Abstract
Background
New daily persistent headache (NDPH) is a rare primary headache disorder characterized by daily and persistent sudden onset headaches. The pathogenesis of NDPH remains unclear, and there are few white matter imaging studies related to NDPH. The purpose of this study was to investigate the micro-structural abnormalities of white matter in NDPH and provided insights into the pathogenesis of this disease based on tract-based spatial statistics (TBSS).
Methods
Twenty-one patients with NDPH and 25 healthy controls (HCs) were included in this study. T1 structural and diffusion magnetic resonance imaging (MRI) were acquired from all participants. Differences in the fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) between patients with NDPH and HCs were investigated using TBSS analysis.
Results
Significantly decreased FA, increased MD and RD were found in patients with NDPH compared to HCs. White matter regions overlaid with decreased FA, increased MD and RD were found in 16 white matter tracts from the Johns Hopkins University ICBM-DTI-81 White-Matter Atlas and Johns Hopkins University White-Matter Tractography Atlas. Specifically, these white matter regions included the right anterior thalamic radiation (ATR), body of the corpus callosum (BCC), bilateral cingulum, left hippocampal cingulum (CGH), left corticospinal tract (CST), forceps major, fornix, left inferior fronto-occipital fasciculus (IFOF), bilateral inferior longitudinal fasciculus (ILF), left posterior limb of the internal capsule (PLIC), right retrolenticular part of the internal capsule (RPIC), splenium of the corpus callosum (SCC), right superior longitudinal fasciculus (SLF) and left uncinate fasciculus (UF). After Bonferroni correction, there were no correlations between the FA, MD, AD and RD values and the clinical characteristics of patients with NDPH (p > 0.05/96).
Conclusion
The results of our research indicated that patients with NDPH might have widespread abnormalities in the white matter of the brain.
Similar content being viewed by others
Background
New daily persistent headache (NDPH) is a rare headache disorder characterized by daily headaches that become unremitting soon after onset, typically in individuals with no previous history of headache [1, 2]. Patients with NDPH can recall and accurately describe the exact time of headache onset [3]. According to previous epidemiological studies, the prevalence of NDPH is 0.03 to 0.1% in the general population [4, 5]. The underlying pathogenesis of NDPH remains unclear [6]. In recent years, neuroimaging studies have been increasingly applied to primary headache [6,7,8,9,10] showing, specifically in migraine, widespread white matter abnormalities [11,12,13,14]. Contrariwise, there have been relatively few neuroimaging studies of NDPH, suggesting a lack of association with infarct-like lesions or white matter abnormalities in these patients investigated by conventional magnetic resonance imaging (MRI) [15]. However, no relevant research has been carried out to determine whether there are changes in the white matter micro-structure in patients with NDPH.
The diffusion tensor imaging (DTI) is an MRI-based technique that measures the micro-structural integrity of white matter fiber bundles, making it an effective tool able to identify subtle tissue changes that affect the structural connectivity integrity of the brain and the interregional transmission of information [16, 17]. The four main metrics evaluated using DTI are fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) [18]. FA measures the directionality and coherence of water diffusion, reflecting fiber density, axonal diameter, and myelination in white matter. MD measures the average molecular motion, or diffusion, within a voxel, and it can increase with tissue damage, reflecting a potential decrease in barriers to diffusion. RD and AD are measures of diffusivity perpendicular and parallel to the fibers, respectively, providing insights into myelin and axon integrity [19]. The tract-based spatial statistics (TBSS) is a spatial statistical analysis method based on the white matter skeleton [20]. By constructing an average FA skeleton map of the images and projecting all FA of the subjects onto this skeleton map, the FA values of each voxel on the skeleton map is very close to the FA values of the nearest white matter fiber bundle center. TBSS can fully reflect the subtle structural changes in white matter, which has become an accurate and widely used method for exploring white matter fiber tracts [21].
DTI data analysis technology based on TBSS has been widely used in central nervous system diseases in the past decades, and can effectively assess the micro-structural integrity of white matter [16, 22,23,24]. In this study, we hypothesized that: 1) patients with NDPH have significant white matter micro-structure changes compared with healthy controls (HCs); 2) diffusivity metrics of the white matter are significantly correlated with disease characteristics in patients with NDPH.
