Abstract
Background and aims
Many patients with intracerebral hemorrhage (ICH) develop intraventricular hemorrhage (IVH), which is associated with higher mortality and worse clinical outcome. External ventricular drains (EVDs) are often placed, but there is little data on how much patients benefit from this intervention. We explored the use, timing, and location of EVD in ICH patients and any association with clinical outcome.
Results
During the study period, 2870 patients presented with primary ICH, and 2486 were included in analyses. Overall, patients were 73 (± 13) years old; 54% were male, and 46% had associated IVH. An EVD was placed in 29% of patients with IVH and 4% of those without. IVH patients with EVD were younger (67 ± 13 vs 74 ± 13, p < 0.001), had larger IVH volumes (17 mL vs 8 mL, p < 0.001), and lower GCS scores (7 vs 10, p < 0.001), compared to those without EVD. Ninety-day mortality was available in 2486 (100%) patients, while 90-day mRS was available in 1673 (67.3%). In univariate analysis, EVD placement was associated with lower likelihood of 90-day mortality (53% vs 59%, p = 0.048) but higher likelihood of poor outcome (88% vs 85%, p < 0.001) in those for whom this was available. Those with poor outcomes underwent faster EVD placement (0.46 days vs. 0.96 days, p = 0.01). In multivariate analysis, EVD placement was associated with lower 90-day mortality (OR 0.19, 95% CI 0.053–0.657, p = 0.009), but not with lower odds of poor outcome (OR 1.64, 95% CI 0.508–5.309, p = 0.4). In multivariate analysis, days to EVD placement was associated with lower 90-day mortality (OR 0.69, 95% CI 0.49–0.96, p = 0.027).
Conclusion
IVH is relatively common after ICH. After controlling for potential confounds, EVD placement is associated with lower mortality, but not clearly with better neurologic outcome. In addition, more rapid EVD placement is associated with higher mortality, potentially reflecting early development of herniation or obstructive hydrocephalus.
Similar content being viewed by others
Introduction
Spontaneous intracerebral hemorrhage (ICH) accounts for 10–15% of strokes globally and is the deadliest stroke subtype [1,2,3,4]. One common complication of ICH is extension of blood into the ventricles, causing associated intraventricular hemorrhage (IVH) in approximately 45% of patients [5].
Patients with ICH with IVH extension are at substantially higher risk of poor outcome [5, 6]. This may be due to thalamus and reticular activating system damage, obstructive hydrocephalus, or inflammatory reactions10. The blood in the ventricles also commonly correlates with increased intracranial pressure (ICP), which may contribute to worse outcome [5].
One common treatment for severe IVH is to insert an extraventricular drain (EVD), which can drain cerebrospinal fluid (CSF) and blood from the ventricles and reduce ICP [7, 8]. The value of EVD has never been shown in a randomized trial, and the evidence base is limited to small observational studies [7, 9, 10]. As a result, the magnitude of any benefit from EVD is not clear, nor whether there is a subset of patients that benefit the most.
We therefore sought to examine EVD use in a large cohort of ICH patients and hypothesized that EVD use would be associated with improved outcome and lower mortality at 90 days. We also explored whether any particular subgroup may benefit.
Results
During the study period, 2870 patients presented with ICH. Three-hundred eighty-four were excluded for inhospital ICH, insufficient data, and possible confounding factors, leaving 2486 for analysis (Fig. 1). Table 1 shows the demographics of this cohort. Overall, patients were 73 + / − 13 years old; 54% were male, and 46% had IVH. An EVD was placed in 29% of patients with IVH and 4% of those without.
Table 2 shows the characteristics of patients with IVH, stratified by EVD placement. Of those patients with IVH, EVD placement was more common in those who were younger (age 67 vs 75, p < 0.001), had smaller ICH volumes (20 vs 37 cm3, p < 0.001), were less likely to have lobar placement of ICH (26% vs 50%, p < 0.001), had larger IVH volumes (17 vs 8 cm3, p < 0.001), and had lower initial GCS scores (7 vs 10, p < 0.001).
