Abstract
Objective
To ascertain whether intravenous infusion of calcitonin gene-related peptide (CGRP) can induce migraine-like headache in people with persistent post-traumatic headache attributed to mild traumatic brain injury (TBI) and no pre-existing migraine.
Methods
A non-randomized, single-arm, open-label study at a single site in Denmark. Eligible participants were aged 18 to 65 years and had a known history of persistent post-traumatic headache attributed to mild TBI for ≥ 12 months. All participants received continuous intravenous infusion of CGRP (1.5 µg/min) over 20 min. A headache diary was used to collect outcome data until 12 h after the start of CGRP infusion. The primary end point was the incidence of migraine-like headache during 12-hour observational period.
Results
A total of 60 participants completed the study protocol and provided data for the analysis of the primary end point. The median age was 32.5 (IQR, 25.5–43.0) years; 43 participants (72%) were female. Following CGRP infusion, 43 (72%) of 60 participants developed migraine-like headache during the 12-hour observational period. The median time to peak headache intensity was 40 min (IQR, 20–60), and the median peak headache intensity was 6 (IQR, 5–8) on the 11-point numeric rating scale.
Conclusion
Intravenous infusion of CGRP is a potent inducer of migraine-like headache in people with persistent post-traumatic headache attributed to mild TBI. This observation underscores the importance of CGRP in the genesis of migraine-like headache that is often experienced by individuals who are afflicted by persistent post-traumatic headache. Further research is warranted to ascertain whether other signaling molecules also contribute to the disease mechanisms underlying post-traumatic headache.
Similar content being viewed by others
Introduction
Post-traumatic headache is a disabling neurologic disorder that remains a challenge for clinicians and researchers alike [1]. Although most people recover from acute post-traumatic headache within three months of onset following traumatic injury to the head [2,3,4], some experience persistence of cephalic pain beyond the 3-month mark and are then diagnosed with persistent post-traumatic headache [5,6,7]. The latter is often associated with exacerbations of cephalic pain that resemble the clinical features of migraine. The frequency of these exacerbations varies among patients [8], and little is known about the underlying molecular signaling mechanisms [9]. However, increased attention is being paid to the possible involvement of the signaling molecule calcitonin gene-related peptide (CGRP) [10]. A recent randomized, double-blind, placebo-controlled trial found that intravenous infusion of CGRP induced migraine-like headache in 21 (70%) of 30 participants with persistent post-traumatic headache, compared with 6 (20%) of 30 participants after placebo infusion [11]. This finding provides compelling evidence of CGRP involvement in the genesis of migraine-like headache among those with persistent post-traumatic headache [12]. However, the results must be replicated in a larger sample before firm conclusions can be drawn.
In this non-randomized, single-arm, open-label study, we seek to confirm that intravenous infusion of CGRP induces migraine-like headache in most people with persistent post-traumatic headache attributed to mild traumatic brain injury (TBI).
Methods
Study oversight
The study protocol was approved by the relevant ethics committee and data protection agency. All participants provided written informed consent before any protocol-related procedures or assessments were performed. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Study population
Eligible participants were aged 18 to 65 years and had a known history of persistent post-traumatic headache attributed to mild TBI for ≥ 12 months that also met the diagnostic criteria outlined in the International Classification of Headache Disorders, 3rd edition (ICHD-3) [13]. Key exclusion criteria were any known history of > 1 TBI or whiplash injury. Potential participants were also excluded if they had any history of a primary headache disorder, except for infrequent episodic tension-type headache (TTH). Electronic medical records were reviewed to ensure eligibility for study inclusion.
Study design
Participants were enrolled in a non-randomized, single-arm, open-label study that was conducted at the Danish Headache Center. All received continuous intravenous infusion of CGRP (1.5 µg/min) in the antecubital fossa over 20 min using a time- and volume-controlled infusion pump.
The CGRP dose is identical to the one used in previous experimental studies that included people with persistent post-traumatic headache, migraine, and cluster headache [11, 14,15,16]. Independent hospital pharmacy staff were responsible for drug preparation. Participants were told that CGRP might induce headache; no information was provided on its possible onset, clinical features, or accompanying symptoms.