Methods
Participants
An observational study with a cross-sectional design was conducted. Fifty-four participants, including 24 patients with NDPH and 30 HCs, were consecutively enrolled. Between October 2020 and October 2022, a total of 24 patients with NDPH were recruited from the headache outpatient unit at Beijing Tiantan Hospital (Capital Medical University). The inclusion criteria for patients with NDPH were as follows: 1) NDPH diagnosis based on the International Classification of Headache Diseases, 3rd edition (ICHD-3) [1]; 2) 14–60 years of age; 3) feasibility of MRI scan; 4) without preventive treatment for at least 3 months; and 5) without the history of excessive use of acute treatment drugs. The general exclusion criteria for patients with NDPH and HCs were as follows: 1) combined with other types of primary headache and pain disorders; 2) pregnancy or breastfeeding; 3) combined with other neurological, cardio-cerebrovascular, and endocrine system diseases; 4) any drug or alcohol abuse history; 5) first degree relative with headaches;6) poor quality of MRI data (significant susceptibility artifact or incomplete raw MRI data); and 7) significant brain lesions or white matter hyperintensities (Fazekas score > 1, especially at the level of the lateral ventricular body).
Demographic data and neuropsychological tests
Demographics, body mass index (BMI), headache disease duration (years), Visual Analogue Scale (VAS), Patient Health Questionnaire-9 (PHQ-9) scores, Headache Impact Test-6 (HIT-6) scores, Generalized Anxiety Disorder-7 (GAD-7) scores and Pittsburgh Sleep Quality Index (PSQI) scores were collected in patients with NDPH. Anxiety and depression symptoms were measured by the PHQ-9 and the GAD-7, respectively. The PSQI was a useful tool to evaluate the quality and patterns of sleep. The HIT-6 was also used to assess impact intensity of headache. The score of PHQ-9 was commonly used to screen for depression with a recommended cut-off score of 10 [25]. The scores of 10 or higher on the GAD-7 indicated generalized anxiety disorder [26]. The PSQI score ≥ 7 was defined as poor quality of sleep [27].
This was a sub-study of the ongoing China HeadAche DIsorders RegiStry Study (CHAIRS, unique identifier: NCT05334927) and was approved by the local ethics committee of Beijing Tiantan Hospital, Capital Medical University (number: KY2022-044). Informed consent was obtained from each participant before their participation, in accordance with the principles of the Declaration of Helsinki.
MRI acquisition
The GE 3.0 Tesla MR scanner (Signa Premier, GE Healthcare) with a 48-channel head coil was used at the National Neurological Center of Beijing Tiantan Hospital to acquire 3D T1 structural and diffusion MRI data. The participants were instructed to remain motionless with their eyes closed during the MRI acquisition. T1 structural images were acquired using the following parameters: MP-RAGE sequence, preparation time = 880 ms, recovery time = 400 ms, acceleration factor = 2, acquisition time = 4:00, field of view = 250 × 250 mm2, flip angle = 8°, slices = 192, and 1 × 1 × 1mm3 of spatial resolution. The following DTI parameters were used: repetition time = 5285 ms, echo time = 85 ms, data matrix = 104 × 104, field of view = 208 × 208 mm2, slice thickness = 2 mm, slices = 78, gradient direction = 108, and diffusion sensitivity coefficients (b) = 0, 1000, and 2000s/mm2.
MRI data processing and analysis
Before pre-processing, two experienced neuroradiologists visually inspected the DTI images to screen for noise artifacts. The image data were analyzed using the FMRIB software library (FSL, version 4.1.8; http://www.fmrib.ox.ac.uk/fsl). The dcm2niigui was used to convert the DICOM format of all the diffused and T1-weighted data into the NIFTI format. With the topup tool, distortions due to susceptibility were corrected by using anterior-to-posterior and posterior-to-anterior phase-encoding directions. Head motion and eddy current distortions were correct by eddy_openmp command. Brain masks from the b0 image of each participant were created using FSL's BET (Brain Extraction Tool). Based on a diffusion tensor model, the FSL toolbox DTIFIT was used to fit the pre-processed image to generate the FA, MD, AD, and RD values. The different measures of the white matter micro-structure were extracted from diffusion images. The FA is a summary measure of micro-structural integrity of white matter. Diffusivity perpendicular to the axonal fibers, as measured by the RD, is strongly correlated with dysmyelination and demyelination processes. Diffusivity parallel to the axonal fibers is calculated by AD and the MD represents average diffusivity of molecular motion.
With the nonlinear registration tool of FNIRT (FMRIB’s Nonlinear Registration Tool), the FA, MD, AD, and RD images of each participant were registered to the FMRIB58_FA template in the Montreal Neurological Institute (MNI) space. By using FA threshold value of 0.2, a mean FA image was calculated from all the participants’ images and was thinned to create a mean FA skeleton. Each participant’s aligned FA map was projected onto the skeleton using the maximum FA values detected in the direction surrounding the tract. Finally, the AD, RD and MD skeleton were created by using the projection parameters obtained in the previous steps. Because TBSS only analyzed the voxels closest to the white matter fiber skeleton, it can effectively reduce statistical errors caused by imperfect registration. In addition, the non-parametric test was used in the statistical analysis, which did not require image smoothing; thus, the partial volume problem had been reduced.
