Abstract
Background
Polycystic ovary syndrome (PCOS) is an endocrine disease in which related to obesity, metabolic disorders and is considered as one of the main causes of infertility in women. This trial was investigated the effects of green cardamom on the expression of genes implicated in obesity and diabetes among obese women with PCOS.
Methods
One hundred ninety-four PCOS women were randomly divided two groups: intervention (n = 99; 3 g/day green cardamom) and control groups (n = 95). All of them were given low calorie diet. Anthropometric, glycemic and androgen hormones were assessed before and after 16-week intervention. The reverse transcription-polymerase chain reaction (RT-PCR) method was used to measure fat mass and obesity-associated (FTO), peroxisome proliferative activating receptor- (PPAR-), carnitine palmitoyltransferase 1A (CPT1A), acetyl-CoA carboxylase beta (ACAB), leptin receptor (LEPR), ghrelin, and lamin A/C (LAMIN) genes expression in each group.
Results
Anthropometric indices were significantly decreased after intervention in both two studied groups. Glycemic indices and androgen hormones were significantly improved in the intervention group compared to the control group. The expression levels of FTO, CPT1A, LEPR, and LAMIN were significantly downregulated compared to control group (P < 0.001), as well as, PPAR-y was significantly upregulated in the intervention group after intervention with green cardamom compared to control group (P < 0.001).
Conclusion
This current study showed that the administration of green cardamom is a beneficial approach for improving anthropometric, glycemic, and androgen hormones, as well as obesity and diabetes genes expression in PCOS women under the low-calorie diet.
Trial registration
This trial was registered with the Iranian Clinical Trials Registry (registration number: IRCT20200608047697N1). 1 August, 2020; https://www.irct.ir/trial/48748
Similar content being viewed by others
Background
Polycystic ovary syndrome (PCOS) is a common and complex endocrine disease in women and is considered one of the main causes of infertility in the reproductive years [1]. Women with PCOS often refer to medical care for menstrual disorders, clinical manifestations of hyperandrogenism, and infertility. PCOS is associated with hyperandrogenism, hyperinsulinemia, changes in the hypothalamic–pituitary–ovarian axis, ovulation disorders, and irregular menstruation, as well as mood swings, anxiety, and depression [2]. These patients are often prone to metabolic disorders characterized by weight gain and obesity, insulin resistance, type 2 diabetes, and cardiovascular disease [3]. The etiology of PCOS is not fully understood. However, some studies have found insulin resistance to be effective in its pathogenesis [4, 5]. Additionally, oxidative stress and an increase in inflammatory cytokines have also been reported to contribute to PCOS development [2].
Recent studies have found that genetic factors play a key role in the development of obesity and insulin resistance in patients with PCOS [6]. For instance, the fat mass and obesity associated (FTO) gene increases adipose tissue, especially in the abdomen area, as well as hyperandrogenism tends to an increase in PCOS' incidence [7,8,9]. Peroxisome proliferative activating receptors (PPARs) are part of the nuclear hormone receptors [10]. The PPAR-γ gene is vital in maintaining normal ovarian function because the PPAR-γ gene isoforms regulate metabolism, hormones related to reproduction, and ovarian function [10, 11].
Green cardamom consists of the whole dried fruit of Elettaria cardamomum (Linn), which belongs to the ginger family [12]. As a seasoning, green cardamom contains polyphenols such as flavonoids (lutolin), flavonols (quercetin and camperfor), and anthocyanins, all of which have antioxidant and anti-inflammatory properties [13]. Green cardamom might affect insulin sensitivity, inflammation and liver stasis by suppressing oxidative stress [14]. So far, several studies have been conducted on the benefits of green cardamom, such as antimicrobial, anti-cancer, anti-inflammatory, and antioxidant activities in animal models [14, 15]. Interventional studies with green cardamom in humans show a reduction in metabolic and inflammatory diseases, for instance, obesity, diabetes and pre-diabetes, cardiovascular disease, and hypertension [16, 17].
