Abstract
Background
Pilea umbrosa (Urticaceae) is used by local communities (district Abbotabad) for liver disorders, as anticancer, in rheumatism and in skin disorders.
Methods
Methanol extract of P. umbrosa (PUM) was investigated for the presence of polyphenolic constituents by HPLC-DAD analysis. PUM (150 mg/kg and 300 mg/kg) was administered on alternate days for eight weeks in rats exposed with carbon tetrachloride (CCl4). Serum analysis was performed for liver function tests while in liver tissues level of antioxidant enzymes and biochemical markers were also studied. In addition, semi quantitative estimation of antioxidant genes, endoplasmic reticulum (ER) induced stress markers, pro-inflammatory cytokines and fibrosis related genes were carried out on liver tissues by RT-PCR analysis. Liver tissues were also studied for histopathological injuries.
Results
Level of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), peroxidase (POD) and glutathione (GSH) decreased (p < 0.05) whereas level of thiobarbituric acid reactive substance (TBARS), H2O2 and nitrite increased in liver tissues of CCl4 treated rat. Likewise increase in the level of serum markers; alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and total bilirubin was observed. Moreover, CCl4 caused many fold increase in expression of ER stress markers; glucose regulated protein (GRP-78), x-box binding protein1-total (XBP-1 t), x-box binding protein1-unspliced (XBP-1 u) and x-box binding protein1-spliced (XBP-1 s). The level of inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) was aggregated whereas suppressed the level of antioxidant enzymes; γ-glutamylcysteine ligase (GCLC), protein disulfide isomerase (PDI) and nuclear erythroid 2 p45-related factor 2 (Nrf-2). Additionally, level of fibrosis markers; transforming growth factor-β (TGF-β), Smad-3 and collagen type 1 (Col1-α) increased with CCl4 induced liver toxicity. Histopathological scrutiny depicted damaged liver cells, neutrophils infiltration and dilated sinusoids in CCl4 intoxicated rats. PUM was enriched with rutin, catechin, caffeic acid and apigenin as evidenced by HPLC analysis. Simultaneous administration of PUM and CCl4 in rats retrieved the normal expression of these markers and prevented hepatic injuries.
Conclusion
Collectively these results suggest that PUM constituted of strong antioxidant chemicals and could be a potential therapeutic agent for stress related liver disorders.
Similar content being viewed by others
Background
Hepatic fibrosis is a pathological condition characterized by activation of hepatic stellate cells (HSCs), extracellular matrix (ECM) proteins accumulation and formation of pseudo lobules or nodules [1]. Due to its frequent recurrence, it affects the people all around the world and causes up to one million deaths in almost 184 countries [2, 3]. The clinical manifestation associated with chronic liver disease includes inflammation, infection, cirrhosis and metabolic disorders which arise as a consequence of exogenous chemicals, drugs and chemotherapeutic agents resulting in over production of reactive oxygen species (ROS). Therefore, if hepatic fibrosis left untreated at earlier stages, it can lead to irreversible cirrhosis or fulminant hepatic carcinoma [4,5,6]. Carbon tetrachloride (CCl4) has been extensively used in animal models to elucidate the mechanism of hepatic injury and for analyzing biomarkers discovery. It stimulates cytochrome P-450 enzyme (CYP) system in endoplasmic reticulum (ER) to metabolize into trichloromethyl radicals (•CCl3) and trichloromethyl peroxy radical (•CCl3O2). These radicals inaugurate lipid peroxidation and compel oxidative stress that ultimately causes liver cell damage. Furthermore, CCl4-induced liver toxicity is accompanied by elevation of inflammatory cytokines such as interleukin (IL-1β), IL-6, tumor necrosis factor (TNF-α), and chemokines e.g. monocyte chemo attractant protein-1 (MCP-1) which causes severe hepatocyte inflammation [7, 8].
Under transient conditions, both ROS and antioxidant defense systems work in an equal proportion. On the other hand, the chronic stress which causes augmentation of ROS in subcellular organelles, as in ER lumen, disturbs the process of protein folding and causes aggregation of unfolded or misfolded proteins, is referred as ER stress. In order to decrease the stress, cells eventually deploy the unfolded protein response (UPR) by restricting aggregation of unfolded protein over transient stress exposition. Activated UPR causes up-regulation of intra-ER chaperones mainly the glucose-regulated protein (GRP-78) associated with protein folding. It is released from ER and provokes three sensory cascades: inositol-requiring protein 1 alpha (IRE1-α), protein kinase RNA-like ER-associated kinase (PERK), and activating transcription factor (ATF-6). These sensory cascades re-establish homeostasis synergistically by promoting transcription of genes for protein folding and terminating general translation [8]. However, if underlying mechanism fails in adaptation of the cells towards ER stress, UPR leads to apoptosis of susceptible cells. Prolonged activation of UPR stimulates IRE1-α branch endoribonuclease activity which triggers cleavage of x box-binding protein-1 (XBP-1) mRNA to generate an effective transcription factor x-box binding protein1-spliced (XBP-1 s). It subsequently transcribes other proteins and causes ER-associated degradation thereby reducing ER stress [9]. Therefore, some recent reports suggested that significantly increased transcription level of GRP-78, XBP-1u and XBP-1 s can be considered as ER stress markers [10,11,12].
In response to liver fibrosis, activated HSCs and Kupffer cells participate in synthesis of ECMs such as collagen type-1 [13]. Alongside, damage cells release inflammatory cytokines and growth factors, which are involved in promoting HSCs and eventually aggravate liver fibrosis. Reports indicated that inflammatory cells and fibrosis activates the transforming growth factor-β (TGF-β)/ Smad signaling pathway. Upon binding of TGF-β with its transmembrane serine/threonine kinase type-I receptor (TβRI), Smad2 and Smad3 get phosphorylated which in turn activates Smad4. This complex is recruited towards the nucleus and binds with TGF-β-responsive genes promoter to encode genes for collagen type-1 and other ECM proteins. Therefore, evidence suggested that TGF-β1/Smad signaling is a potential target to prevent HSCs activation, which subsequently inhibits ECM synthesis and ameliorates hepatic fibrosis. Other factors which diminish ER stress are implicated in reducing hepatic fibrosis involve chaperones such as 4-PBA [14] and has been declared as anti-fibrotic agent, under the influence of UPR. Nevertheless, fibrosis triggered by the complex mechanism of UPR requires a deeper understanding of the mechanism that plays a role in activating HSCs that can contribute to evade fibrosis.