Statistical analysis
The sample size was based on a cross-sectional study design and the FA values of the white matter tracts in the NDPH and HC groups. A sample size of 46 (25 HCs and 21 patients with NDPH) achieved 86% power to reject the null hypothesis of equal means when the mean difference was 0.025, with a standard deviation for both groups of 0.027 at a two-sided alpha of 0.05. Stata 16.0 software (StataCorp LLC, TX, USA) was used for all clinical data analyses. Age, BMI, and other continuous variables were reported as mean ± standard deviation (SD) or median (interquartile range) and analyzed using independent sample t-tests or Mann–Whitney tests, respectively. Differences between genders were compared using the chi-square test. Statistical significance was set at p < 0.05. We executed voxel-wise cross-subject statistics of two-sample t-tests between patients with NDPH and HCs, which was carried out by FSL Randomize tool (version 2.1) using 5,000 permutations with the threshold-free cluster enhancement (TFCE) option. Although age and gender did not show significant differences between groups, they were added as covariates to minimize their potential effects. Multiple comparison correction was carried out with the family wise error (FWE) method applying the TFCE option in the FSL permutation-based inference tool by nonparametric statistics called “randomise”. Statistical maps were set at p < 0.05 (two-sided, FWE corrected) with a cluster size of > 100 voxels [13]. Pearson’s correlation analysis was conducted to examine correlations between FA, MD, AD, and RD values and the clinical characteristics (disease duration of NDPH [years], VAS scores, HIT-6 scores, PHQ-9 scores, GAD-7 scores, and PSQI scores). The level of statistical significance was set at p < 0.05/N (Bonferroni-corrected for multiple comparisons; N corresponds to the number of correlation analyses tested).
Results
Demographics and clinical characteristics
Demographic and clinical data of the enrolled participants were presented in Table 1. We recruited 54 participants with 24 patients with NDPH and 30 HCs in our initial cohort. Three participants with NDPH were excluded owing to the poor quality of the MRI data (n = 1) and existing white matter hyperintensities (n = 2). Five HCs were also excluded because of the poor quality of the MRI data (n = 3) and existing white matter hyperintensities (n = 2) (Fig. 1). Finally, a total of 46 participants (21 patients with NDPH and 25 HCs) were included, and no differences in age, gender ratio, or BMI were found between the two groups (Table 1).
TBSS analysis of FA
Compared to HCs, the NDPH group showed significantly decreased FA in several white matter tracts (P FWE < 0.05). These white matter tracts included the bilateral corona radiata (CR), left anterior limb of the internal capsule (ALIC), bilateral anterior thalamic radiation (ATR), corpus callosum (CC), bilateral cingulum, bilateral external capsule (EC), fornix, forceps minor, bilateral inferior fronto-occipital fasciculus (IFOF), bilateral inferior longitudinal fasciculus (ILF), bilateral posterior thalamic radiation (PTR), bilateral retrolenticular part of the internal capsule (RPIC), and bilateral superior longitudinal fasciculus (SLF) (Fig. 2) (P FWE < 0.05).
TBSS analysis of MD
Compared to HCs, patients with NDPH presented with significantly higher MD in several white matter tracts. These white matter tracts included the bilateral CR, bilateral ALIC, bilateral ATR, CC, bilateral cerebral peduncle (CP), bilateral corticospinal tract (CST), cingulum, bilateral EC, forceps minor, forceps major, fornix, bilateral IFOF, bilateral ILF, bilateral posterior limb of the internal capsule (PLIC), right PTR, right RPIC, bilateral SLF, and bilateral uncinate fasciculus (UF) (Fig. 3) (P FWE < 0.05).
TBSS analysis of RD
Compared to the HCs, patients in the NDPH group exhibited higher RD in several brain regions. These regions included the bilateral CR, bilateral ALIC, bilateral ATR, right CST, CC, cingulum, bilateral EC, forceps minor, forceps major, fornix, genu of the corpus callosum (GCC), bilateral IFOF, bilateral ILF, left PLIC, bilateral PTR, bilateral RPIC, splenium of the corpus callosum (SCC), bilateral superior CR, bilateral SLF, and left UF (Fig. 4) (P FWE < 0.05).