On the experimental day, participants were scheduled to arrive between 08:00 AM and 13:00 PM. A site investigator would then perform a semi-structured interview to collect data on demographics, medical history, and full clinical course. Following this, participants underwent a physical and neurologic examination, in addition to a 12-lead electrocardiogram. Eligibility on the experimental day was contingent upon the participant reporting no intake of acute medications (e.g., simple analgesics, triptans) within 48 h of infusion start and a baseline headache intensity of ≤ 5 on an 11-point numeric rating scale (NRS, 0 being no headache, 10 being the worst imaginable headache). Furthermore, it was required that participants did not report, if relevant, their usual migraine-like headache at baseline, with the latter defined as the time of infusion start (T0).
Participants were placed in a supine position at the time of infusion start and during the in-hospital phase (0–90 min), a site investigator would collect data on headache characteristics, use of rescue medication, adverse events, and hemodynamics (i.e., blood pressure and heart rate) every 10 min until 60 min after the start of infusion and then again at 90 min after infusion start. Hereafter, participants would be discharged with a headache diary that they needed to fill out hourly until 12 h after infusion start.
Statistical analysis
Descriptive statistics were used to summarize demographic and clinical characteristics, and the Shapiro-Wilk test was performed to evaluate whether the variables followed a normal distribution. The median value with interquartile ranges (IQR) or the mean with standard deviations (SD) were then used as appropriate. The primary end point was the incidence of migraine-like headache during the 12-hour observational period after the start of CGRP infusion. Predefined criteria were used to assess whether the participants developed migraine-like headache during the 12-hour observational period (Table 1). Secondary end points included median time to peak headache intensity, median peak headache intensity, and baseline corrected area under the curve (AUC) for headache intensity scores (0–12 h). The sample size was based on the number of individuals who participated in a clinical trial and were also interested in undergoing provocation with CGRP in the present study. All analyses were performed with R (v4.1.2).
Results
Characteristics of the Study Population
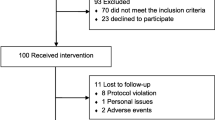
The study included 60 participants who received intravenous infusion of CGRP and provided data for the analysis of the primary end point (Fig. 1; Table 2). The median age was 32.5 (IQR, 25.5–43.0) years; 43 participants (72%) were female, and 21 participants (35%) reported current use of preventive medications. In addition, the median number of monthly headache days was 28 (IQR, 23.0–28.0), while the median number of monthly headache days of moderate to severe intensity was 13.5 (IQR, 4.8–23.3). Most participants (52 [87%]) had a migraine-like phenotype while a ‘pure’ TTH-like phenotype was less common (8 [13%]).
Migraine-like headache
Intravenous infusion of CGRP induced migraine-like headache in 43 (72%) of 60 participants during the 12-hour observational period (Supplemental File 1). The median time to peak headache intensity was 40 min (IQR, 20–60), and the median peak headache intensity was 6 (IQR, 5–8) on NRS among all study participants (Fig. 2). Furthermore, migraine-like headache was experienced by 39 (75%) of 52 participants who had a migraine-like phenotype, while 4 (50%) of 8 participants with a ‘pure’ TTH-like phenotype reported migraine-like headache. Thirteen (22%) of 60 participants took rescue medication during the 12-observational period.
Among those who developed a migraine-like headache (n = 43), the median time to peak headache intensity was 40 min (IQR, 20–60), and the median peak headache intensity was 7 (IQR, 6–8) on NRS. Most (n = 38) reported migraine-like headache within 90 min after the start of infusion. Moreover, migraine-like headache was reported by 19 (90%) of 21 participants who had current use of preventive medications and 24 (62%) of the remaining 39 participants who did not use preventive medications at the time of study inclusion.
Adverse events and hemodynamics
The most common adverse event was flushing (n = 59) followed by warm sensations (n = 58) and palpitations (n = 42). Less common adverse events were abdominal discomfort (n = 3), tiredness (n = 2), increased appetite (n = 2), and tension in the jaw (n = 2). In terms of hemodynamics, mean arterial blood pressure decreased while the heart rate increased during the 90-minute in-hospital phase after the start of infusion (Fig. 3a and b).
Discussion
In this open-label study of patients with persistent post-traumatic headache, 43 (72%) of 60 participants developed migraine-like headache during the 12-hour observational period after intravenous infusion of CGRP. This finding accords well with a recent randomized, double-blind, placebo-controlled trial in which the 21 (70%) of 30 participants with persistent post-traumatic headache developed migraine-like headache after CGRP infusion [11]. The similar induction rates of migraine-like headache also suggest that there are limited to no nocebo effects in provocation experiments with CGRP in patients with persistent post-traumatic headache. However, the median time to peak headache intensity was 40 min after the start of CGRP infusion in the present study, compared with 120 min in the aforementioned randomized, double-blind, placebo-controlled trial [11]. It remains unclear whether the more rapid time to peak headache intensity is, in part, attributable to nocebo effects, which could also the higher peak headache intensity in the present study, compared with placebo-controlled trials.