Compounds with antioxidant aptitude related to the prevention and treatment of oxidative damage induced by intracellular ROS level have been studied. Various taxa within the Urticaceae family have been explored for its medical potential against a wide array of diseases including cardiovascular, inflammatory, metabolic and respiratory disorders [15,16,17]. Among them some well-known plants such as Elatostema umbellatum, Pilea microphylla and Urtica dioica owing phenolic contents have been documented with antioxidant properties [18]. Pilea umbrosa Blume commonly known as Sikri booti is a perennial herb. It is 30–50 cm in height often covered with hairs. It is found at an altitude of 1200–2500 m in Himalayan forest of Pakistan. The plant has been reported for its therapeutic use in liver disorders, as anticancer and in rheumatism [19]. The plant is also used to cure skin diseases by traditional people. The current study has been disbursed to assess the protective potential of methanol extract of P. umbrosa on CCl4 induced hepatotoxicity in Sprague Dawley male rats. Therefore, the protecting proceeding and underlying mechanisms were evaluated, to assess the rationale of P. umbrosa in treating hepatic disorders and suggesting its clinical use.
Materials and methods
Plant material collection and identification
Fresh plant of Pilea umbrosa was collected from mountain top of Mushkpuri, Abbottabad district (7550 ft. altitude) of Pakistan in October 2017. The plant sample was recognized and validated by Dr. Syed Aneel Ahmad Gilani at Pakistan Museum of Natural History, Islamabad. The voucher specimen (#129917) was submitted at Herbarium of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan.
Extraction and isolation
The bulk of P. umbrosa plant was made free of dirt and dried for 9 days. Thereafter, it was crushed by Willy mill into fine powder (1 kg) of 80 mesh size. Methanol extract (PUM) using standard ratio of 1:4 was obtained twice by immersing in 95% of methanol for a week. The material was repeatedly shaken and mixed until all the constituents get dissolved in solvent. It is then filtered and concentrated under vacuum with rotary evaporator at (25 °C ± 5) to get PUM (50 g) and stored at 4 °C.
HPLC-DAD analysis of PUM
HPLC-DAD analysis of PUM was carried out with the help of Agilent technology-1200 series, Germany, equipped with UV detector and reverse phase analytical column Zorbex plus RSC8 (Agilent U.S.A); particle size 5 μm; capacity 25 ml for separation. The mobile phase composed entirely of elution solvent A as acetonitrile (5%); methanol (10%); water (85%); acetic acid (1%) and elution solvent B as acetonitrile (40%); methanol (60%); acetic acid (1%) following gradient program [20]. The flow rate was maintained at 1 ml/min keeping injection volume at 1 ml. Prior to analysis, samples solutions (10 mg/ml) were diluted by methanol (HPLC grade > 99.9%) (Sigma Aldrich) and filtered via 0.45 μm membrane filters. The column was renovated after every turn for 10 min. The standards; rutin and gallic acid were eluted at 257 nm, catechin at 279 nm, caffeic acid and apigenin at 325 nm, myricetin, quercetin and kaempferol eluted at 368 nm, respectively. The compounds were analyzed in triplicates and quantified at different wavelength using standard’s peaks.
Ethical statement
Animal studies were performed at primate facility after ethical approval (Bch# 0323) by review board of ethics committee of Quaid-i-Azam University, Islamabad, Pakistan. The scientific animal experimentation was performed according to the standard guidelines of National Institute of Health, Islamabad, Pakistan.
Acute toxicity study
In a preliminary study, the acute toxicity study was determined by using the procedure approved by [11]. Female rats (n = 5) were given 50 mg/kg body weight (bw) dose of PUM and were investigated for any deterioration and weight loss and no signs of distress were seen. Afterward, the procedure was aspired at different concentrations (100, 500, 1000, 1500 and 3000 mg/kg) of PUM to 5 female rats (for each respective dose) and left for 3 weeks to observe mortality. Since doses were well tolerated by rats, therefore one tenth (300 mg/kg bw) and one twentieth (150 mg/kg bw) of the highest dose was chosen for the appraisal of hepatoprotective effect of PUM. At the end of procedure, the rats administered with 1500 mg/kg and 3000 mg/kg were dissected in order to collect blood via cardiac puncture for hematological parameters.
Animal treatment
Forty-two male Sprague-Dawley rats (weighing 125 ± 5 g) acquired from primate facility of Quaid-i-Azam University were employed for experimentation. The rats were kept at ambient temperature (22 ± 2 °C) under 12 h-dark and l2 h-light cycles, relative humidity of (50% ± 5%) and retained under pathogen free condition to verify the absence of exclusion criteria. Rats had acquired access to standard rodent diet and at liberty to water, unless otherwise noted.
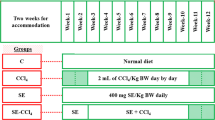
Experimental design
Before commencement of experimentation, 42 rats were divided into seven groups (six rats in each). The procedure was carried out on alternate days for 8 weeks (24 doses). Group I rats served as a control, received 1 ml/kg bw of 0.9% saline along with regular chow diet and kept under standard conditions. Next cohort of rats (Group II) was injected only with intra-peritoneal treatment of 1 ml/kg bw of CCl4 (Sigma-Aldrich) diluted with olive oil (CCl4: olive oil; 3:7 v/v) thrice a week on alternate days, to induce hepatotoxicity. Group III was treated with silymarin as a standard drug at 200 mg/kg bw concentration after 24 h of CCl4 intra-peritoneal injection to rat. Group IV and V were administered with (150 mg/kg bw and 300 mg/kg bw) PUM by gavage after 24 h of intra-peritoneal injection of CCl4 to rat. Whereas, Group VI and VII were treated alone with PUM extract at 150 mg/kg and 300 mg/kg bw dose, respectively on alternate days of 2 weeks. Each rat was observed for any visible sign of deterioration at different time points from dose administration. Following experiment completion, the treatment was discontinued for 3 days (substituted for standard chow). Thereafter, the rats were euthanized and sacrificed; their livers were removed, weighed and sectioned into two pieces. The first section was immediately snapped frozen at − 40 °C for molecular and enzymatic analysis, while other section was fixed with 5% formalin for histological examination. Total blood sample was collected for serum analysis.
Organ weight and body weight analysis
Rats in all the seven groups were precisely weighed at the beginning and after the completion of experimental procedure and percent increase of body weight was determined for each rat separately. Following dissection; liver was excised, washed with 0.9% saline and its absolute weight was determined. Likewise, relative weight of liver was calculated for each rat of individual group as liver weight/body weight × 100.
Biochemical parameters in serum
In order to evaluate hepatotoxicity serum was collected by spinning blood samples at low speed (3000×g for 20 min at 4 °C). Thereafter, level of ALT, AST, ALP, albumin and total bilirubin in serum were determined with an AMP Diagnostic kits (Krenngasse 8010 Graz, Austria) by using clinical biochemical analyzer (Beckman, USA), according to the standard protocol.