TBSS analysis of overlapping maps of FA, MD and RD
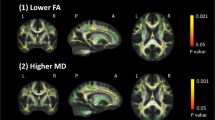
As shown in the Fig. 5, the overlap of the decrease of FA with increase of MD and RD in patients with NDPH group compared to HCs group was shown in red, which included the right ATR, body of the corpus callosum (BCC), bilateral cingulum, left hippocampal cingulum (CGH), left CST, forceps major, fornix, left IFOF, bilateral ILF, left PLIC, right RPIC, SCC, right SLF, and left UF (P FWE < 0.05).
The correlations between FA of overlapping tracts and clinical characteristics in patients with NDPH
For Pearson’s correlation analysis, we selected the regions showing overlapping differences in FA, MD and RD values between patients with NDPH and HCs (Table 2). The FA, MD, AD, and RD values of these regions were extracted. There were no significant correlations between the FA, MD, AD, and RD values of the overlapping tracts and the clinical characteristics (pain intensity, disease duration, HIT-6 score, GAD-7 score, PHQ-9 score, and PSQI score) after Bonferroni correction (p > 0.05/96).
Discussion
Our study first performed TBSS analysis to investigate abnormalities in white matter micro-structure in patients with NDPH. The initial results revealed that patients with NDPH exhibited significantly decreased FA, increased MD and RD compared to HCs. White matter regions overlaid with decreased FA, increased MD and RD were found in the right ATR, BCC, bilateral cingulum, left CGH, left CST, forceps major, fornix, left IFOF, bilateral ILF, left PLIC, right RPIC, SCC, right SLF, and left UF (Table 2).
Compared with voxel-based analysis method in DTI [28, 29], TBSS method is better in image alignment and without the smoothing problem [30, 31]. Several DTI-derived metrics based on TBSS, including FA, MD, AD, and RD, are sensitive to changes in white matter micro-structural. A low FA values may be caused by several conditions, including axonal loss, inflammation, demyelination, and gliosis [18]. The MD is an inverse measure of the membrane density and reflects both cellular swelling and cellular density [32]. AD is a measure of diffusivity parallel to axonal fibers, and a reduced AD may indicate axonal loss [18]. The RD increases in white matter with disrupted myelination [19]. Changes in the axonal diameters or density may also influence RD.
Given that FA is a summary measure of micro-structural integrity, MD and RD represent inverse measures of the membrane density and white matter with de- or dys-myelination [19]. Our findings may reveal micro-structural white matter abnormalities, especially disrupted myelination or decreased cellular density (such as gliosis) in patients with NDPH compared with HCs (shown in red in Fig. 5). Since the activation of glial cells and neuro-glial interactions may be key mechanism underlying chronic pain [33], our results may suggest that white matter abnormalities in this study may be connected with the chronic and unremitting pain state in patients with NDPH. Moreover, the white matter tracts that exhibited abnormal diffusive metrics in this TBSS study are mainly involved with the transmission and integration of sensory, cognitive, and/or emotional information. Consistent with previous findings of DTI study in other primary headache, especially migraine, we can firmly believe that the diffuse white matter micro-structural changes can disrupt the pain perception and lead to chronic pain [11,12,13,14].
The IFOF and UF are primarily associated with functions related to language and emotion [34, 35], which might potentially be relevant in the context of chronic pain experience in NDPH. The IFOF courses between the frontal and occipital lobes and may be involved in the language system and provide anatomic connections for visuospatial attention [34]. Meanwhile, the UF connects parts of the limbic system (temporal pole, amygdala, and anterior parahippocampus) with inferior portions of the frontal lobe (orbitofrontal cortex) [35]. The UF links emotion and cognition to the ventral limbic pathway [36]. Evidence from previous researches suggested that these tracts were involved in several cognitive and emotional processes, such as semantic processing of language, auditory working memory, and sound recognition [37]. Furthermore, studies have found micro-structural white matter abnormalities in these regions to be associated with major depressive disorder and other psychiatric disorders [38,39,40]. However, it is crucial to clarify that the patients with NDPH in this study did not have any pre-existing psychiatric disorders before the onset of their headaches. Some patients with NDPH developed symptoms of depression and/or anxiety as a result of enduring persistent headaches. Interestingly, Chen et al. have proposed that a reduction in myelin density in the IFOF and UF might contribute to the pathophysiology of major depressive disorder (MDD) [41]. Although distinct differences exist between MDD and NDPH, these findings underscore the possibility that micro-structural abnormalities in the white matter of the IFOF and UF may also be relevant to the neurobiology of NDPH. In the context of NDPH, it would be speculative, but interesting, to consider whether these micro-structural changes in the IFOF and UF could be linked to the chronic pain experience. Future studies investigating the potential associations between these white matter changes and specific symptom domains in patients with NDPH could provide valuable insights. For example, comparing NDPH patients with and without comorbid affective symptoms might elucidate whether these tract changes are more pronounced in patients with additional emotional distress.