Considering that most studies in this field have been conducted mostly in cell and animal models, and there are few human studies in other fields other than polycystic ovary syndrome, this current randomized clinical trial was aimed to evaluate the effects of green cardamom supplementation on the expression of genes implicated in obesity and diabetes among obese women with PCOS.
Material and methods
Study design
This randomized, double-blind, placebo-controlled clinical trial study was conducted to evaluate the effects of green cardamom supplementation on obesity and diabetes gene expression among obese women with PCOS referring to gynecology and female infertility clinics. CONSORT statement for randomized clinical trials [18] was used to design of the current study. According to the increase in insulin sensitivity in the previous study [19] with 95% power and 5% significance, the sample size was considered to be 70 subjects for each group. For more reassurance and possible dropouts, we entered 100 subjects in each group. The trial was ethically approved by the Ethics Committee of Kermanshah University of Medical Sciences (Ethical NO: IR.KUMS.REC.1399.375) and registered with the Iranian Clinical Trials Registry (registration number: IRCT20200608047697N1). A written consent form was completed for all subjects after explaining the objectives of the study, grant no: 990412.
Participants, recruitment, and randomization
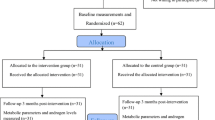
Study subjects were recruited from gynecology and female infertility clinics in Kermanshah, western Iran. Inclusion criteria Women with PCOS are diagnosed according to Rotterdam criteria if there are at least two factors: (1) oligomenorrhea or amenorrhea; (2) biochemical or clinical signs of increased androgens in the blood; and (3) having polycystic ovaries based on ultrasonography report, as well as age 18–45 years, body mass index (BMI) ≥ 30 kg/m2, and willingness to cooperate in this study. We did not include pregnant and lactating women, women with diseases such as autoimmune diseases, gastrointestinal, liver, thyroid, and unstable cardiovascular diseases, severe depression (due to inability to answer questions), mental illness, severe respiratory disease (asthma and chronic bronchitis), consumption of any vitamins, minerals, other dietary supplements, allergies to green cardamom, green cardamom tea, and green cardamom products. Furthermore, we did not include women receiving medications for the mentioned diseases that might interfere with green cardamom. Initially, 219 subjects were assessed. Subsequently, twenty subjects were excluded on account of the coronavirus exposure, inaccessible remote residence, and pregnant. Finally, subjects were randomly divided into two groups of placebo (n = 99) and intervention group (n = 100) using the random number table method (Fig. 1).
Intervention
All studied subjects underwent a weight loss diet that reduced their daily calorie intake by 400–500 kcal per day based on their adjusted ideal weight. According to previous studies, the dose of cardamom powder was three grams per day. This dose of cardamom improved the lipid profile, increased total antioxidant status, decreased systolic and diastolic blood pressure, improved inflammatory markers and liver enzymes, and no toxicity was observed with this dose [17, 20]. Therefore, patients in the intervention group were given three 1000 mg cardamom capsules of Karen Company three times a day to reduce possible gastrointestinal side effects with meals. On the other hand, patients in the placebo group received three placebo tablets containing starch powder three times a day with the same shape, color, and size of cardamom supplement. Each supplement pack was coded by a representative’s company, so the researcher and the subjects were not aware of the content of the packs.
Measurements
All subjects were asked to provide demographic information, including age and marital status. Furthermore, we collected their anthropometric indices, dietary intake, physical activity, biochemical indices, and expression of obesity and diabetes genes before and after intervention.
Anthropometric indices
In the current study, height was measured with a wall-mounted stadiometer (Seca, Hamburg, Germany) while their shoulders, hips, and heels were in contact with the wall. The subjects’ weight and body fat mass were measured using bioelectrical impedance analysis on a body analyzer device (Inbody Co, Seoul, Korea) in standing position with light clothing and no shoes. The non-stretched and flexible tape was used to measure waist circumference (WC) at the level of the iliac crest with a precision of 0.1 cm [21]. BMI was calculated by dividing weight in kilograms by height in meters squared.