Biochemical parameters of liver
The frozen liver samples were vigorously homogenized in 1 mM EDTA and (100 mM) potassium phosphate buffer (pH = 7.4); it was retained for 20 min at 4 °C before centrifugation at 1500×g for getting supernatant (crude enzymes). Total protein in tissue was estimated following the standard procedure of [21]. Absorbance of reaction mixture was quantified by spectrophotometer at 595 nm. Bovine serum albumin (BSA) standard curve was generated to analyze the total protein from tissue homogenate.
Antioxidant enzyme status in liver
The activity of antioxidant enzymes such as CAT, POD, SOD and glutathione (GSH) was determined by standard procedure of [22,23,24,25], respectively. Absorbance was recorded against blank (same reagent without enzyme extracts) at respective wavelength for each assay. The amount of enzyme activities was estimated (1 unit/min) as decrease in absorbance value of 0.01/min of enzyme activity and presented as U/mg of protein for CAT, POD, SOD activity and μM GSH/ mg of protein for glutathione assay.
Measurement of lipid peroxidation (TBARS) assay
Level of thiobarbituric acid (TBARS) was determined by using the method of [26]. The activity was recorded as nM TBARS/min/mg tissue at 37 °C by applying molar extinction coefficient (1.56 × 105 M− 1 cm− 1).
Hydrogen peroxide assay (H2O2)
By the outline of H2O2–intermediate horseradish peroxidase supported oxidative reaction of phenol red with H2O2 assay was performed [27]. At the end of reaction, absorbance of supernatant was determined at 610 nm in comparison to blank reagent. H2O2 amount was recorded as nM H2O2/min/mg tissue, whereas oxidation of phenol red by H2O2 was taken as a standard curve.
Nitrite assay
For the measurement of nitric oxide metabolite Griess reagent based [28] methodology was used in this study. Following the reaction procedure, the absorbance of resultant mixture was measured at 540 nm. For defining the concentration of nitrite in tissue samples sodium nitrite curve was used.
RNA extraction
Total RNA was extracted using TRIzol reagent (Invitrogen) from adherent cells of hepatic tissues, as described else [29]. The resulting pellet was eluted in 50 μl of RNAse free water of molecular grade and preserved at − 40 °C. RNA was quantified by using Nano drop, integrity and purity of RNA was assessed by analyzing the absorption ratios 260/280 (~ 2.0) and 260/230 (2.0–2.2).
cDNA synthesis
Equimolar concentrations of RNA were successfully converted to cDNA using 2000 ng of total RNA by using Revert Aid First Strand cDNA Synthesis Kit. The incubation was performed in Thermal Gene Cycler (Biometra). The PCR conditions were adjusted as: 42 °C/60 min, 70 °C/5 min and 4 °C/5 min in Thermal Gene Cycler (Biometra). Besides, controls in the absence of reverse transcriptase and non-template were also employed to ensure fidelity of process. The primer sets of target genes, annealing temperatures and expected product size used in real-time PCR reactions are enlisted in Table 1.
Real-time PCR (RT-qPCR) assays
The mRNA expression analysis was performed with real time Thermal Cycler (iCycler, Bio-Rad) according to the manufacturer’s protocol. Briefly, the reaction mixture was prepared by using 2X Scientific Maxima SYBR Green/ROX qPCR master Mix, 1 nl cDNA and 1 nM primers. Real time PCR cycling parameters were adjusted as: 95 °C for 5 min, followed by 40 cycles of (95 °C/15 s; 55 °C/15 s and 72 °C/60 s) with heat-denaturing PCR products a terminal melt curve analysis was done over 35 °C gradient at 0.2 °C/s from 60 °C to 95 °C. To ensure PCR fidelity melting curve analysis and agarose gel electrophoresis was performed. Expression level of genes was estimated via internal normalization by beta actin as reference gene. Relative quantification of target gene was performed by using the 2−ΔΔCt method. Results were expressed as relative mRNA abundance over values obtained from control animals. The data was assembled with the aid of Microsoft Excel and statistically analyzed using REST software on ΔCts with the formulas: ΔCt = Ct_Mean (target) - Ct_Mean (control), ΔΔCt = ΔCt_treated- ΔCt_control.
Histopathology analysis
For histological analysis, paraffin embedded 5 μm thin sections of liver tissue were stained with hematoxylin and eosin (H&E). The slides were well examined to analyze the protective aptitude of PUM against CCl4 intoxicated liver and results were interpreted using the histological scoring system by adding the scores of steatosis (0–3), leukocyte infiltration (0–3), fatty degeneration (0–3). perivascular and lobular necrosis (0–3), cellular hypertrophy (0–3) and sinusoidal obstruction (0–3). Thorough examination of the slides was performed under compound microscope (DIALUX 20 EB) at 40X magnification and photographed with HDCE-50 B camera. Injuries induced with CCl4 in liver of rat and amelioration with extract were graded as; +/−; very minor, +; lower, ++/−; medium, ++; severe and +++; very severe grade.
Statistical analysis
Results are displayed for continuous variables as the means ± standard deviation on GraphPad Prism v.6.0. One-way analysis of variance (ANOVA) on Statistics 8.1 was performed for verification of differences among groups. For biochemical markers, results were analyzed using Microsoft Excel 2010. Tukey HSD Post hoc comparison among the various treatment groups was carried at P < 0.05.
Results
HPLC-DAD analysis of PUM
Eight analytes were subjected to HPLC-DAD method. PUM prototype compounds were identified based on the comparison of retention time and absorption spectra with those of standard (Fig. 1). HPLC spectrogram indicated that among 8 standards 4 compounds were verified as rutin (6.68 ± 0.04 μg/mg), catechin (1.95 ± 0.03 μg/mg), apigenin (0.302 ± 0.01 μg/mg) and caffeic acid (3.94 ± 0.015 μg/mg) in PUM. Result also showed that rutin was the most abundant compound, followed by caffeic acid and catechin, while apigenin was the least abundant compound.
Acute toxicity and hematological profile
PUM (4% obtained yield) wan administered at a dose of (150 mg/kg and 300 mg/kg bw) disclosed no obvious symptoms of toxicity and mortality in rats. The tested doses did not show any weight loss, physical or clinical changes in female rats. However, after being treated with single dose, plant extract significantly (P < 0.05) elevated (~ 2 folds) white blood cells (WBCs) at 1500 mg/kg bw and 3000 mg/kg bw treated rats (Table 2). Red blood cells (RBCs) showed non-significant differences (P > 0.05) among treated and control rats. However, hemoglobin (g/dl) level significantly (P < 0.05) increased at higher dose. Similarly, lymphocytes and mean corpuscular hemoglobin concentration (MCHC) showed substantial increase (~ 2–3 folds) in treated rats, whereas neutrophil count revealed non-significant differences (P > 0.05) among treated and control group.