The current study revealed the significant involvement of the CC, forceps major, cingulum, and PLIC in NDPH, notably their roles in the pain processing network. The CC, as a key interhemispheric commissural pathway, connects most neocortical areas and plays a central role in the integration of perceptual, cognitive, and volitional information across the hemispheres. The callosal fibers arising from the SCC that interconnect the parieto-occipital regions are called the forceps major. The fibers projecting into the primary somatosensory cortex are contained in the BCC. The SCC is the most posterior part of the corpus callosum, which connects somatosensory perception between the parietal lobe of the two hemispheres and the visual center in the occipital lobe [42]. Lots of experimental and clinical studies [43,44,45] have found that the CC and its components, including the forceps major, are involved in pain modulation and processing within the central nervous system. The cingulum, a core part of the limbic system, has been implicated in several brain functions, including pain perception [46, 47]. In this study, we found micro-structural white matter abnormalities in the left PLIC. The PLIC contains projection fibers of facial and somatosensory signals that arrive at the thalamus. Given the critical role of the PLIC, such abnormalities could be crucial for understanding the pain processing mechanisms in NDPH. Moreover, some previous studies have reported patients with primary headache, such as migraine and cluster headache showed micro-structure changes in various white matter regions, including the CC, forceps major and minor, cingulum, and internal and external capsules [13, 14, 48]. This finding further corroborates the notion that these regions could be integral to the pathophysiology of headache disorders, including NDPH. In summary, the CC, cingulum, and internal capsule (IC) play indispensable roles in pain processing in patients with NDPH. Further studies are needed to explore the correlation between white matter abnormalities in these regions and NDPH symptomatology. This could help illuminate the shared pathophysiological mechanisms across headache and painful disorders, and guide future therapeutic approaches.
As a part of the limbic system, the fornix originates from the hippocampus and stretches to the diencephalon and basal forebrain. Although the fornix itself is not primarily associated with pain perception, it plays a crucial role in cognitive processes, such as memory, learning, and emotional response, which can significantly influence the perception and processing of pain [49]. The SLF is the main long association fiber pathway in the suprasylvian area. The temporal pole and the dorsolateral occipital lobe are connected by the ILF, which lies within the inferior temporal lobe [50]. Of significant note, Rahimi et al. documented alterations in the white matter of these tracts in patients with migraine [51]. This suggests that the SLF and ILF might contribute to the neuropathology of migraines, perhaps by influencing the perception and processing of pain associated with these conditions. Further investigation is required to clarify the extent and implications of these changes in the context of headache disorders, such as NDPH.
Previous TBSS studies on migraine and cluster headache have also played an important role in exploring the pathophysiological changes in primary headache. These findings demonstrated alterations in FA, MD, AD, and RD across these types of headache. Specifically, there were decreased FA, MD and AD values in several white matter regions in patients with episodic migraine compared to the HCs [14]. In addition, the RD and MD values of white matter were significantly higher in chronic migraine than in HCs [13]. Furthermore, the decreased FA values and the increased AD, MD, and RD values were found in patients with cluster headache compared with the HCs [48]. However, significantly decreased FA, increased MD and RD were found in patients with NDPH compared to HCs. The above studies indicated that the pattern of white matter micro-structural changes in patients with NDPH was slightly different from that in patients with other types of primary headaches. Notably, while previous studies have reported associations between white matter hyperintensities or increased risk for ischemic stroke and migraine [52, 53], such hyperintensities are absent in NDPH. This absence further points to distinctive white matter changes in NDPH compared to other primary headaches, and warrants further exploration for a comprehensive understanding of these neurological conditions.
A few limitations of the study should be noted. First, this study was a cross-sectional design research, so it was hard to draw causal conclusions. Second, the study's small sample size might have biased our results and our preliminary results need to be verified with more data in the future. Third, there were few adolescents in the NDPH group. However, our control group did not include adolescents due to the practical limitations of the study. Fourth, psychotic comorbidities, especially depression and anxiety, could affect brain structure even at low grade and untreated levels. Further research is needed to determine whether this type of comorbidities may cause the micro-structural abnormalities detected in this study.
Conclusion
Our results suggested that patients with NDPH can be related to the widespread white matter abnormalities in the brain. Significantly decreased FA, increased MD and RD were found in patients with NDPH compared to HCs in 16 white matter regions from the Johns Hopkins University ICBM-DTI-81 White-Matter Atlas and Johns Hopkins University White-Matter Tractography Atlas. Furthermore, the subtle structural changes in white matter were primarily involved in emotion, cognition and pain modulation. In addition, our TBSS results suggested disrupted myelination or decreased cellular density in the above white matter regions of patients with NDPH. However, more comprehensive experimental designs are required to determine the exact role of these white matter changes in pathology of NDPH. We hope that our results can improve the understanding of the mechanism of NDPH and provide therapeutic strategies and potential diagnostic information for patients with NDPH.