Dietary assessment
A 3-day food record (2 days of weekdays and the weekend) was completed to evaluate the dietary intake, micro- and macronutrients, and the amount of vitamin D intake through diet before and after 16-week intervention by a trained dietitian. The energy and nutrients of their dietary intake were calculated by NUTRITIONIST IV software using the United States Department of Agriculture Food Composition Table, which was modified for Iranian foods [22].
Physical activity
The International Physical Activity Questionnaire (IPAQ)-short form before and after the intervention was completed by an interviewer for all studied subjects. The validity and reliability of the questionnaire had previously been confirmed in Iran [23].
Biochemical indices
At the beginning of the follicular phase (third day of the menstrual cycle), 10 cc of fasting venous blood was collected after 12 h of fasting overnight from all subjects. The blood samples were centrifuged, and the serum was stored at – 80 °C until analysis. Fasting blood sugar (FBS) was measured by an enzymatic method (Pars Azmoon Co., Iran). Fasting insulin concentrations and vitamin D3 were measured by Electrochemiluminescence (ECL) method. Glycated hemoglobin (Hb) A1C was analyzed by ion exchange chromatography. The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the following formula:
HOMA-IR: [Fasting serum insulin (μU/ml) × fasting glucose (mmol/l)]/22.5 [24].
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels were measured by radioimmunoassay (RIA) technique by a LKB gamma counter. Testosterone, prolactin, thyroid stimulating hormone (TSH), androstenedione, and dehydroepiandrosterone (DHEA) were measured in these subjects by Monobinde kit and SGHB by ILB kit using ELISA device. Measurement of ghrelin by ELISA method using Human Acylated Ghrelin kit (SPI Bio Company, France). Leptin was measured based on ELISA with a dual antibody method (Sandwich) prepared by the company DRG.
Expression of obesity and diabetes genes
Blood samples were stored in coated vials of ethylenediaminetetraacetic acid (EDTA) to evaluate the expression of FTO, PPAR-γ, carnitine palmitoyltransferase 1A (CPT1A), acetyl-CoA carboxylase beta (ACACB), leptin receptor (LEPR), ghrelin (GHRL), and lamin A/C (LAMIN) genes. Using Ficoll-Histopaque solution gradient (Ficoll-paque, Miltenyi Biotec GmbH, Germany), peripheral blood mononuclear cells (PBMC) were separated during density gradient centrifugation (Ficoll-paque, Miltenyi Biotec GmbH, Germany). Using Trisor Regaent kit (YTzol pure RNA, Iran), total RNA from PBMC cells was extracted. One microgram of the extracted RNA was applied for complementary DNA synthesis (cDNA) by Prime Script-RT reagent kits (Takara Bio Ink. Tokyo, Japan). Dedicated primers purchased from Metabion (Metabion, Germany) are presented in Table 1. The fold change formula was used to calculate the data after normalizing it to the rate of 18SrRNA expression as a housekeeping control gene. All samples were done in three versions.
Statistical analysis
SPSS (SPSS Inc. Chicago, IL, USA version 19) [25] was used to analyze data from this current trial. Kolmogorov–Smirnov test was applied to the data normality. Basic characteristics of studied subjects described by mean ± standard deviation (SD), percent frequency, and chart. To compare qualitative variables, the chi-square test was used. Mann–Whitney U, and independent sample t test were used to evaluate the quantitative variables difference between the two groups. The difference in quantitative variables within the studied groups was analyzed by paired sample t test or Wilcoxon. Per protocol analyses were performed on only those adhering to the protocol. A significance level of less than 0.05 was considered.
Results
One hundred and ninety-nine of the subjects fulfilled the inclusion criteria and participated in the study, but five subjects dropped out for the following reasons: one due to coronavirus infection in the intervention group, three due to coronavirus infection, and one due to pregnancy in the placebo group. Therefore, 194 subjects (intervention group 99 subjects, placebo group 95 subjects) completed the trial (Fig. 1). Ultimately, statistical analyses were performed on all 194 participants.