Organ and body weight analysis
The effect of PUM on body weight as well as on absolute and relative weight of liver was determined and presented in Table 3. Upon treatment with CCl4, significant (P < 0.05) increase in the absolute and relative weight of liver was observed while with a notable decrease in body weight. By contrast, simultaneous administration of silymarin and CCl4 restored the liver and body weight towards the control rats. The administration of PUM (150 mg/kg and 300 mg/kg bw) in CCl4 treated rats significantly (P < 0.05) restored the body weight towards the control rats. In addition, co-administration of PUM normalized the absolute and relative weight of liver as compared to CCl4 treated group. Likewise, all rats treated with only PUM dose of 150 mg/kg bw and 300 mg/kg bw manifested a significant (P < 0.05) increment in body weight when compared with the control group.
Serum analysis of rat
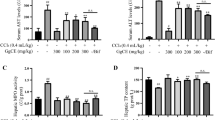
Compared to control group, treatment with CCl4 significantly (P < 0.05) increased the level of serum enzymes; ALP, ALT, AST and total bilirubin (Table 4). On the other hand, a notable decline in albumin concentration was observed with CCl4 administration to rat. Treatment with silymarin (200 mg/kg bw) significantly (P < 0.05) inhibited the CCl4 elevated level of serum markers, while raising the concentration of albumin protein. As expected, simultaneous administration of either low or high dose of PUM (150 mg/kg, 300 mg/kg bw) with CCl4 had shown a pronounced effect (P < 0.05) in decreasing the level of these serum enzymes, yet an increase in albumin level was also observed.
Antioxidant status in PUM-treated rats
Investigation on antioxidant status in the liver of treated rats is illustrated in (Fig. 2a-d). In CCl4 treated group, we noticed a tissue specific damage accompanied by significant (P < 0.05) decline in level of antioxidant enzymes such as CAT, SOD, POD and GSH. However, rats administered with standard drug silymarin along with CCl4 protected liver injury, as evidenced by significantly (P < 0.05) increased level of these antioxidants. Exposure with PUM at a dose of 150 mg/kg/300 mg/kg combined with CCl4, significantly (P < 0.05) raised their level towards the control rats. However, administration with either low or high dose of PUM (150 mg/kg bw, 300 mg/kg bw) exhibited slightly lower activity of these enzymes from control group.
Effect of PUM on antioxidant enzymes in rat liver. CAT; catalase activity, POD; peroxidase activity, SOD; superoxide dismutase activity, GSH; glutathione content. I; Control group, II; CCl4 (1 ml/ kg bw) treated group, III; CCl4 + Silymarin (200 mg/kg bw) treated group, IV; CCl4 + PUM (150 mg/kg bw) treated group, V; CCl4 + PUM (300 mg/kg bw) treated group, VI; PUM (150 mg/kg bw) treated group, VII; PUM (300 mg/ kg bw) treated group. Mean ± SD (n = 6). Bars with different alphabet letter indicate significance with each other at P < 0.05
Biochemical indices of liver in PUM-treated rats
From the Table 5, we found significant (P < 0.05) increase in the level of biochemical indices such as total protein, H2O2, nitrite and TBARS in the liver of CCl4-treated groups except the level of total protein. Administration of silymarin 200 mg/kg demonstrated a significant (P < 0.05) restoration in these biochemical markers by relieving hepatotoxicity induced by CCl4. A similar restoration trend was noticed when exposed with PUM at a dose of 300 mg/kg bw as compared to low dose (150 mg/kg bw), suggesting nearly similar effect of PUM to silymarin at maximum dose. Moreover, exposure to either dose of PUM alone did not affect the levels of examined biochemical markers across treated groups.
Effect of PUM extract on CCl4-induced hepatic ER stress
To explore the effect of PUM on CCl4-mediated hepatic ER stress, we detected the mRNA expression of hepatic ER stress related proteins. As revealed in (Fig. 3a-e), the mRNA level of GRP78, XBP-t, XBP1-u and XBP1-s PDI was decreased significantly (P < 0.05) in CCl4 intoxicated group compared with the control group. However, after treatment with silymarin along with CCl4, a significant (P < 0.05) increase was obtained in the level of ER stress related protein mRNA expressions. However, co-administration of PUM extract 300 mg/kg with CCl4 had shown considerably significant (P < 0.05) effect in alleviating the ER stress related protein mRNA expression. Furthermore, non-significant differences were found among both PUM doses tested at 150 mg/kg and 300 mg/kg bw on ER stress markers when compared with control or each other. These results indicated that PUM significantly (P < 0.05) decreased the level of ER stress markers upregulated in UPR pathway.
Graphical representation of different treatments of PUM on mRNA expressions of various genes involved in multiple pathways i.e., ER stress, inflammation and fibrosis. I; Control group, II; CCl4 (1 ml/ kg bw) treated group, III; CCl4 + Silymarin (200 mg/kg bw) treated group, IV; CCl4 + PUM (150 mg/kg bw) treated group, V; CCl4 + PUM (300 mg/kg bw) treated group, VI; PUM (150 mg/kg bw) treated group, VII; PUM (300 mg/ kg bw) treated group. PUM; Pilea umbrosa methanol extract. Mean ± SD (n = 3). Bars with different alphabet letter indicate significance with each other at P < 0.05
Effect of PUM on CCl4-induced hepatic inflammation and antioxidant enzymes
As shown in (Fig. 3f-h), the expression of hepatic inflammatory markers including cytokines TNF-α, IL-6 and chemokine MCP-1 in treated and control groups were determined. CCl4 treatment significantly (P < 0.05) up-regulated the mRNA level of TNF-α, IL-6 and MCP-1,when compared with the control group. Treatment with silymarin at 200 mg/kg bw along with CCl4 had significantly (P < 0.05) down regulated the mRNA level of these markers relative to the CCl4 group. Similarly, co-administration of PUM (300 mg/kg) with CCl4 had remarkably diminished the mRNA level of TNF-α, IL-6 and MCP-1 thereby reducing hepatic inflammation. Similarly, PUM treatment alone had not shown any significant effect (P > 0.05) in altering the level of these markers relative to the control group. In addition, the mRNA expression level of antioxidant enzymes GCLC and Nrf-2 was significantly (P < 0.05) reduced after CCl4 intoxication (Fig. 3). However, the effect of PUM at 300 mg/kg was found effective in increasing the expression of antioxidant enzymes regarding to CCl4 treated group.
Effect of PUM extract on CCl4-induced hepatic fibrosis
In order to investigate the effect of PUM on hepatic fibrosis, we detected the hepatic mRNA expressions of TGF-β, Smad-3 and Col1-α. As presented in (Fig. 3), the mRNA expression level of TGF-β, Smad-3 and Col1-α in CCl4 intoxicated group were significantly aggregated (P < 0.05) relevant to the control group. Simultaneous administration of silymarin at 200 mg/kg bw with CCl4 significantly reduced the expression level of these markers. Interestingly, PUM co-treatment at 300 mg/kg with CCl4 also significantly reduced the mRNA expression of these genes. It was observed that rats treated solely with PUM extract (150 mg/kg bw, 300 mg/ kg bw) had shown non-significant decrease on mRNA expression levels of fibrosis markers from control group indicating the hepatoprotective effect of PUM extract.