Availability of data and materials
Data can be made available upon request.
Abbreviations
- AD:
-
Axial diffusivity
- ALIC:
-
Anterior limb of internal capsule
- ATR:
-
Anterior thalamic radiation
- BCC:
-
Body of corpus callosum
- BMI:
-
Body mass index
- CC:
-
Corpus callosum
- CGH:
-
Hippocampal cingulum
- CP:
-
Cerebral peduncle
- CR:
-
Corona radiata
- CST:
-
Corticospinal tract
- DTI:
-
Diffusion tensor imaging
- EC:
-
External capsule
- FA:
-
Fractional anisotropy
- FSL:
-
FMRIB software library
- FWE:
-
Family wise error
- GAD-7:
-
Generalized anxiety disorder-7
- GCC:
-
Genu of corpus callosum
- HCs:
-
Healthy controls
- HIT-6:
-
Headache Impact Test-6
- IC:
-
Internal capsule
- ICHD-3:
-
International classification of headache diseases, 3rd edition
- IFOF:
-
Inferior fronto-occipital fasciculus
- ILF:
-
Inferior longitudinal fasciculus
- MD:
-
Mean diffusivity
- MDD:
-
Major depressive disorder
- MNI:
-
Montreal neurological Institute
- MRI:
-
Magnetic resonance imaging
- NDPH:
-
New daily persistent headache
- PHQ-9:
-
Patient health questionnaire-9
- PLIC:
-
Posterior limb of internal capsule
- PSQI:
-
Pittsburgh sleep quality index
- PTR:
-
Posterior thalamic radiation
- RD:
-
Radial diffusivity
- RPIC:
-
Retrolenticular part of internal capsule
- SCC:
-
Splenium of corpus callosum
- SD:
-
Standard deviation
- SLF:
-
Superior longitudinal fasciculus
- TBSS:
-
Tract-based spatial statistics
- TFCE:
-
Threshold-free cluster enhancement
- UF:
-
Uncinate fasciculus
- VAS:
-
Visual analogue scale
References
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1): 1–211. https://doi.org/10.1177/0333102417738202
Gelfand AA, Robbins MS, Szperka CL (2022) New daily persistent headache-a start with an uncertain end. JAMA Neurol 79(8):733–734. https://doi.org/10.1001/jamaneurol.2022.1727
Tyagi A (2012) New daily persistent headache. Ann Indian Acad Neurol 15(Suppl 1):S62-65. https://doi.org/10.4103/0972-2327.100011
Aaseth K, Grande RB, Kvaerner KJ et al (2008) Prevalence of secondary chronic headaches in a population-based sample of 30–44-year-old persons. The Akershus study of chronic headache. Cephalalgia 28(7):705–713. https://doi.org/10.1111/j.1468-2982.2008.01577.x
Yamani N, Olesen J (2019) New daily persistent headache: a systematic review on an enigmatic disorder. J Headache Pain 20(1):80. https://doi.org/10.1186/s10194-019-1022-z
Peng KP, Rozen TD (2023) Update in the understanding of new daily persistent headache. Cephalalgia 43(2):3331024221146314. https://doi.org/10.1177/03331024221146314
Schwedt TJ, Chiang CC, Chong CD et al (2015) Functional MRI of migraine. Lancet Neurol 14(1):81–91. https://doi.org/10.1016/s1474-4422(14)70193-0
Jordan JE, Flanders AE (2020) Headache and Neuroimaging: Why We Continue to Do It. AJNR Am J Neuroradiol 41(7):1149–1155. https://doi.org/10.3174/ajnr.A6591
Lee MC, Tracey I (2013) Imaging pain: a potent means for investigating pain mechanisms in patients. Br J Anaesth 111(1):64–72. https://doi.org/10.1093/bja/aet174
Russo A, Silvestro M, Tessitore A et al (2018) Advances in migraine neuroimaging and clinical utility: from the MRI to the bedside. Expert Rev Neurother 18(7):533–544. https://doi.org/10.1080/14737175.2018.1486708
Planchuelo-Gómez Á, García-Azorín D, Guerrero ÁL et al (2020) White matter changes in chronic and episodic migraine: a diffusion tensor imaging study. J Headache Pain 21(1):1. https://doi.org/10.1186/s10194-019-1071-3
Szabó N, Kincses ZT, Párdutz Á et al (2012) White matter microstructural alterations in migraine: a diffusion-weighted MRI study. Pain 153(3):651–656. https://doi.org/10.1016/j.pain.2011.11.029
Coppola G, Di Renzo A, Tinelli E et al (2020) Patients with chronic migraine without history of medication overuse are characterized by a peculiar white matter fiber bundle profile. J Headache Pain 21(1):92. https://doi.org/10.1186/s10194-020-01159-6
Yu D, Yuan K, Qin W et al (2013) Axonal loss of white matter in migraine without aura: a tract-based spatial statistics study. Cephalalgia 33(1):34–42. https://doi.org/10.1177/0333102412466964
Rozen TD (2016) New daily persistent headache: A lack of an association with white matter abnormalities on neuroimaging. Cephalalgia 36(10):987–992. https://doi.org/10.1177/0333102415612766