The mean age in the intervention and placebo groups was 32.99 ± 5.57 and 33.81 ± 5.42 years, respectively, and there was no difference between the two studied groups (P = 0.073). Moreover, there was no difference between the two studied groups in terms of physical activity, marital status, weight, BMI, WC, and BFM. Table 2 presents the basic characteristics of the two studied groups.
The mean of the calorie and nutrient did not differ between the two studied groups before and after the intervention. According to the given low calorie diet to all subjects, the calorie and nutrient had differences within each of the studied groups before and after intervention (Table 3).
After the intervention, all participating women underwent re-ultrasound. PCOS was improved in terms of cyst size and number, decreasing 54.1% in the intervention group and 35.5% in the placebo group, which was significantly different between the two studied groups (P = 0.031).
Table 4 showed that the mean of weight, BMI, WC, BFM, and FBS were significantly decreased after intervention in both of the two studied groups. Also, we observed that HbA1c, insulin, HOMA-IR, leptin, androstenedione, DHEA, and LH were significantly decreased in intervention group after intervention with green cardamom, as well as FSH, were significantly increased in this group.
Figure 2 indicates the expression levels of the obesity and diabetes genes in both two studied groups. Among the measurement of the obesity and diabetes genes, the expression level of FTO, CPT1A, LEPR, and LAMIN were significantly downregulated in the intervention group after intervention with green cardamom (P < 0.001). Furthermore, PPAR-γ was significantly upregulated in this group (P < 0.001).
Discussion
In this study, three gram per day green cardamom supplementation with a low-calorie diet in PCOS obese women resulted in an improvement in glycemic indices, including FBS, HbA1c, insulin, and HOMA-IR compared with placebo intake. In addition, when compared to placebo intake, androgen hormones and ultrasonography reports of ovarian function were improved in the intervention group. Furthermore, we observed that green cardamom intervention in PCOS women was associated with decreased expression of FTO, CPT1A, LEPR, and LAMIN, but PPAR-γ was considerably upregulated in this group.
PCOS is a complex disease with genetic and environmental components, and genes related to obesity and insulin metabolism appear to be involved in the etiology of this syndrome [26]. Insulin resistance and hyperinsulinemia affect 65–70% of women with PCOS, and obesity also accelerates the clinical manifestations of this syndrome in susceptible women [1].
In the current study, weight, BMI, WC, and BFM were significantly decreased in both of the two studied groups under the low calorie diet. Furthermore, all glycemic indices, including FBS, HbA1c, insulin, and HOMA-IR were significantly improved after 16 weeks intervention. Yaghooblou et al. [27] in their trial on pre-diabetic women, they observed that after 2 months of intervention with 3 g of cardamom, weight, BMI, WC, and insulin sensitivity were significantly decreased compared to the control group. However, other glycemic indices, including FBS, insulin, and HOMA-IR, had not changed after intervention. Another trial by Aghasi et al. [28] showed that HbA1c, insulin, and HOMA-IR were significantly decreased after green cardamom supplementation. For ethical consideration, we gave both groups the low calorie diet. Therefore, it seems changes in anthropometric indices after intervention in both groups are normal. Green cardamom is rich in flavonoids and isoflavones that contribute in reducing insulin resistance by decreasing adipose tissue storage [13, 14].
Our results indicated that after intervention with the green cardamom, endocrine outcomes including leptin, androstenedione, DHEA, and LH were significantly reduced in the intervention group, as well as, FSH were significantly increased in this group. A literature review of 33 studies showed a decrease in LH, prolactin, insulin, and testosterone after the administration of herbal medicine to women with PCOS [29]. In a study on overweight and obese PCOS women, Khorshidi et al. [6] reported that after quercetin supplementation, the levels of LH, testosterone, and SHBG were significantly decreased. Obesity, especially abdominal obesity, as well as insulin resistance, exacerbate hyperandrogenism. Obesity is mainly associated with increased levels of free fatty acids (FFA), which increase FFA, reducing insulin sensitivity [30]. Finally, abdominal obesity and insulin resistance synergistically affect the production of androgen hormones [1]. On the other hand, increasing adipose tissue causes the production of the hormone leptin. Leptin is a hormone encoded by the obesity gene (LPER) on human chromosome 7 [31]. High levels of this hormone are seen in some women with PCOS, which prevents the conversion of androgens to estrogen and subsequent follicular atresia [1, 31]. Therefore, it seems that green cardamom, with anti-inflammatory properties and reduced fat storage, has beneficial effects in improving the status of androgen hormones.
