a positive conjunctival swab result. On the contrary, a patient without ocular symptoms can also have detectable presence of SARS-CoV-2 virus on the ocular surface.
Similar content being viewed by others
Introduction
Since December 2019 until now, a new disease called Coronavirus disease 2019 (COVID-19) had emerged in Wuhan, China. The disease had quickly spread across the globe and become a pandemic that pose a highly serious threat to millions of people. The etiology of the disease is severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), which causes infection of the respiratory tract that can lead to multiple organ infections and result in death. It has similarities in receptors and other properties with the former member of coronavirus (SARS-CoV-1) [1,2,3,4].
SARS-CoV-2 gains entry to host cells through angiotensin-converting enzyme 2 (ACE-2) receptors, which are expressed in many organs. Conjunctival and corneal cells were also shown to have ACE-2 receptors, thus providing possible route of entry [5,6,7]. Previous studies have found that the SARS-CoV-2 could be detected in tear and conjunctival tissues both in the presence and absence of ocular symptoms [1,2,3,4]. In addition, the rate of positivity in ocular secretion also varied between 0 to 28% across studies [8, 9].
Several studies have investigated the correlation between SARS-CoV-2 infection and its ocular manifestations, however many results remained controversial [1,2,3]. The ocular manifestations reported ranged from simple conjunctivitis with symptoms of red eyes, epiphora, conjunctival secretion, and chemosis to keratoconjunctivitis, posterior uveitis, retinitis, and optic neuritis, which affect visual acuity [4,5,6, 10]. The prevalence of conjunctivitis in studies widely varied, ranging from 2 to 31% [7, 11, 12]. Another study also reported that 11.64% of COVID-19 cases experienced forms of ocular symptoms [6, 13].
As viral transmission is possible through body fluid and mucous membranes, the role of ocular surface and conjunctival secretion as possible reservoir and route of transmission in patients infected with COVID-19 remains under discussion [1, 2, 4, 14, 15]. Therefore, our study aimed to focus on exploring the ocular manifestations in COVID-19 patients and detecting the presence of SARS-CoV-2 in conjunctival secretion through reverse transcriptase-polymerase chain reaction (RT-PCR) procedures. To the best of our knowledge, this is the first Indonesian study, which investigated the spectrum of ocular manifestations and results of conjunctival swab in COVID-19 patients in two general hospitals in Jakarta, Indonesia.
Methods
Definitions
A suspect case of COVID-19 was defined as patient who has either one of the criteria below: (i) patient with acute respiratory tract infection (ARTI) who has a travel history or stay in a country or region in Indonesia which reported local transmission of the disease within 14 days, (ii) patient with signs and symptoms of ARTI who has a contact with a confirmed or probable case of COVID-19 within 14 days, (iii) patient with severe ARTI/pneumonia who needs to be hospitalized and has no other probable etiologies. A probable case of COVID-19 was defined as suspect case with severe ARTI/ARDS or death with clinical symptoms of COVID-19 and has not been confirmed with RT-PCR examination. A confirmed case of COVID-19 was defined as patient with/without symptoms of COVID-19, who has been confirmed with naso-oropharyngeal (NOP) RT-PCR examination [16, 17].
Study design
This cross-sectional study was conducted in two COVID-19 referral hospitals in Jakarta, which are Cipto Mangunkusumo and Persahabatan Hospital. The data was collected from July 2020 to March 2021. This study has been approved by the Ethics Committee in both institutions and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants. We included patients who were hospitalized in either of these hospitals. The inclusion criteria of this study were children or adult who fulfilled the indicator of suspected cases of COVID-19, patients with positive result of COVID-19 rapid test or NOP RT-PCR test, with or without ocular symptoms. We excluded patients who were critically ill to have the examination and conjunctival swab performed. Recorded audio informed consent was obtained from patients and/or legal guardians for underaged children. This study has obtained ethical clearance from the ethics committee of the Faculty of Medicine, University of Indonesia (ethical approval number: KET-467/UN2.F1/ETIK/PPM.00.02/2020).