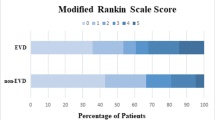
For the remainder of the analyses, patients made comfort measures only (CMO) within 24 h (692 patients) were excluded. Tables 3 and 4 show factors associated with neurologic outcome and mortality within patients with IVH. While mRS was available at discharge in 95% of patients, 90-day mRS was only available in 67%. In univariate analysis of those not made CMO, EVD placement in patients with IVH was associated with higher likelihood of poor outcome (88% vs 85%, p = 0.04) and lower mortality (53% vs 59%, p = 0.003). However, due to concern that this finding may have been confounded by indication (with EVD placement directed towards the highest risk patients), we performed a multivariable analysis to control for disease severity. Tables 5 and 6 show that in patients with both ICH and IVH, EVD placement was independently associated with lower 90-day mortality (OR 0.187, 95% CI 0.053–0.657, p = 0.009) but was not independently associated with lower 90-day poor outcome (OR 1.642, 95% CI 0.508–5.309, p = 0.4). We also examined characteristics of EVD placement such as timing and early hydrocephalus. In multivariate analysis controlling for age, initial ICH and IVH volume, and GCS at admission, longer time to EVD placement was associated with lower 90-day mortality (OR 0.687, 95% CI 0.49–0.96, p = 0.027), and hydrocephalus was positively associated with 90-day mortality (OR 1.973, 95% CI 1.12–3.47, p = 0.018).
In an exploratory analysis, we examined whether EVD placement was associated with disproportionate benefit in any particular subgroup (Fig. 2). We found that EVD placement was associated with disproportionate reduction in mortality in younger patients, patients with greater ICH volumes, patients with smaller IVH volumes, and patients with higher mRS prior to the index event.
a Forest plot of EVD and 90-day mortality: a subgroup analysis. Abbreviations: CI, confidence interval; EVD, external ventricular drain; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage. b Forest plot of EVD and 90-day poor outcome: a subgroup analysis.Abbreviations: CI, confidence interval; EVD, external ventricular drain; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; mRS, modified Rankin scale
Discussion
Overall, we found that in patients with ICH and IVH, EVD placement was independently associated with lower mortality, but not with better 90-day neurologic outcome. Other factors associated with 90-day mortality and outcome included age, ICH and IVH volume, ICH location, and initial GCS score.
We noted several factors associated with IVH incidence. These included a past medical history of hypertension and diabetes, higher initial systolic blood pressure, larger ICH volume, basal ganglia location, and lower initial GCS score. Other authors have found similar findings [5, 11]; however, some have also found older age to be associated with IVH [5]. We are unaware of prior findings suggesting a linkage between diabetes and IVH. We also confirmed prior findings that IVH predicts poor outcome [9, 12,13,14].
Few other studies have examined the link between EVD placement and other clinical markers of ICH and outcome. Herrick et al. (2014) found that EVD placement is primarily in patients with greater IVH volumes, younger age, lower admission GCS score, and basal ganglia location [9]. Similarly, we found that EVDs are placed in those with smaller ICH volume, larger IVH volume, basal ganglia location, higher initial blood pressure, and lower initial GCS. Not surprisingly, those with withdrawal of care were less likely to undergo EVD placement.
Our finding that EVD placement was associated with worse neurologic outcome in univariate analysis, but not after multivariate analysis, suggests that its use is preferentially directed towards the most severely injured patients. We note that Nieukamp et al. (2014) reached similar conclusions from their metanalyses of published literature [14]. We can thus infer that some of the findings on univariate analysis may be due to confounding by indication. The fact that controlling for disease severity reverses this finding suggests that clinical providers may be appropriately targeting those patients for EVD placement who are likely to truly benefit. Overall, our data suggest that the current use of EVD in clinical practice is justified and likely lowering mortality.
Whether lowering mortality leads to an increase in “good” neurologic outcome is unclear. Other clinical trials of surgical therapy have found reductions in mortality, without a corresponding improvement in neurologic outcome [11, 15,16,17], suggesting that some interventions may save lives but with severe associated morbidity, rather than leading to “good” recovery. Another study has similarly found that EVD subjects exhibited lower mortality but worse outcome [18]. It is not clear whether EVD placement falls into this category or whether any benefit from its use comes from bundling it with multiple other interventions.