The current study found that after a green cardamom intervention, FTO, CPT1A, LEPR, and LAMIN were downregulated while PPAR-γ was upregulated in PCOS women. Limited data are available evaluating the effects of the green cardamom on obesity and diabetes genes expression (Fig. 3).
Results of a meta-analysis by Liu et al. showed that the expression level of FTO gene was related to a higher risk of PCOS in which the FTO gene appears to be involved in the pathogenesis of PCOS by increasing fat mass and eventually obesity [32]. In animal models, the expression level of CPT1A was associated with increasing BMI, WC, and hypertriglyceridemia [33, 34]. It has been established that LEPR polymorphism is linked to obesity, insulin resistance, and dyslipidemia, and that serum leptin in PCOS women is elevated due to a high quantity of adipose tissue [35, 36]. Excessive production of inflammatory markers in adipose tissue is mediated by the LAMIN gene (mapped on the long arm of chromosome 1) through macrophages, which leads to diabetes development [37]. Nasri et al. [38] discovered that administering omega three fatty acids with anti-inflammatory properties could up-regulate PPAR-γ in PCOS women (P = 0.005) in a randomized clinical trial. Heshmati et al. [39] in their study showed that 4.5 g/day curcumin supplementation was related to PPAR-γ coactivator 1a gene up-regulation in PCOS women (P = 0.011). Similarly, Daneshi et al. [17] reported that 3 g/day cardamom supplementation could increase the level of Irisin, which can improve PPAR-γ coactivator 1a secretion in overweight and obese with non-alcoholic fatty liver disease patients. The PPAR-γ gene has a role in regulating metabolism, reproductive hormones, and ovarian function [10, 11]. Due to its anti-inflammatory and antioxidant properties, green cardamom plays an important role in reducing inflammation and improving insulin resistance [14]. Our other study showed that green cardamom consumption was associated with decreased levels of inflammatory factors and down regulation genes [40].
In conclusion, this is the first study to evaluate the effect of green cardamom supplementation on obesity and diabetes gene expression in PCOS women.
This study demonstrated that a green cardamom intervention improved anthropometric indices, glycemic indices, and sexual hormones, as well as the expression level of obesity and diabetes genes FTO, CPT1A, LEPR, LAMIN, and PPAR-γ genes in PCOS women.
Availability of data and materials
Data will be available upon request from the corresponding author.
Change history
27 January 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12263-023-00720-7
Abbreviations
- ACACB:
-
Acetyl-CoACarboxylase Beta
- BMI:
-
Body mass index
- CPT1A :
-
Carnitine palmitoyltransferase 1A
- DHEA:
-
Dehydroepiandrosterone
- EDTA:
-
Ethylenediaminetetraacetic acid
- FTO:
-
Fat mass and obesity associated
- FBS:
-
Fasting blood sugar
- FSH:
-
Follicle-stimulating hormone
- GHRL:
-
Ghrelin
- (Hb) A1C :
-
Glycated hemoglobin
- HOMA-IR:
-
Homeostatic Model Assessment for Insulin Resistance
- IPAQ:
-
International Physical Activity Questionnaire
- LAMIN:
-
Lamin A/C
- LEPR:
-
Leptin receptor
- LH:
-
Luteinizing hormone
- PBMC:
-
Peripheral blood mononuclear cells
- PPARs:
-
Peroxisome proliferative activating receptors
- PCOS:
-
Polycystic ovary syndrome
- SD:
-
Standard deviation
- TSH:
-
Thyroid stimulating hormone
- WC:
-
Waist circumference
References
Zeng X, Xie Y-J, Liu Y-T, Long S-L, Mo Z-C. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–21. https://doi.org/10.1016/j.cca.2019.11.003.