Sample collection
Cases were reported by one ophthalmologist from each hospital delegated for this task. Patient history was taken including the demographic information, contact history, clinical symptoms. Ocular signs and symptoms were also recorded. Conjunctival swab were done simultaneously by the ophthalmologist following appropriate infection control and prevention measures. Conjunctival swab was used to collect the conjunctival secretion and tears from the patient. A single conjunctival swab was collected from both eyes using a sterile dacron flocked swab in the lower eyelid fornix without topical anesthesia. The swab was then broken off and placed into a viral transport media inside the sampling tube and stored in a 4 degrees Celsius environment. RT-PCR test of the conjunctival swab was performed to assess the presence of SARS-CoV-2 RNA in the sample and was processed in clinical microbiology laboratory in both hospitals.
As our study began at the early phase of the pandemic, qualitative antibody testing from blood was the only rapid test available and performed with rapid test kit for Covid-19 IgM/IgG. A total of 10µL of blood collected from finger pin-prick was added to the sample pad, then two drops of sample buffer were added. After a 10-min incubation period, results were interpreted in which the presence of only the control line was negative, the presence of the control line with IgM and/or IgG line was considered positive. NOP RT-PCR swab test was also performed in all patients. The NOP RT-PCR swab were performed based on standardized guidelines for COVID-19 diagnosis by the Indonesian Health Ministry [17].
Data collection
We collected information on patients’ demographic (age, gender), history of contact, underlying medical condition, systemic condition (COVID-19 symptoms, body temperature), ocular manifestations (ocular symptoms, diagnosis, uncorrected visual acuity), supporting examination result, RT-PCR of NOP swab, and RT-PCR of conjunctival swab through questionnaire-styled history taking, physical examination, and patients’ medical records. The questionnaire was filled in by the ophthalmologist in charge in each hospital. The detail of the questionnaire can be seen in Appendix 1. According to guidelines by the National Institute of Health (NIH), severity of systemic symptoms of our study was classified as mild, moderate, or severe. None of the patients in this study were asymptomatic or critical. Guidelines by the NIH stated that asymptomatic or pre-symptomatic patients were those who tested positive for SARS-CoV-2 but experienced no symptoms of Covid-19. Patients with mild illness showed varying symptoms and signs of Covid 19 but without dyspnea, shortness of breath or abnormal chest imaging. Patients with moderate illness showed signs of lower respiratory infection either by physical examination or imaging, with an oxygen saturation (SpO2) ≥ 94% on room air at sea level. Patients with severe illness have SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg, a respiratory rate > 30 breaths/min, or lung infiltrates > 50%. Patients who were critical included those with respiratory failure, septic shock, and/or multiple organ dysfunction[17, 18].
Statistical analyses
All statistical analyses were performed using SPSS software version 24.0 for Mac (SPSS Inc, Chicago, IL, USA). Categorical data were reported in the form of frequency (percentage). Continuous variables were reported in the form of mean ± SD or median (range). Normality of the data was evaluated with Shapiro–Wilk test. Independent t-test was conducted to evaluate significant difference between the groups. A P-value of less than 0.05 was considered statistically significant.
Results
A total of 53 patients who were defined as suspect, probable or confirmed cases of Covid-19 were recruited for this study. About 46 out of 53 patients (86.79%) tested positive for either Covid-19 antibody rapid test or NOP swab, while 4 out of 13 patients with positive antibody rapid test tested negative for both NOP and conjunctival swabs. Of all 42 patients tested positive for NOP swab, two out of them (4.76%) were also tested positive for conjunctival swab test. Summary of the result was listed in Table 1. The number of days between NOP and conjunctival swabs taken ranged from 0 to 7 days with median of 2 days.
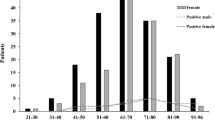
Forty-two patients with positive NOP swab ranged from 1 to 69 years old with a mean age of 33.69 ± 22.38 years old. Twenty-six out of 42 patients (61.9%) were male and 10 patients (38.1%) were female. A total of 30 out of 42 patients (71.4%) experienced several systemic symptoms including fever, dyspnea, cough, anosmia, myalgia, and gastrointestinal symptoms, while others remain asymptomatic. Twenty-one out of 42 patients (50%) had systemic comorbidities, with diabetes, hypertension, and coronary artery disease being the most common one. Others include hyperthyroidism, chronic kidney disease, leukemia and systemic lupus erythematosus.