Tae WS, Ham BJ, Pyun SB et al (2018) Current clinical applications of diffusion-tensor imaging in neurological disorders. J Clin Neurol 14(2):129–140. https://doi.org/10.3988/jcn.2018.14.2.129
Chanraud S, Zahr N, Sullivan EV et al (2010) MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev 20(2):209–225. https://doi.org/10.1007/s11065-010-9129-7
Alexander AL, Lee JE, Lazar M et al (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4(3):316–329. https://doi.org/10.1016/j.nurt.2007.05.011
Song SK, Sun SW, Ramsbottom MJ et al (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17(3):1429–1436. https://doi.org/10.1006/nimg.2002.1267
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Stämpfli P, Sommer S, Manoliu A et al (2019) Subtle white matter alterations in schizophrenia identified with a new measure of fiber density. Sci Rep 9(1):4636. https://doi.org/10.1038/s41598-019-40070-2
Barnea-Goraly N, Chang KD, Karchemskiy A et al (2009) Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry 66(3):238–244. https://doi.org/10.1016/j.biopsych.2009.02.025
Chao YP, Liu PB, Wang PN et al (2022) Reduced inter-voxel white matter integrity in subjective cognitive decline: diffusion tensor imaging with tract-based spatial statistics analysis. Front Aging Neurosci 14:810998. https://doi.org/10.3389/fnagi.2022.810998
Thiel K, Meinert S, Winter A, et al (2022) Reduced fractional anisotropy in bipolar disorder v. major depressive disorder independent of current symptoms. Psychol Med:1–11. https://doi.org/10.1017/s0033291722001490
Negeri ZF, Levis B, Sun Y et al (2021) Accuracy of the Patient Health Questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis. BMJ 375:n2183. https://doi.org/10.1136/bmj.n2183
Löwe B, Decker O, Müller S et al (2008) Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care 46(3):266–274. https://doi.org/10.1097/MLR.0b013e318160d093
Li J, Yao YS, Dong Q et al (2013) Characterization and factors associated with sleep quality among rural elderly in China. Arch Gerontol Geriatr 56(1):237–243. https://doi.org/10.1016/j.archger.2012.08.002
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11(6 Pt 1):805–821. https://doi.org/10.1006/nimg.2000.0582
Bookstein FL (2001) “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage 14(6):1454–1462. https://doi.org/10.1006/nimg.2001.0770
Jones DK, Symms MR, Cercignani M et al (2005) The effect of filter size on VBM analyses of DT-MRI data. Neuroimage 26(2):546–554. https://doi.org/10.1016/j.neuroimage.2005.02.013
Smith SM, Johansen-Berg H, Jenkinson M et al (2007) Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 2(3):499–503. https://doi.org/10.1038/nprot.2007.45
Harsan LA, Poulet P, Guignard B et al (2006) Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 83(3):392–402. https://doi.org/10.1002/jnr.20742
Ji RR, Berta T, Nedergaard M (2013) Glia and pain: is chronic pain a gliopathy? Pain 154 Suppl 1(01):S10-s28. https://doi.org/10.1016/j.pain.2013.06.022
Conner AK, Briggs RG, Sali G et al (2018) A connectomic atlas of the human cerebrum-chapter 13: tractographic description of the inferior fronto-occipital fasciculus. Oper Neurosurg (Hagerstown) 15(suppl_1):S436–S443. https://doi.org/10.1093/ons/opy267
Olson IR, Von Der Heide RJ, Alm KH et al (2015) Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci 14:50–61. https://doi.org/10.1016/j.dcn.2015.06.003
Singh S, Singh K, Trivedi R et al (2016) Microstructural abnormalities of uncinate fasciculus as a function of impaired cognition in schizophrenia: a DTI study. J Biosci 41(3):419–426. https://doi.org/10.1007/s12038-016-9631-z
Papagno C (2011) Naming and the role of the uncinate fasciculus in language function. Curr Neurol Neurosci Rep 11(6):553–559. https://doi.org/10.1007/s11910-011-0219-6
Szeszko PR, Robinson DG, Ashtari M et al (2008) Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology 33(5):976–984. https://doi.org/10.1038/sj.npp.1301480
Li X, Lu W, Zhang R et al (2021) Integrity of the uncinate fasciculus is associated with the onset of bipolar disorder: a 6-year followed-up study. Transl Psychiatry 11(1):111. https://doi.org/10.1038/s41398-021-01222-z