Histopathological analysis
Histopathological examination indicated normal liver morphology in control group (Fig. 4a). Liver section treated with CCl4 showed severe injuries including focal area of severe perivascular and lobular necrosis with neutrophil infiltration, cellular hypertrophy and sinusoidal obstruction (Fig. 4b). Co-treatment with silymarin recovered liver morphology and showed slight perivascular necrosis along with clear and distinct hepatocyte appearance (Fig. 4c). However, at 150 mg/kg bw of PUM extract, few non-patterned areas with mild necrosis, less neutrophil infiltration and steatosis were seen, but there were no extensive changes found on histological features of liver section treated with 300 mg/kg bw in CCl4 treated group (Fig. 4d & e). Treatment with PUM alone at either low or high dose had shown quite normal histological features as in control group (Fig. 4f & g). Thus, histological examination indicated the hepatoprotective effect of PUM with less necrosis and leukocyte infiltration.
Protective potential of PUM on liver histopathology of rats (40× magnification with hematoxylin-eosin stain). a; Control group, b; CCl4 (1 ml/kg bw) treated group, c; CCl4 + Silymarin (200 mg/kg bw) treated group, d; CCl4 + PUM (150 mg/kg bw) treated group, e; CCl4 + PUM (300 mg/kg bw) treated group, f; PUM (150 mg/kg bw) treated group, g; PUM (300 mg/kg bw) treated group, h; hepatic injuries ranges from 0 to 3. PUM; Pilea umbrosa methanol extract. Mean ± SD (n = 6). Bars with different alphabet letter indicate significance with each other at P < 0.05
Studies on markers of liver injuries
Different liver injuries obtained after CCl4 administration to rat and the ameliorative potential of PUM is shown in Table 6. Liver injuries such as steatosis, leukocyte infiltration, fatty degeneration, perivascular and lobular necrosis, cellular hypertrophy and sinusoidal obstruction were recorded in very severe range after CCl4 treatment to rat. Amelioration of CCl4-induced liver injuries in rat was recorded in a lower to medium range with co-treatment of silymarin to rat. On the other hand medium to severe grade and lower to medium grade liver injuries were recorded with co-administration of PUM (150 mg/kg bw and 300 mg/kg bw), respectively. However, different liver injuries in PUM (150 mg/kg bw) and PUM (300 mg/kg bw) alone treated rats were recorded in very minor grade or in lower grade.
Discussion
Exposure to xenobiotics provokes liver dysfunction which subsequently leads to inflammation, ER stress and induces fibrosis. In order to overcome these detrimental effects, certain natural antioxidants having scavenging activity have been reported recently showing beneficial effects on the human body [30, 31]. Plants rich in polyphenols and flavonoids exhibit a wide range of biological effects such as antioxidant, hepatoprotective, anticancer, anti-diabetic and anti-inflammation [32]. In this regard, we have analyzed the hepatoprotective effects of PUM plant against CCl4–induced damaging effects on the rat liver. HPLC analysis confirmed the presence of phytoconstituents in an appreciable amount, such as polyphenols and flavonoids compounds in PUM including rutin, catechin, caffeic acid and apigenin. These secondary metabolites have been reported in varieties of medicinal properties. Rutin revealed admirable hepatoprotective, antiplatelet, anti-inflammatory and antioxidant activities [33]. Catechin and caffeic acid showed protection against neurological disorders, inflammation and apoptosis due to anti-oxidant potential whereas for apigenin anti-inflammatory property was reported [34]. The presence of these secondary metabolites in admiring amount in PUM revealed its potential anti-oxidant and hepatoprotective effect.
The present study declared that based on hematological parameters, PUM is considered as non-toxic when examined on rat model and had not posed an adverse effect on count of WBC, RBC, MCV, HB, HCT and platelets at maximum dose of 3000 mg/kg. Moreover, mortality and any obvious symptoms of abnormal physical behavior also had not been observed. Hence, these findings are supported by the previous studies which were performed with methanol extract of other plant species [35].
Screening of liver and relative body weight serve as an indicator for the general health status of animals [36]. Data indicated that the group treated with CCl4 led to an increase in liver weight and decrease in ratio of liver/body weight compared to control. On the contrary, treatment with PUM along with CCl4 showed an increase in liver and body weight near to control in a dose dependent manner. Initially, we observed weight loss in rats exposed with CCl4 but after treatment with PUM both liver and body weight of rat was restored to normal.
Upon biotransformation, CCl4 converts into free radicals and causes hepatic injury or leakage in cell membrane which results in the discharge of liver enzymes into the blood stream. The serum level of AST, ALT, ALP, bilirubin and albumin that are used as marker for screening of liver function [37] were altered after treatment with CCl4 as compared to control. Whereas treatment with PUM to CCl4 intoxicated rats had dose dependently restored their level towards the control group. Therefore, these results confirmed that the PUM has significant preventive potential for hepatic injuries.
Antioxidant enzymes; CAT, POD and SOD or a molecule such as glutathione in liver tissue play important role against reactive oxygen species and show curative activity against liver inflammation [38]. These biochemical markers have the ability to scavenge superoxide anion and hydrogen peroxide thereby reduces their toxic effects. SOD protects hepatic cells by dismutation of superoxide radical into hydrogen peroxide and oxygen [39]. CAT neutralizes the toxicity of H2O2 and decomposes it into water and oxygen. Glutathione forms a disulfide bond with H2O2 and decreases its concentration. Altogether, these serum markers are associated with the first line of defense against free radical mediated stress [40]. In CCl4 treated group, we detected a drastic decrease in the level of these antioxidant enzymes which corresponds to disease severity. On the other hand, CCl4 generated free radicals might also be involved in inactivation of antioxidant enzymes [41]. In contrast, the group administered with PUM prior to CCl4 treatment revealed significant dramatic increase in antioxidant enzymes parallel to the amount raised in the silymarin treated group. We could demonstrate that these results confirm the significant effect of PUM in the protection of liver by increasing antioxidant enzymes to minimize inflammation. These results were in line with our serum markers findings.