Laboratory results collected, which include blood count, procalcitonin, ureum, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST) as well as C-reactive protein were not significantly different between patients with positive conjunctival swab versus patients with negative conjunctival swab as shown in Table 2.
Approximately 14 out of 42 patients (33.33%) had infection related ocular complaints including red eye, epiphora and eye discharge. During physical examination, some patients also presented with palpebral edema, chemosis of conjunctiva, and decreased visual acuity. Details of the clinical and ocular characteristics of patients with positive NOP swab and infection related ocular signs and symptoms were listed in Table 3. None of the patients with ocular complaints tested positive in conjunctival swab. On the contrary, none of the two patients who tested positive in conjunctival swab experienced any ocular symptoms. Clinical characteristics of the patients who tested positive for conjunctival swab were summarized in Table 4.
Discussion
The mucous membrane of the upper respiratory tract is the main portal entry of SARS-CoV-2. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is initiated after type 2 transmembrane protease (TMPRSS2) cleaves the viral spike glycoprotein, which then binds to the angiotensin-converting enzyme 2 (ACE-2) receptor. Presence of ACE-2 receptors and TMPRSS2 in the ocular surface makes it a potential port of entry for SARS-CoV-2[19,20,21].
We found 2 cases of patients with positive conjunctival swabs and NOP swabs. Ocular symptoms were not observed in these patients. NOP and conjunctival swabs were taken 0 and 1 day apart. The low rate of viral detection in conjunctival samples for our study (2 out of 42 patients) with positive NOP samples (4.76%) might be due to several causes. First, majority of the patients in our sample group might be exposed to the virus via the upper respiratory tract pathway instead of the ocular surface. As the virus would descend from the upper to the lower respiratory tract, viral load was therefore hardly detected in the conjunctival samples [22,23,24]. The second probable reason was a fast clearance rate of the virus from the ocular surface due to drainage via the nasolacrimal pathway, evaporation, and its low volume [7, 25]. The third reason was the fact that ocular secretions were known to have a strong local immune system (lactoferrin and immunoglobulins) against coronavirus[7, 24, 25]. Lastly, the low number of ACE-2 receptors to have been shown in conjunctival tissue (< 50% lower than other tissues), thus their ability to bind to the virus is very poor [25, 26]. It is also possible that the viruses appear at specific period of time in the ocular surface, thus requiring price collection time in order to gain positive results [10, 27]. Other studies have supported these findings, reporting the low rates of positive conjunctival swab results ranged from 0 to 15.6% [1, 2, 16, 20, 28, 29]. Factors including technical errors, difficulties in sampling, handling, and processing, the stage of the disease, low sensitivity of RT-PCR, and variable time sampling may also cause variations of positivity rate [30].
Fourteen out of 42 patients experienced infection related ocular complaints including red eye, epiphora and eye discharge. However, conjunctival samples were negative for SARS-CoV-2. Only one patient, who was clinically diagnosed as Herpes Zooster Ophthalmicus, was given topical antiviral (acyclovir) after the conjunctival samples were collected. Others were prescribed topical antibiotic and/or topical anti-inflammatory eyedrops based on the findings. Symptoms were relieved and the patients recovered without ocular complications. The relatively low number of ocular manifestations in COVID-19 patients were supported also by a systematic review and meta-analysis conducted by Aggarwal et al., which reported that only 8.35–11.64% of COVID-19 patients would present with at least one ocular symptom [31].
A low percentage of patients with conjunctivitis who had positive conjunctival swab results was reported [20]. A study by Ranzenigo et al. [32], Ozturk et al. [5], and Sonmez et al. [13] revealed none of the patients in the study with ocular symptoms tested positive for conjunctival swab and tear samples. This was also supported by meta-analysis done by Aggarwal et al., which reported low positivity rate of only 3.5% in patients with or without ocular symptoms [31]. These studies along with our results suggested that the occurrence of conjunctivitis is a systemic response rather than local activity of the virus on the ocular surface [5, 10, 13].