While previous projects have examined EVD placement and outcome, this analysis includes a substantially larger cohort than prior efforts [7, 9, 10], lending more power to adjust for multiple factors related to clinical outcome. Other papers previously examining EVD usage in large populations have focused on assessing EVD placement and complications but have not looked directly at clinical outcome [5]. We were able to include comparable data from this relatively large population of EVD recipients over a time span of 19 years because EVD placement standards and guidelines for treatment of ICH with IVH at the study center did not change greatly over the study duration. We also investigated variables related to EVD placement such as timing of EVD placement and early presence of hydrocephalus. Hydrocephalus was positively associated with 90-day mortality, meaning that the presence of hydrocephalus on the first CT was correlated with a higher likelihood of death within 90 days. Time to EVD placement was negatively associated with 90-day mortality, meaning that the later an EVD was placed, the more likely patients were to survive past 90 days. This may mean that acute EVD places additional stressors on patients with intracerebral hemorrhages, but it could also indicate that the most severe cases are given priority for EVD placement as a final measure to attempt to improve outcome.
There were several limitations to the scope of this study. First, as this was an observational study, EVD placement was at the direction of clinical providers, and there may have been confounding by indication. Clinicians may have selected those patients most likely to have good outcome to undergo EVD placement. We attempted to control for this with multivariable analysis, but unmeasured confounders may still have been present. Second, we did not directly assess hydrocephalus, the expansion of fluid in the ventricles that is thought to cause many of the problems associated with IVH. We also did not assess the position of EVD placement, the experience of the neurosurgeon involved, or the actual amount of CSF drainage, all of which may have an impact on its efficacy. The EVD placement decision was made by individual clinicians, raising the risk of confounding by indication. Many patients did not have 3-month functional outcome available; as a result, poor outcome may be overrepresented as those who died were more likely to have available outcomes (Social Security Death Index) than those who did not. We were also unable to control for thrombolytic treatments received. Finally, as this was a retrospective single-center study, the results may not be generalizable to other institutions with different patient populations or practice patterns.
Conclusion
EVD placement appears independently associated with lower mortality after ICH with IVH. Future prospective studies should evaluate which patients benefit most from this intervention.
Methods
Study population
We performed a retrospective analysis of a prospectively collected cohort of consecutive patients with primary ICH presenting to a single academic medical center between the years 2000 and 2019 [19, 20]. Patients with secondary ICH including trauma, aneurysm, ischemic stroke, tumor, arteriovenous malformation, or other causes were excluded. Patients without information regarding IVH the presence and EVD placement were also excluded. Demographic and clinical data were collected prospectively. Data on EVD placement were collected via medical record review. Patients consenting to future contact were prospectively assessed via 3-month telephone interviews to determine clinical outcome, and mortality data were collected via serial queries of the Social Security Death Index. Good outcome was defined as 90-day modified Rankin score (mRS) of 0–3. This study was performed with the approval of our institutional review board. Patients provided informed consent, or need for consent was waived for retrospective review.
Clinical data
Baseline data collection was performed as described previously [19, 20]. Briefly, clinical variables collected included age, sex, medical history of vascular risk factors, current medications, and characteristics upon presentation. Intraventricular thrombolysis was performed very infrequently (< 5 times) and so was not included in the analysis. Do not resuscitate (DNR) orders and comfort measures only (CMO) orders were captured when they occurred within the first 24 h. Time to EVD placement was measured as a whole number of days. Since accurate times were not consistently available in the records, we rounded to the nearest day. Subjects were recorded as intubated if they were intubated at any time during their hospitalization for this event. The enrolling hospital had no formal clinical policy regarding EVD placement. Hydrocephalus was captured if specifically diagnosed by the neuroradiology or clinical teams. Discharge modified Rankin scale (mRS) was calculated on the day of discharge, and follow-up mRS was assessed through follow-up telephone calls 3 months after the index ICH or through records of death before 3 months. Mortality was assessed by periodic review of the Social Security Death Index.
Neuroimaging acquisition and analysis
For all subjects, the first available CT was used to calculate ICH and IVH volumes. Volumes were read independently using Analyze Direct 11.0 software — a semiautomated computer-assisted technique with excellent interrater reliability [19, 21]. Trained raters were blinded to all clinical information while assessing scans.