Generally, CCl4 delivers chloride (Cl־) ion that reacts with poly unsaturated fatty acids and causes their peroxidation which eventually causes deformity in hepatocyte cell membrane. This shift generates highly reactive aldehydes (TBARS) an indicator of oxidative injuries, that later on triggers accumulation of ECM and fibroblast proliferation [42, 43]. Radicals of nitric oxide and H2O2 also damage HSCs and Kupffer cells implying lipid peroxidation thereby facilitating inflammation and fibrogenesis [44]. In the present study, except protein concentration the level of nitrite radical, TBARS and H2O2 were elevated in rat group challenged with CCl4. However, PUM treatment in combination with CCl4 effectively restored the level of these liver biochemical markers closer towards the control rats, thus prevented lipid peroxidation [45]. Therefore, based on these findings we can suggest that PUM has anti-inflammatory activity which is most likely corresponds to its high content of polyphenol and flavonoid such as rutin, caffeic acid and catechin. These findings are consistent with previously published study [46]. In addition, a growing body of reports have been emphasizing the hepatoprotective mechanism of various genera from CCl4 induced lipid peroxidation and cell damage [47].
In response to stress mediated by ROS, homeostasis of ER becomes dysfunctional, which triggers accumulation of unfolded proteins due to which ER prompts a protective response named as unfolded-protein response (UPR) intended to restore homeostasis. Recent studies have demonstrated that upon ER stress, UPR through its IRE1-α branch elicits cleavage of XBP-1, generating XBP-1 s transcription factor that eventually associated with ERAD and transcription of chaperones (associated with protein folding) such as protein disulfide isomerase (PDI). In prolonged stress if the damage is not repairable, ER provoked inflammatory and cell death pathways through activation of caspases [48, 49]. As evidenced by our previous study, methanol extract of PUM effectively enhanced liver protection activity with activation of GRP-78 and XBP-1 s of UPR1 pathway at molecular level. Therefore, we attempted to reveal the effect of PUM against ER stress. Findings indicated that consistent with above mentioned mechanism, CCl4 causes down regulation of GRP-78 and XBP-1 s and PDI, hence indicated ER stress, whereas upregulation of these markers after applying PUM confirmed the hepatoprotective activity of this plant via activation of IRE1-α branch of UPR pathway (Fig. 3a-e). Moreover stimulation of PERK branch of UPR phosphorylate nuclear erythroid 2 p45-related factor 2 (Nrf-2), a transcription factor for antioxidant enzymes such as glutathione-S-transferase has been implicated in this study [50]. In addition, an antioxidant enzyme regulator γ-glutamylcysteine ligase (GCLC) considered as a centralized enzyme regulator for glutathione synthesis has also been observed. Therefore, in CCl4 intoxicated group the level of Nrf-2 and GCLC were remarkably reduced when compared with the control group. However, PUM treatment reciprocated their level towards the control rat and suggesting that protective effect of PUM is critical because of its increased antioxidant enzyme level.
Besides, UPR has also been associated with release of pro-inflammatory cytokines through IRE1-α mediated activation of nuclear factor kappa B (NF-кB) and c-jun N-terminal kinase (JNK) pathways. In addition upon chronic liver injury, Kupffer cells excessively release pro-inflammatory cytokines (IL-6 and TNF-α) that subsequently activate HSCs to produce MCP-1 (chemokine) which aggregate inflammation [31]. Upon CCl4 treatment, we observed an increase in the level of IL-6, TNF-α and MCP-1. Treatment with PUM has tremendous effect to forestall the level of these inflammatory mediators. These results confirmed the anti-inflammatory activity of PUM which is consistent with our previous finding [10].
Next, we attempted to analyze the role of PUM in evading hepatic fibrosis. TGF-β/Smad signaling pathway causes liver fibrosis by hyper activation of TGF-β, Smad-3 and Col1-α [51]. A previous report showed inhibition of fibrosis by deactivation of HSCs over TGF-β/Smad interference [14]. These results indicated that PUM extract showed anti-fibrotic potential by suppressing the mRNA expression of TGF-β, Smad-3 and Col1-α. Current study hence revealed that PUM is considerably resilient to fibrosis by inhibiting TGB-β/Smad pathway.
Histopathology examination of liver revealed the non-toxic property of PUM in control group depicting normal hepatocytes architecture whereas the photomicrograph of CCl4 intoxicated group showed liver injury with necrosis, massive fibrosis, neutrophils infiltration and vacuolar degeneration. The high dose of PUM (300 mg/kg) and silymarin treatment depicted symmetrical arrangement of liver sinusoids along with normal hepatocytes morphology. Furthermore, we observed decrease in inflammation and reduction in dilation of central vein with normal cellular morphology which might be due to rapid regenerative property of PUM in a dose dependent manner (Karp, 2009). This finding revealed the nearly similar efficacy of high dose of PUM to silymarin standard drug. In addition, the non-toxic nature was also confirmed for PUM by microscopic examination of the group treated with PUM solely. This examination also illustrated that PUM does not cause hypertrophy or alteration in cell structure.
Conclusion
Taken together, our study illustrated the existence of a high amount of polyphenolic compounds in PUM. Our studies confirmed the anti-inflammatory, anti-fibrosis and hepatoprotective activity of PUM at biochemical and molecular level, which could be explained on the basis of an appreciable amount of flavonoids, phenolics and other antioxidants present in PUM extract. Therefore, we conclude that administration of defined concentration of PUM extract can reduce liver ailments. Future studies on PUM extract at low to medium concentration (150 mg/kg bw, 300 mg/kg bw) to be used as potential therapeutic agent for liver diseases and isolation of active compounds involved in the above mentioned activities are highly needed.
Availability of data and materials
The data may be available on request.
Abbreviations
- PUM:
-
Methanol extract of Pilea umbrosa
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- ALP:
-
Alkaline phosphatase
- MCV:
-
Mean corpuscular volume
- MCHC:
-
Mean corpuscular hemoglobin concentration
- MCH:
-
Mean corpuscular hemoglobin test
- TLC:
-
Total leukocyte count
- PLT:
-
Platelet count
- ER:
-
Endoplasmic reticulum
- (GRP-78):
-
Glucose regulated protein
- XBP-1 t:
-
x-box binding protein1-total
- XBP-1 u:
-
x-box binding protein1-unspliced
- XBP-1 s:
-
x-box binding protein1-spliced
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- MCP-1:
-
Monocyte chemoattractant protein-1
- GCLC:
-
γ-glutamylcysteine ligase
- PDI:
-
Protein disulfide isomerase
- Nrf-2:
-
Nuclear erythroid 2 p45-related factor 2
- TGF-β:
-
Transforming growth factor-β
- Col1-α:
-
Collagen type 1
References
Asselah T, Marcellin P, Bedossa P. Improving performance of liver biopsy in fibrosis assessment. J Hepatol. 2014;61:193–5.
Mokdad A, Lopez A, Shahraz S, Lozano R, Mokdad A, Stanaway J, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145 Available from: http://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-014-0145-y.
Akcora B, Storm G, Bansal R. Inhibition of canonical WNT signaling pathway by β-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12. Biochim Biophys Acta (BBA)-Molecular Basis Dis. 2018;1864:804–18 Available from: https://www.sciencedirect.com/science/article/pii/S0925443917304507.