Ocular involvement in Covid-19 patients other than conjunctivitis has been described, including keratitis, uveitis, retinal pathologies, and neuro-ophthalmological involvement [25, 32, 33]. These clinical manifestations often occur in patients who required mechanical ventilation and in patients with more severe illness [20, 34, 35]. As our patients who had positive conjunctival swab results and mild symptoms of systemic Covid-19, ocular symptoms were not observed.
Laboratory results of patients with positive conjunctival swab results was not significantly different from patients with negative conjunctival swab results as shown in Table 3. A previous study by Atum et al. also found no significant difference in laboratory result characteristics between the two groups [36]. However, this might be due to the small number of patients who had positive conjunctival swab results and might not be statistically representative of the cohort.
Viral entry through the ocular route is a cause of concern as detectable viral presence in the ocular surface is not always symptomatic and eye-hand-eye as well as hand-eye transmission are possible. It might necessitate further protective measures such as the use of eye protection in conjunction with wearing masks, googles, gloves, and face shields, especially for health workers and ophthalmologist [6, 19, 21, 22]. Viral entry into the ocular surface can be caused by direct exposure to droplets, aerosol, contact with contaminated ophthalmic instruments or hand to eye contact after touching a contaminated surface [6, 19, 23, 37].
This study also had several limitations. First, the sample size was relatively small and it also came from only two medical centers. Second, the information regarding the COVID-19 vaccination history of patients is unavailable, as the data was taken in the early phase of the pandemic, in which the vaccination policy had not yet been fully implemented in Indonesia. Third, only one sample of conjunctival swab was taken from each patient. For further studies, we suggest to do the follow-up of COVID-19 patients, isolate the live virus, quantify conjunctival viral load, and take multiple conjunctival samples at variable days in order to better understand the correlation between the presence of SARS-CoV-2 virus and ocular manifestations in COVID-19 patients.
Conclusion
The presence of ocular symptoms might not warrant a positive conjunctival swab result while the presence of SARS-CoV-2 virus on the ocular surface might not cause ocular symptoms. The positivity rate of conjunctival RT-PCR swab was 4.76% in patients with confirmed SARS-CoV-2 infection. As ocular symptoms often occur as a systemic response rather than local viral activity, conjunctival swabs were negative for our patients who experienced symptoms of conjunctivitis. More extensive studies need to be carried out to identify the role of SARS-CoV-2 infection in the ocular surface.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome novel coronavirus 2
- SARS-CoV-1:
-
Severe acute respiratory syndrome novel coronavirus 1
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- NOP:
-
Naso-oropharyngeal
- NIH:
-
National Institute of Health
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- TMPRSS2:
-
Type 2 transmembrane protease
- (ACE-2) receptor:
-
Angiotensin-converting enzyme 2
- CD 147:
-
Cluster differentiation 147
- CTSL:
-
Cathepsin L
References
Güemes-Villahoz N, Burgos-Blasco B, Arribi-Vilela A, Arriola-Villalobos P, Rico-Luna CM, Cuiña-Sardiña R et al (2021) Detecting SARS-CoV-2 RNA in conjunctival secretions: Is it a valuable diagnostic method of COVID-19? J Med Virol 93(1):383–388
Kumar K, Prakash AA, Gangasagara SB, Rathod SBL, Ravi K, Rangaiah A et al (2020) Presence of viral RNA of SARS-CoV-2 in conjunctival swab specimens of COVID-19 patients. Indian J Ophthalmol 68(6):1015–1017