Machado V, Escalda C, Proença L, Mendes JJ, Botelho J. Is there a bidirectional association between polycystic ovarian syndrome and periodontitis? A systematic review and meta-analysis. J Clin Med. 2020;9(6):1961. https://doi.org/10.3390/jcm9061961.
Zhang J, Xu J-H, Qu Q-Q, Zhong G-Q. Risk of Cardiovascular and Cerebrovascular Events in Polycystic Ovarian Syndrome Women: A Meta-Analysis of Cohort Studies. Front Cardiovasc Med. 2020;7:552421. https://doi.org/10.3389/fcvm.2020.552421.
Minocha N. Polycystic ovarian disease or polycystic ovarian syndrome: how to identify and manage-a review. Arch Pharma Pract. 2020;11(2):102–6.
Paganini C, Peterson G, Stavropoulos V, Krug I. The overlap between binge eating behaviors and polycystic ovarian syndrome: an etiological integrative model. Curr Pharm Des. 2018;24(9):999–1006. https://doi.org/10.2174/1381612824666171204151209.
Khorshidi M, Moini A, Alipoor E, Rezvan N, Gorgani-Firuzjaee S, Yaseri M, et al. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytother Res. 2018;32(11):2282–9. https://doi.org/10.1002/ptr.6166.
Song DK, Lee H, Oh J-Y, Hong YS, Sung Y-A. FTO gene variants are associated with PCOS susceptibility and hyperandrogenemia in young Korean women. Diabetes Metab J. 2014;38(4):302–10. https://doi.org/10.4093/dmj.2014.38.4.302.
Wehr E, Schweighofer N, Möller R, Giuliani A, Pieber TR, Obermayer-Pietsch B. Association of FTO gene with hyperandrogenemia and metabolic parameters in women with polycystic ovary syndrome. Metabolism. 2010;59(4):575–80. https://doi.org/10.1016/j.metabol.2009.08.023.
Liu AL, Xie HJ, Xie HY, Liu J, Yin J, Hu JS, et al. Association between fat mass and obesity associated (FTO) gene rs9939609 A/T polymorphism and polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med Genet. 2017;18(1):89. https://doi.org/10.1186/s12881-017-0452-1.
Xu Y, Wu Y, Huang Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS: a meta-analysis. Arch Gynecol Obstet. 2017;296(4):661–77. https://doi.org/10.1007/s00404-017-4480-z.
Prabhu YD, Gopalakrishnan AV. γ-Linolenic acid ameliorates DHEA induced pro-inflammatory response in polycystic ovary syndrome via PPAR-γ signaling in rats. Reprod Biol. 2020;20(3):348–56. https://doi.org/10.1016/j.repbio.2020.05.004.
Ashokkumar K, Murugan M, Dhanya M, Warkentin TD. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]–a critical review. J Ethnopharmacol. 2020;10(246):112244. https://doi.org/10.1016/j.jep.2019.112244.
Azimi P, Ghiasvand R, Feizi A, Hariri M, Abbasi B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud Fall-Winter. 2014;11(3–4):258–66. https://doi.org/10.1900/RDS.2014.11.258.
Rahman MM, Alam MN, Ulla A, Sumi FA, Subhan N, Khan T, et al. Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Dis. 2017;16(1):151. https://doi.org/10.1186/s12944-017-0539-x.
Al-Othman AM, Ahmad F, Al-Orf S, Al-Murshed KS, Arif Z. Effect of dietary supplementation of Ellataria cardamomum and Nigella sativa on the toxicity of rancid corn oil in Rats. Int J Pharmacol. 2006;2(1):60–5. https://doi.org/10.3923/ijp.2006.60.65.