Wynn T, Ramalingam T. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028 Available from: https://www.nature.com/nm/journal/v18/n7/abs/nm.2807.html.
Birbrair A, Zhang T, Files D, Mannava S, Smith T, Wang Z, et al. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther. 2014;5:122 Available from: http://stemcellres.com/content/5/6/122.
Wong M, Jiang J, Goggins W, Liang M, Fang Y, Fung F, et al. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017;7:45846 Available from: https://www.nature.com/articles/srep45846.
Hirschfield G, Dyson G, Alexander J, Chapman M, Collier J, Hubscher S, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568–94 Available from: http://gut.bmj.com/lookup/doi/10.1136/gutjnl-2017-315259.
Anand KV, Anandhi R, Pakkiyaraj M, Geraldine P. Protective effect of chrysin on carbon tetrachloride (CCl 4 )—induced tissue injury in male Wistar rats. Toxicol Ind Health. 2011;27:923–33 Available from: http://journals.sagepub.com/doi/10.1177/0748233711399324.
Leng Y-P, Qiu N, Fang W, Zhang M, He Z-M, Xiong Y. Involvement of Increased Endogenous Asymmetric Dimethylarginine in the Hepatic Endoplasmic Reticulum Stress of Type 2 Diabetic Rats. PLoS One. 2014;9:97125 Available from: http://dx.plos.org/10.1371/journal.pone.0097125.
Batool R, Khan M, Zai J, Ali S, Maryam S, Naz I, et al. Brachychiton populneus (Schott & Endl.) r.Br. Ameliorate carbon tetrachloride induced oxidative stress through regulation of endoplasmic reticulum stress markers and inflammatory mediators in Sprague-Dawley male rats. Biomed Pharmacother. 2018;107:1601–10.
Zai JA, Khan MR, Mughal ZUN, Batool R, Naz I, Maryam S, et al. Methanol extract of: Iphiona aucheri ameliorates CCl4 induced hepatic injuries by regulation of genes in rats. Toxicol Res (Camb). Royal Society Chemistry. 2019;8:815–32 [cited 2020 Jun 13] Available from: https://academic.oup.com/toxres/article/8/6/815/5709551.
Ustuner D, Kolac UK, Ustuner MC, Tanrikut C, Ozdemir Koroglu Z, Burukoglu Donmez D, et al. Naringenin Ameliorate Carbon Tetrachloride-Induced Hepatic Damage Through Inhibition of Endoplasmic Reticulum Stress and Autophagy in Rats. J Med Food. Mary Ann Liebert Inc. 2020; [cited 2020 Jun 13];jmf.2019.0265. Available from: https://www.liebertpub.com/doi/10.1089/jmf.2019.0265.
de Galarreta M, Navarro A, Ansorena E, Garzon A, Modol T, Lopez-Zabalza M, et al. Unfolded protein response induced by Brefeldin A increases collagen type I levels in hepatic stellate cells through an IRE1α, p38 MAPK and Smad-dependent pathway. Biochim Biophys Acta (BBA)-Molecular Cell Res. 2016;1863:2115–23 Available from: https://www.sciencedirect.com/science/article/pii/S0167488916301306.
Jiang Y, Wang C, Li Y, Wang X, An J, Wang Y. Mistletoe alkaloid fractions alleviates carbon tetrachloride-induced liver fibrosis through inhibition of hepatic stellate cell activation via TGF-β/Smad interference. J Ethnopharmacol. 2014;158:230–8 Available from: https://www.sciencedirect.com/science/article/pii/S0378874114007387.
Momo CE, Oben J, Tazoo D, Dongo E. Antidiabetic and hypolipidaemic effects of a methanol/methylene-chloride extract of Laportea ovalifolia (Urticaceae), measured in rats with alloxan-induced diabetes. Ann Trop Med Parasitol. 2006;100:69–74.
Luo X, Li L, Zhang S, Lu JL, Zeng Y, Zhang H, et al. Therapeutic effects of total coumarins from Urtica dentata hand on collagen-induced arthritis in Balb/c mice. J Ethnopharmacol. 2011;138:523–9.
Ngugi C, Oyoo-Okoth E, Mugo-Bundi J, Orina P, Chemoiwa E, Aloo P. Effects of dietary administration of stinging nettle (Urtica dioica) on the growth performance, biochemical, hematological and immunological parameters in juvenile and adult Victoria Labeo (Labeo victorianus) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. Elsevier Ltd. 2015;44:533–41. Available from:. https://doi.org/10.1016/j.fsi.2015.03.025.
Chahardehi A, Ibrahim D, Sulaiman S. Antioxidant activity and total phenolic content of some medicinal plants in Urticaceae family. J Appl Biol Sci. 2009;2:25–9.
Oudhia P. Useful biodiversity and traditional healing links from Pankaj Oudhia’s medicinal plant Database-24 - səhifə 87; 2016.
Araujo Leon J-A, Ruiz Ciau D-V, Coral Martinez T-I, Cantillo Ciau Z-O. Comparative fingerprint analyses of extracts from the root bark of wild Hippocratea excelsa and “Cancerina” by high-performance liquid chromatography. J Sep Sci. 2015;38:3870–5. Available from:. https://doi.org/10.1002/jssc.201401480.
Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75 Available from: https://www.cabdirect.org/cabdirect/abstract/19511404458.
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6 Available from: https://www.sciencedirect.com/science/article/pii/S0076687984050163.
Tan T, Cheng L, Bhat R, Rusul G, Easa A. Composition, physicochemical properties and thermal inactivation kinetics of polyphenol oxidase and peroxidase from coconut (Cocos nucifera) water obtained from immature, mature and overly-mature coconut. Food Chem. 2014;142:121–8 Available from: https://www.sciencedirect.com/science/article/pii/S0308814613009680.
Kakkar P, Das B, Viswanathan P. A modified spectrophotometric assay of superoxide dismutase. 1984; Available from: http://nopr.niscair.res.in/handle/123456789/19932.
Liu J, Gao S, Luo G, Yan G, Shen J. Artificial imitation of glutathione peroxidase with 6-selenium-bridged β-cyclodextrin. Biochem Biophys Res Commun. 1998;247:397–400 Available from: https://www.sciencedirect.com/science/article/pii/S0006291X9898545X.
Iqbal M, Wright D. Host resistance to insecticides can confer protection to endo-larval parasitoids. Bull Entomol Res. 1996;86:721–3 Available from: https://www.cambridge.org/core/product/identifier/S0007485300039249/type/journal_article.
Pick E, Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytic stimuli. Cell Immunol. 1981;59:301–18 Available from: https://www.sciencedirect.com/science/article/abs/pii/0008874981904111.
Grisham M, Johnson G, Lancaster J. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268:237–46 Available from: https://www.sciencedirect.com/science/article/pii/S0076687996680264.
Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–5 Available from: http://www.nature.com/doifinder/10.1038/nprot.2006.83.