Valente P, Iarossi G, Federici M, Petroni S, Palma P, Cotugno N et al (2020) Ocular manifestations and viral shedding in tears of pediatric patients with coronavirus disease 2019: a preliminary report. J aapos 24(4):212–215
Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L et al (2020) Characteristics of ocular findings of patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province. China JAMA Ophthalmol 138(5):575–578
Ozturk M, Kumova D, Alacam S, Erdogan H, Onder F (2021) Detection of coronavirus in tear samples of hospitalized patients with COVID-19. Int Ophthalmol 40(3):348–350. https://doi.org/10.1097/ICO.0000000000002562
Zhong Y, Wang K, Zhu Y, Lyu D, Yu Y, Li S et al (2021) Ocular manifestations in COVID-19 patients: a systematic review and meta-analysis. Travel Med Infect Dis 44:102191
Thapa B (2021) Role of eyes and eyes protection amidst SARS-CoV-2 infection. JNMA J Nepal Med Assoc 59(233):108–110
Chawhan A, Athale A, Khan K, Agarwal S, Paul R, Iyer K et al (2021) Detection of SARS-CoV-2 RNA in a conjunctival swab sample in real-time-polymerase chain reaction positive COVID-19 patients and its association with comorbidity and severity at a designated COVID-19 hospital in Central India. Indian J Ophthalmol 69(12):3633
Gasparini MS, Dos Santos LM, Hamade AM, Gross LG, Favarato AP, de Vasconcellos JP et al (2021) Identification of SARS-CoV-2 on the ocular surface in a cohort of COVID-19 patients from Brazil. Exp Biol Med (Maywood) 246(23):2495–2501
Hadrawi M, Malak M, Almahmoudi F, Mogharbel A, Rozy O, Hanafi S et al (2021) Testing the sensitivity of conjunctival swabs from confirmed COVID-19 patients. Clin Ophthalmol 15:2489–2496
Li X, Chan JF, Li KK, Tso EY, Yip CC, Sridhar S et al (2021) Detection of SARS-CoV-2 in conjunctival secretions from patients without ocular symptoms. Infection 49(2):257–265
Vallejo-Garcia JL, Balia L, Raimondi R, Rustioni G, Camesasca FI, Borgia A et al (2021) Conjunctivitis as a sign of persistent SARS-COV-2 infection? An observational study and report of late symptoms. Eur J Ophthalmol 32(2):11206721211056594. https://doi.org/10.1177/11206721211056594
Sonmez A, Aydin Kurna S, Aslan FG, Kaplan FB, Acikalin B, Eker P (2022) SARS-COV-2 viral load in tears of patients with COVID-19 in the early symptomatic stages: comparison of two different tear sampling methods. Int Ophthalmol 42(8):2425–2438
Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A et al (2020) Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol 55(4):e125–e129
Xia J, Tong J, Liu M, Shen Y, Guo D (2020) Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol 92(6):589–594
Zhou Y, Zeng Y, Tong Y, Chen C (2020) Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. medRxiv. https://doi.org/10.1101/2020.02.11.20021956.
Indonesia KKR (2020) Pedoman dan Pencegahan Pengendalian COVID-19
Health NIo. Clinical Spectrum of SARS-CoV-2 Infection 2021 [updated 19 October 2021. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/.
Barnett BP, Wahlin K, Krawczyk M, Spencer D, Welsbie D, Afshari N et al (2020) Potential of Ocular Transmission of SARS-CoV-2: A Review. Vision (Basel) 4(3):40. https://doi.org/10.3390/vision4030040
Sopp NM, Sharda V (2021) An eye on COVID-19: a meta-analysis of positive conjunctival reverse transcriptase-polymerase chain reaction and SARS-CoV-2 conjunctivitis prevalence. Optom Vis Sci 98(5):429–436
de Freitas SD, de Sousa LB, Câmara NOS, de Freitas D, de Oliveira LA (2021) SARS-COV-2 and ocular surface: from physiology to pathology, a route to understand transmission and disease. Front Physiol 12:612319
Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM et al (2021) Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596(7871):276–280. https://doi.org/10.1038/s41586-021-03777-9
Kharel Sitaula R, Khatri A, Janani MK, Mandage R, Sadhu S, Madhavan HN et al (2020) Unfolding COVID-19: lessons-in-learning in ophthalmology. Clin Ophthalmol 14:2807–2820
Deng W, Bao L, Gao H, Xiang Z, Qu Y, Song Z et al (2020) Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun 11(1):4400