Daneshi-Maskooni M, Keshavarz SA, Qorbani M, Mansouri S, Alavian SM, Badri-Fariman M, et al. Green cardamom increases Sirtuin-1 and reduces inflammation in overweight or obese patients with non-alcoholic fatty liver disease: a double-blind randomized placebo-controlled clinical trial. Nutr Metab. 2018;15(1):63. https://doi.org/10.1186/s12986-018-0297-4.
Daneshi-Maskooni M, Keshavarz SA, Qorbani M, Mansouri S, Alavian SM, Badri-Fariman M, et al. Green cardamom supplementation improves serum irisin, glucose indices, and lipid profiles in overweight or obese non-alcoholic fatty liver disease patients: a double-blind randomized placebo-controlled clinical trial. BMC Complement Altern Med. 2019;19(1):59. https://doi.org/10.1186/s12906-019-2465-0.
Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295–309. https://doi.org/10.7326/0003-4819-148-4-200802190-00008.
Fatemeh Y, Siassi F, Rahimi A, Koohdani F, Doostan F, Qorbani M, et al. The effect of cardamom supplementation on serum lipids, glycemic indices and blood pressure in overweight and obese pre-diabetic women: a randomized controlled trial. Diabetes Metabol Disord. 2017;16(1):1–9. https://doi.org/10.1186/s40200-017-0320-8.
Aghasi M, Ghazi-Zahedi S, Koohdani F, Siassi F, Nasli-Esfahani E, Keshavarz A, et al. The effects of green cardamom supplementation on blood glucose, lipids profile, oxidative stress, sirtuin-1 and irisin in type 2 diabetic patients: a study protocol for a randomized placebo-controlled clinical trial. BMC Complement Altern Med. 2018;18(1):18. https://doi.org/10.1186/s12906-017-2068-6.
Mahan L, Raymond J. Krause's food & the nutrition care process; Clinical: Biochemical, Physical, and Functional Assessment. 14 ed: Elsevier Health Sciences, 2016. 114 p
Ghaffarpour M, Houshiar-Rad A, and Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy 7. 1999:213
Gholami Fesharaki M, AzadMarzabadi E. Evaluation of the reliability and validity of Azad-Fesharaki’s physical activity questionnaire (AFPAQ). J Arak Univ Med Sci. 2011;14(3):36–44.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. https://doi.org/10.1007/BF00280883.
George D, Mallery P. IBM SPSS statistics 19 step by step. Boston: Mass; 2012.
El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome: an updated overview. Front Physiol. 2016;7:124. https://doi.org/10.3389/fphys.2016.00124.
Fatemeh Y, Siassi F, Rahimi A, Koohdani F, Doostan F, Qorbani M, et al. The effect of cardamom supplementation on serum lipids, glycemic indices and blood pressure in overweight and obese pre-diabetic women: a randomized controlled trial. J Diabetes Metab Disord. 2017;29(16):40. https://doi.org/10.1186/s40200-017-0320-8.
Aghasi M, Koohdani F, Qorbani M, Nasli-Esfahani E, Ghazi-Zahedi S, Khoshamal H, et al. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: a randomized double-blind placebo controlled clinical trial. J Sci Food Agric. 2019;99(8):3933–40. https://doi.org/10.1002/jsfa.9617.
Arentz S, Abbott JA, Smith CA, Bensoussan A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement Altern Med. 2014;14(1):511. https://doi.org/10.1186/1472-6882-14-511.
Sirotkin AV, Fabian D, Babeľová J, Vlčková R, Alwasel S, Harrath AH. Body fat affects mouse reproduction, ovarian hormone release, and response to follicular stimulating hormone. Reprod Biol. 2018;18(1):5–11. https://doi.org/10.1016/j.repbio.2017.12.002.
Chow LS, Mashek DG, Wang Q, Shepherd SO, Goodpaster BH, Dubé JJ. Effect of acute physiological free fatty acid elevation in the context of hyperinsulinemia on fiber type-specific IMCL accumulation. J Appl Physiol. 2017;123(1):71–8. https://doi.org/10.1152/japplphysiol.00209.2017.