Shin S, Cho I, Kim S. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3β inhibition downstream of poly(ADP-ribose) polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–95 Available from: http://molpharm.aspetjournals.org/content/76/4/884.short.
Dong GZ, Jang EJ, Kang SH, Cho IJ, Park SD, Kim SC, et al. Red ginseng abrogates oxidative stress via mitochondria protection mediated by LKB1-AMPK pathway. BMC Complement Altern Med. 2013;13:64 Available from: http://bmccomplementalternmed.biomedcentral.com/articles/10.1186/1472-6882-13-64.
Apostolou A, Stagos D, Galitsiou E, Spyrou A, Haroutounian S, Portesis N, et al. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem Toxicol. 2013;61:60–8 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23380202.
Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT-Food Sci Technol. 2008;41:1060–6.
Jain A, Manghani C, Kohli S, Nigam D, Rani V. Tea and human health: the dark shadows. Toxicol Lett. 2013;220:82–7 Available from: https://www.sciencedirect.com/science/article/pii/S0378427413001653.
Slichter S. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfus Med Rev. 2004;18:153–67 Available from: https://www.sciencedirect.com/science/article/pii/S0887796304000197.
Hilaly E, Israili Z, Lyoussi B. Acute and chronic toxicological studies of Ajuga Iva in experimental animals. J Ethnopharmacol. 2004;91:43–50 Available from: https://www.sciencedirect.com/science/article/pii/S0378874103004185.
Yachi R, Igarashi O, Kiyose C. Protective effects of vitamin E analogs against carbon tetrachloride-induced fatty liver in rats. J Clin Biochem Nutr. 2010:1008040046 Available from: https://www.jstage.jst.go.jp/article/jcbn/advpub/0/advpub_10-35/_article/-char/ja/.
Sutti S, Bruzzi S, Albano E. The role of immune mechanisms in alcoholic and nonalcoholic steatohepatitis: a 2015 update. Expert Rev Gastroenterol Hepatol. 2016;10:243–53 Available from: http://www.tandfonline.com/doi/full/10.1586/17474124.2016.1111758.
Karbownik M, Tan D, Reiter R. Melatonin reduces the oxidation of nuclear DNA and membrane lipids induced by the carcinogen δ-aminolevulinic acid. Int J Cancer. 2000;88:7–11 Available from: http://doi.wiley.com/10.1002/1097-0215%2820001001%2988%3A1%3C7%3A%3AAID-IJC2%3E3.0.CO%3B2-T.
Timmerman K. Molecular characterization of corn glutathione S-transferase isozymes involved in herbicide detoxication. Physiol Plant. 1989;77:465–71. Available from:. https://doi.org/10.1111/j.1399-3054.1989.tb05668.x.
Yang J, Schmelzer K, Georgi K, Hammock B. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray oonization tandem mass spectrometry. Anal Chem. 2009;81:8085–93. Available from:. https://doi.org/10.1021/ac901282n.
Jang H, Park M, Kim H, Lee Y, Hwang K, Park J, et al. Black rice (Oryza sativa L.) extract attenuates hepatic steatosis in C57BL/6 J mice fed a high-fat diet via fatty acid oxidation. Nutr Metab (Lond). 2012;9:27 Available from: http://nutritionandmetabolism.biomedcentral.com/articles/10.1186/1743-7075-9-27.
Shenoy K, Somayaji S, Bairy K. Hepatoprotective effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Indian J Pharmacol. 2001;33:260–6 Available from: http://medind.nic.in/ibi/t01/i4/ibit01i4p260.pdf.
Sahreen S, Khan M, Khan R, Alkreathy H. Protective effects of Carissa opaca fruits against CCl4-induced oxidative kidney lipid peroxidation and trauma in rat. Food Nutr Res. 2015;59:28438 Available from: http://foodandnutritionresearch.net/index.php/fnr/article/view/801.
Fusco D, Colloca G, Rita M, Monaco L, Cesari M. Effects of antioxidant supplementation on the aging process. Clin Interv Aging. 2007;2:377–87 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2685276/pdf/cia-2-377.pdf.
Ali S, Khan MR, Shah SA, Batool R, Maryam S, Majid M, et al. Protective aptitude of Periploca hydaspidis Falc against CCl 4 induced hepatotoxicity in experimental rats. Biomed Pharmacother. 2018;105:1117–32.
Harris T, Bettaieb A, Kodani S, Dong H, Myers R, Chiamvimonvat N, et al. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. Elsevier Inc. 2015;286:102–11. Available from:. https://doi.org/10.1016/j.taap.2015.03.022.
Cheng Y, Yang JM. Survival and death of endoplasmic-reticulum-stressed cells: Role of autophagy. World J Biol Chem. 2011;2:226 Available from: http://www.wjgnet.com/1949-8454/full/v2/i10/226.htm.
Benbrook D, Long A. Integration of autography, proteasomal degradation, unfolded protein responce and apoptosis. Exp Oncol. 2012;34:286–97 Available from: http://dspace.nbuv.gov.ua/handle/123456789/139052.
Cullinan S, Zhang D, Hannink M, Arvisais E, Kaufman R, Diehl J. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–209 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC230321/pdf/0593.pdf.
Xiao J, Ho C, Liong E, Nanji A, Leung T, Lau TY, et al. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 2014;53:187–99 Available from: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s00394-013-0516-8&casa_token=GoBUhtxMfAsAAAAA:eu5myIAJSElkF9zxpRxD23SvsnsW3go0DUi9XZvn-CnHxux7uicgljgYfcc4F2UOXpScYet6HjZCoJQ0.
Acknowledgements
The authors acknowledge the Faculty of Biological Sciences, Quaid-i-Azam University Islamabad for providing the research facilities.
Funding
The project was funded by the Department of Biochemistry Quaid-i-Azam University Islamabad Pakistan.
Author information
Authors and Affiliations
Contributions
Irum Naz, Jawaid Ahmed Zai, Riffat Batool, Zartash Zahra and Aemin Tahir contributed in the experimentation and data acquisition. Irum Naz writes the manuscript. Muhammad Rashid Khan designs the experiment and edited the manuscript. The authors assign Muhammad Rashid Khan for correspondence and publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal studies were performed at primate facility after ethical approval (Bch#362) by review board of ethics committee of Quaid-i-Azam University, Islamabad, Pakistan. Consent to participate is not applicable.
Consent for publication
All the authors agreed for publication of this manuscript and its distribution according to the policy of the journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Naz, I., Khan, M.R., Zai, J.A. et al. Pilea umbrosa ameliorate CCl4 induced hepatic injuries by regulating endoplasmic reticulum stress, pro-inflammatory and fibrosis genes in rat. Environ Health Prev Med 25, 53 (2020). https://doi.org/10.1186/s12199-020-00893-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12199-020-00893-2