Karabela Y, Karabela SN, Ozbas M, Kasikci H, Kart YK (2021) Investigation of SARS-CoV-2 in tear and conjunctival secretions of hospitalized patients with clinically-confirmed COVID-19 pneumonia. BMC Infect Dis 21(1):918
Shemer A, Einan-Lifshitz A, Itah A, Dubinsky-Pertzov B, Pras E, Hecht I (2021) Ocular involvement in coronavirus disease 2019 (COVID-19): a clinical and molecular analysis. Int Ophthalmol 41(2):433–440
Kaya H, Caliskan A, Okul M, Sari T, Akbudak IH (2020) Detection of SARS-CoV-2 in the tears and conjunctival secretions of Coronavirus disease 2019 patients. J Infect Dev Ctries 14(9):977–981
Cao K, Kline B, Han Y, Ying GS, Wang NL (2020) Current evidence of 2019 Novel Coronavirus Disease (COVID-19) ocular transmission: a systematic review and meta-analysis. Biomed Res Int 2020:7605453
Zhang X, Chen X, Chen L, Deng C, Zou X, Liu W et al (2020) The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf 18(3):360–362
Mahmoud H, Ammar H, El Rashidy A, Ali AH, Hefny HM, Mounir A (2020) Assessment of Coronavirus in the conjunctival tears and secretions in patients with SARS-CoV-2 infection in Sohag Province Egypt</p>. Clin Ophthalmol 14:2701–2708
Aggarwal K, Agarwal A, Jaiswal N, Dahiya N, Ahuja A, Mahajan S et al (2020) Ocular surface manifestations of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS One 15(11):e0241661
Ranzenigo M, Bruzzesi E, Galli L, Castagna A, Ferrari G (2021) Symptoms and signs of conjunctivitis as predictors of disease course in COVID-19 syndrome. J Ophthalmic Inflamm Infect 11(1):35
Szczęśniak M, Brydak-Godowska J (2021) SARS-CoV-2 and the eyes: a review of the literature on transmission, detection, and ocular manifestations. Med Sci Monit 27:e931863
Kaur P, Sehgal G, Shailpreet, Singh KD, Singh B (2021) Evaluation and comparison of conjunctival swab polymerase chain reaction results in SARS-CoV-2 patients with and without ocular manifestations. Indian J Ophthalmol 69(8):2211–2214. https://doi.org/10.4103/ijo.IJO_587_21
Ekici Gok Z, Gok A, Acun Delen L, Kasapoglu US, Gurbuz E, Mutlu K (2021) Evaluation of eye care and ocular findings in critically ill COVID-19 patients. Int J Clin Pract 75(12):e14909. https://doi.org/10.1111/ijcp.14909
Atum M, Boz AAE, Çakır B, Karabay O, Köroğlu M, Öğütlü A et al (2020) Evaluation of conjunctival Swab PCR results in patients with SARS-CoV-2 infection. Ocul Immunol Inflamm 28(5):745–748
Cheong KX (2020) Systematic review of ocular involvement of SARS-CoV-2 in Coronavirus Disease 2019. Curr Ophthalmol Rep 8(4):185–194
Acknowledgements
None.
Funding
This work was supported by PUTI Saintekes Grant by Universitas Indonesia (Contract number: NKB-2300/UN2.RST/HKP.05.00/2020).
Author information
Authors and Affiliations
Contributions
MS analyzed the data and wrote the paper, HD, DF and DAD collected conjunctival swab samples from patients, JDB, DE, AAV, PP, NDP recruited patients for the study, BB and BH analyzed samples from the conjunctival swabs, JC and NS obtained informed consent and clinical data of patients, RSS conceptualized, coordinated and headed the study. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Recorded audio informed consent was obtained from patients and/or legal guardians for underaged children. This study has obtained ethical clearance from the ethics committee of the Faculty of Medicine, University of Indonesia (ethical approval number: KET-467/UN2.F1/ETIK/PPM.00.02/2020).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix1.
COVID-19 Questionnaire Form.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Susiyanti, M., Daniel, H., Faridah, D. et al. Incidence and clinical characteristic of ocular surface manifestation: an evaluation of conjunctival swab results in Corona Virus 2019 (COVID-19) patients in Jakarta, Indonesia. J Ophthal Inflamm Infect 13, 20 (2023). https://doi.org/10.1186/s12348-023-00343-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12348-023-00343-4