Liu AL, Xie HJ, Xie HY, Liu J, Yin J, Hu JS, et al. Association between fat mass and obesity associated (FTO) gene rs9939609 A/T polymorphism and polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med Genet. 2017;18(1):89. https://doi.org/10.1186/s12881-017-0452-1.
Moody L, Xu GB, Chen H, Pan Y-X. Epigenetic regulation of carnitine palmitoyltransferase 1 (Cpt1a) by high fat diet. Biochim Biophys Acta Gene Regul Mech. 2019;1862(2):141–52. https://doi.org/10.1016/j.bbagrm.2018.12.009.
Warfel JD, Vandanmagsar B, Dubuisson OS, Hodgeson SM, Elks CM, Ravussin E, et al. Examination of carnitine palmitoyltransferase 1 abundance in white adipose tissue: implications in obesity research. Am J Physiol Regul Integr Comp Physiol. 2017;312(5):R816–20. https://doi.org/10.1152/ajpregu.00520.2016.
Li L, Lee K-J, Choi B-C, Baek K-H. Relationship between leptin receptor and polycystic ovary syndrome. Gene. 2013;527(1):71–4. https://doi.org/10.1016/j.gene.2013.05.074.
Daghestani MH, Daghestani MH, Daghistani MH, Bjørklund G, Chirumbolo S, Warsy A. The influence of the rs1137101 genotypes of leptin receptor gene on the demographic and metabolic profile of normal Saudi females and those suffering from polycystic ovarian syndrome. BMC Womens Health. 2019;19(1):10. https://doi.org/10.1186/s12905-018-0706-x.
Kim Y, Bayona PW, Kim M, Chang J, Hong S, Park Y, et al. Macrophage lamin A/C regulates inflammation and the development of obesity-induced insulin resistance. Front Immunol. 2018;9:696. https://doi.org/10.3389/fimmu.2018.00696.
Nasri K, Hantoushzadeh S, Aghadavod E, Taghizadeh M, Asemi Z. The effects of omega-3 fatty acids supplementation on gene expression involved in the insulin and lipid signaling pathway in patients with polycystic ovary syndrome. Horm Metab Res. 2017;49(6):446–51. https://doi.org/10.1055/s-0042-122782.
Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, et al. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr. 2020;14(2):77–82. https://doi.org/10.1016/j.dsx.2020.01.002.
Cheshmeh S, Ghayyem M, Khamooshi F, Heidarzadeh-Esfahani N, Rahmani N, Hojati N, et al. Green cardamom plus low-calorie diet can decrease the expression of inflammatory genes among obese women with polycystic ovary syndrome: a double-blind randomized clinical trial. Eat Weight Disord. 2022;27(2):821–30. https://doi.org/10.1007/s40519-021-01223-3.
Acknowledgements
We wish to thank and acknowledge the valuable contribution of all women in this study and especially want to acknowledge the Kermanshah University of Medical Science for financial support.
Funding
This study was supported by Kermanshah University of Medical Science (Grant No: 990412).
Author information
Authors and Affiliations
Contributions
SM and SCh contributed in conception and design of the research. NE, MG, and EM contributed to data collection. SM and SCh contributed to the acquisition and analysis of the data; SM and SCh contributed to the interpretation of the data. SM, SCh, and YP contributed to draft the manuscript. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (ethics approval number: IR.KUMS.REC.1399.375) and registered with the Iranian Clinical Trials Registry (registration number: IRCT20200608047697N1). Written informed consent was obtained from each studied subject after explaining the purpose of the study. The right of subjects to withdraw from the study at any time and subject’s information is reserved and will not be published.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheshmeh, S., Elahi, N., Ghayyem, M. et al. Effect of green cardamom on the expression of genes implicated in obesity and diabetes among obese women with polycystic ovary syndrome: a double blind randomized controlled trial. Genes Nutr 17, 17 (2022). https://doi.org/10.1186/s12263-022-00719-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12263-022-00719-6