Statistics
Demographics and clinical characteristics were compared in univariate analyses between IVH and non-IVH ICH cases and within IVH patients between EVD and non-EVD patients. Medians between two integer variables were rounded down. Initial multivariable analysis was performed on IVH cases who were not made CMO during their course. The model included variables identified as significant and near significant in univariate analysis (p < 0.1). Highly collinear variables were removed from multivariable analysis. These included intubation, the presence of DNR status within 24 h, and the presence of CMO status within 24 h. Remaining variables were narrowed down via backwards stepwise logistic regression, with mortality and 90-day poor outcome as dependent variables. The SPSS 24 statistical package was used for statistical analysis (IBM Corp., Armonk, NY, USA). Significance level was set at 0.05 for all analyses.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to protected health information, but deidentified data are available from the corresponding author on reasonable request.
References
Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–48. https://doi.org/10.1161/CIRCRESAHA.116.308413. PMID: 28154096.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–44. https://doi.org/10.1016/S0140-6736(09)60371-8. PMID:19427958;PMCID:PMC3138486
Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164(8):880–4. https://doi.org/10.1001/archinte.164.8.880. PMID: 15111374.
Staykov D, Huttner HB, Lunkenheimer J, Volbers B, Struffert T, Doerfler A, Ganslandt O, Juettler E, Schwab S, Bardutzky J. Single versus bilateral external ventricular drainage for intraventricular fibrinolysis in severe ventricular haemorrhage. J Neurol Neurosurg Psychiatry. 2010;81(1):105–8. https://doi.org/10.1136/jnnp.2008.168427. PMID: 20019227.
Hinson HE, Hanley DF, Ziai WC. Management of intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2010;10(2):73–82. https://doi.org/10.1007/s11910-010-0086-6. PMID:20425231;PMCID:PMC3138489.
Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD, STICH Investigators. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–8. https://doi.org/10.1007/3-211-30714-1_16. PMID: 16671427.
Dey M, Jaffe J, Stadnik A, Awad IA. External ventricular drainage for intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2012;12(1):24–33. https://doi.org/10.1007/s11910-011-0231-x. PMID:22002766;PMCID:PMC6777952.
Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60. https://doi.org/10.1161/STR.0000000000000069. Epub 2015 May 28. PMID: 26022637.
Herrick DB, Ullman N, Nekoovaght-Tak S, Hanley DF, Awad I, LeDroux S, Thompson CB, Ziai WC. Determinants of external ventricular drain placement and associated outcomes in patients with spontaneous intraventricular hemorrhage. Neurocrit Care. 2014;21(3):426–34. https://doi.org/10.1007/s12028-014-9959-x. PMID: 24522761.
Kakarla UK, Kim LJ, Chang SW, Theodore N, Spetzler RF. Safety and accuracy of bedside external ventricular drain placement. Neurosurgery. 2008;63(1 Suppl 1):ONS162-6; discussion ONS166-7. https://doi.org/10.1227/01.neu.0000335031.23521.d0. PMID: 18728595.
Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, Dawson J, Gandhi D, Ullman N, Mould WA, Mayo SW, Mendelow AD, Gregson B, Butcher K, Vespa P, Wright DW, Kase CS, Carhuapoma JR, Keyl PM, Diener-West M, Muschelli J, Betz JF, Thompson CB, Sugar EA, Yenokyan G, Janis S, John S, Harnof S, Lopez GA, Aldrich EF, Harrigan MR, Ansari S, Jallo J, Caron JL, LeDoux D, Adeoye O, Zuccarello M, Adams HP Jr, Rosenblum M, Thompson RE, Awad IA, CLEAR III Investigators. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–11. https://doi.org/10.1016/S0140-6736(16)32410-2. Epub 2017 Jan 10. PMID: 28081952; PMCID: PMC6108339.
Hallevi H, Albright KC, Aronowski J, Barreto AD, Martin-Schild S, Khaja AM, Gonzales NR, Illoh K, Noser EA, Grotta JC. Intraventricular hemorrhage: anatomic relationships and clinical implications. Neurology. 2008;70(11):848–52. https://doi.org/10.1212/01.wnl.0000304930.47751.75. PMID:18332342;PMCID:PMC2745649.
Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40(4):1533–8. https://doi.org/10.1161/STROKEAHA.108.535419. Epub 2009 Feb 26. PMID: 19246695; PMCID: PMC2744212.
Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247(2):117–21. https://doi.org/10.1007/pl00007792. PMID: 10751114.
Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, Mayo SW, Bistran-Hall AJ, Gandhi D, Mould WA, Ullman N, Ali H, Carhuapoma JR, Kase CS, Lees KR, Dawson J, Wilson A, Betz JF, Sugar EA, Hao Y, Avadhani R, Caron JL, Harrigan MR, Carlson AP, Bulters D, LeDoux D, Huang J, Cobb C, Gupta G, Kitagawa R, Chicoine MR, Patel H, Dodd R, Camarata PJ, Wolfe S, Stadnik A, Money PL, Mitchell P, Sarabia R, Harnof S, Barzo P, Unterberg A, Teitelbaum JS, Wang W, Anderson CS, Mendelow AD, Gregson B, Janis S, Vespa P, Ziai W, Zuccarello M, Awad IA, MISTIE III Investigators. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021–32. https://doi.org/10.1016/S0140-6736(19)30195-3. Epub 2019 Feb 7. Erratum in: Lancet. 2019 Apr 20;393(10181):1596. PMID: 30739747; PMCID: PMC6894906.
Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, STICH II Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397–408. https://doi.org/10.1016/S0140-6736(13)60986-1 Epub 2013 May 29. Erratum in: Lancet. 2013 Aug 3;382(9890):396. Erratum in: Lancet. 2021 Sep 18;398(10305):1042. PMID: 23726393; PMCID: PMC3906609.
Steiner T, Vincent C, Morris S, Davis S, Vallejo-Torres L, Christensen MC. Neurosurgical outcomes after intracerebral hemorrhage: results of the Factor Seven for Acute Hemorrhagic Stroke Trial (FAST). J Stroke Cerebrovasc Dis. 2011;20(4):287–94. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.12.008. Epub 2010 May 8. PMID: 20452785.
Lovasik BP, McCracken DJ, McCracken CE, McDougal ME, Frerich JM, Samuels OB, Pradilla G. The effect of external ventricular drain use in intracerebral hemorrhage. World Neurosurg. 2016;94:309–18. https://doi.org/10.1016/j.wneu.2016.07.022. Epub 2016 Jul 17 PMID: 27436212.
Morotti A, Boulouis G, Charidimou A, Schwab K, Kourkoulis C, Anderson CD, Gurol ME, Viswanathan A, Romero JM, Greenberg SM, Rosand J, Goldstein JN. Integration of computed tomographic angiography spot sign and noncontrast computed tomographic hypodensities to predict hematoma expansion. Stroke. 2018;49(9):2067–73. https://doi.org/10.1161/STROKEAHA.118.022010. PMID: 30354976; PMCID: PMC6206864.
Rymer MM. Hemorrhagic stroke: intracerebral hemorrhage. Mo Med. 2011;108(1):50–4. PMID: 21462612; PMCID: PMC6188453.
Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-360. https://doi.org/10.1161/CIR.0000000000000350. Epub 2015 Dec 16. Erratum in: Circulation. 2016 Apr 12;133(15):e599. PMID: 26673558.
Acknowledgements
N/A
Funding
The research in this manuscript was not funded.
Author information
Authors and Affiliations
Contributions
ADW, ANG, and BM collected and managed the data. ADW and QL performed the initial statistical analyses. ADW and JNG developed the initial draft of the manuscript. KS, SMG, AV, CA, MEG, AP, and JNG advised on study design and project management. All authors reviewed and edited the manuscript for important intellectual content. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research in this manuscript adhered to ethical guidelines and the Massachusetts General Hospital IRB. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all participants for that data which consisted of more than standard-of-care clinical data obtained from medical records. The Massachusetts General Hospital IRB waived the need for informed consent for the obtaining of clinical care data from medical records.
Consent for publication
Not applicable.
Competing interests
Dr. Goldstein reports having received research and consulting support from CSL Behring, Octapharma, Takeda, Alexion, Pfizer, NControl, and Cayuga. Dr. Anderson receives sponsored research support from the National Institute of Health, American Heart Association, Massachusetts General Hospital, and Bayer AG and has consulted for ApoPharma, Inc. All research support and potential conflicts of interest have been disclosed. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Warren, A.D., Li, Q., Schwab, K. et al. External ventricular drain placement is associated with lower mortality after intracerebral hemorrhage with intraventricular hemorrhage. Int J Emerg Med 15, 51 (2022). https://doi.org/10.1186/s12245-022-00450-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12245-022-00450-4