Koch SBJ, van Zuiden M, Nawijn L et al (2017) Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: a diffusion tensor imaging study. J Psychiatry Neurosci 42(5):331–342. https://doi.org/10.1503/jpn.160129
Chen G, Fu S, Chen P et al (2022) Reduced myelin density in unmedicated major depressive disorder: an inhomogeneous magnetization transfer MRI study. J Affect Disord 300:114–120. https://doi.org/10.1016/j.jad.2021.12.111
Caminiti R, Ghaziri H, Galuske R et al (2009) Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates. Proc Natl Acad Sci U S A 106(46):19551–19556. https://doi.org/10.1073/pnas.0907655106
Horton JE, Crawford HJ, Harrington G et al (2004) Increased anterior corpus callosum size associated positively with hypnotizability and the ability to control pain. Brain 127(Pt 8):1741–1747. https://doi.org/10.1093/brain/awh196
Kim DJ, Lim M, Kim JS et al (2014) Altered white matter integrity in the corpus callosum in fibromyalgia patients identified by tract-based spatial statistical analysis. Arthritis Rheumatol 66(11):3190–3199. https://doi.org/10.1002/art.38771
Emsell L, Adamson C, De Winter FL et al (2017) Corpus callosum macro and microstructure in late-life depression. J Affect Disord 222:63–70. https://doi.org/10.1016/j.jad.2017.06.063
Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6(7):533–544. https://doi.org/10.1038/nrn1704
Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018) The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev 92:104–127. https://doi.org/10.1016/j.neubiorev.2018.05.008
Szabó N, Kincses ZT, Párdutz Á et al (2013) White matter disintegration in cluster headache. J Headache Pain 14(1):64. https://doi.org/10.1186/1129-2377-14-64
Thomas AG, Koumellis P, Dineen RA (2011) The fornix in health and disease: an imaging review. Radiographics 31(4):1107–1121. https://doi.org/10.1148/rg.314105729
Herbet G, Zemmoura I, Duffau H (2018) Functional anatomy of the inferior longitudinal fasciculus: from historical reports to current hypotheses. Front Neuroanat 12:77. https://doi.org/10.3389/fnana.2018.00077
Rahimi R, Dolatshahi M, Abbasi-Feijani F et al (2022) Microstructural white matter alterations associated with migraine headaches: a systematic review of diffusion tensor imaging studies. Brain Imaging Behav 16(5):2375–2401. https://doi.org/10.1007/s11682-022-00690-1
Tana C, Tafuri E, Tana M et al (2013) New insights into the cardiovascular risk of migraine and the role of white matter hyperintensities: is gold all that glitters? J Headache Pain 14(1):9. https://doi.org/10.1186/1129-2377-14-9
McKinley EC, Lay CL, Rosenson RS et al (2021) Risk for ischemic stroke and coronary heart disease associated with migraine and migraine medication among older adults. J Headache Pain 22(1):124. https://doi.org/10.1186/s10194-021-01338-z
Acknowledgements
Our study was technically supported by the National Neurological Imaging Centre of Beijing Tiantan Hospital, Capital Medical University.
Funding
This study was supported by the National Natural Science Foundation of China (grant numbers 32170752, 91849104, and 31770800) and the National Natural Science Foundation of Beijing (Z200024).
Author information
Authors and Affiliations
Contributions
YLM, BBS and YGW designed this research. YLM analyzed the clinical and MRI data and WW verified the results. All authors contributed to the data collection. YLM produced the first draft. The revised manuscript was reviewed and approved by all authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All participants were informed in detail about the study and volunteered to sign an informed consent form. Our study had been registered on Clinical Trial (NCT05334927) and received ethical approval from Beijing Tiantan Hospital, Capital Medical University (no. KY2022-044).
Consent for publication
All authors consent for the publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mei, Y., Wang, W., Qiu, D. et al. Micro-structural white matter abnormalities in new daily persistent headache: a DTI study using TBSS analysis. J Headache Pain 24, 80 (2023). https://doi.org/10.1186/s10194-023-01620-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-023-01620-2