The site and mechanism of action by which CGRP induces migraine-like headache is incompletely delineated [17,18,19,20], albeit multiple animal studies have established that CGRP can modulate nociceptive transmission in concussed rodents [21,22,23,24]. A compelling theory has recently been posited to explain migraine pathogenesis and might also extend, in part, to the genesis of migraine-like headache in people with post-traumatic headache [17]. This theory suggests that CGRP binds to its G protein-coupled receptor on the vascular smooth muscle cell within the walls of intracranial arteries and then initiates downstream intracellular signaling resulting in the opening of specific potassium channels. The latter causes efflux of potassium and accompanying vasodilation [17, 25,26,27]. This, in turn, is thought to provide chemical and mechanical stimuli sufficient to activate and sensitize perivascular nociceptors that project to first-order neurons in the trigeminal ganglion and upper cervical dorsal root ganglia [17]. From here, ascending trigeminal pain pathways relay the nociceptive information to cortical and subcortical regions that are responsible for the perception of migraine-like pain. In support of this theory, ample experimental data have shown that dilators of intracranial arteries (including specific potassium channel openers) can induce migraine attacks in people with migraine [26,27,28,29,30,31,32,33,34]. However, further studies are needed to draw firm conclusions on the link between dilation of intracranial arteries and subsequent activation of perivascular nociceptors in the genesis of migraine-like headache.
Another possible explanation is that CGRP elicits migraine-like headache through direct binding to its receptors on primary afferents that originate from the trigeminal ganglion [35]. Increased attention is being paid to this line of reasoning because animal data have shown that fremanezumab – a monoclonal antibody (mAb) against CGRP – inhibits nociceptive transmission from meningeal nociceptors by decreasing their responsiveness [36]. The same lab also reported that injection of fluorescently-labeled fremanezumab in rodents can be detected in the meninges and its blood vessels as well as in the sensory and autonomic ganglia [37]. However, no fluorescently-labeled fremanezumab was observed within CNS structures, including the brain stem, thalamus, hypothalamus, and cortex. Although these findings do not exclude involvement of the intracranial vasculature in the genesis of migraine-like headache, it seems reasonable to further explore whether CGRP mediates its pro-nociceptive effects via direct receptor-binding on primary afferents of the trigeminal ganglion and (upper cervical dorsal root ganglia). It should also be noted that some intriguing evidence suggest that CGRP-mediated nociceptive signals might be mediated via Schwann cells [38].
The involvement of CGRP in the mechanisms underlying post-traumatic headache is a rapidly expanding field. Recent open-label trial data also suggest that erenumab – a mAb against the CGRP receptor – holds promise for the preventive treatment of persistent post-traumatic headache [39]. However, it is unlikely that CGRP is the only pathogenic driver of migraine-like headache in people afflicted by persistent post-traumatic headache. There is a clear need to study responses to other signaling molecules that are well-established modulators of nociceptive transmission in migraine, e.g. adrenomedullin, amylin, and pituitary adenylate cyclase-activating polypeptide [31, 32, 40]. Establishing the contribution of possible pathogenic drivers should facilitate the identification of novel drug targets which, in turn, can be applied for patient benefit. Concerted efforts are also needed to clarify the pathogenic drivers involved in disease persistence and chronification of post-traumatic headache [9, 41, 42]. The most obvious unanswered scientific question remains why post-traumatic headache remits in some people and persists in others. In addition, the phenotypic and pathophysiologic similarities between post-traumatic headache and migraine should capture more interest, as it remains unclear whether head trauma can evoke migraine in susceptible individuals and perhaps even chronic migraine in those who previously had episodic migraine. If the latter is proven to be true, head trauma would constitute a risk factor for migraine chronification.
Limitations
This study has several limitations. First, some participants reported current use of preventive medications which might affect the likelihood of developing migraine-like headache after CGRP infusion. It would be interesting if larger studies are performed to examine whether the use of specific preventive medications is associated with a lower induction rate of migraine-like headache. Second, the in-hospital phase lasted until 90 min after the start of infusion. Patient were then discharged for the remaining observational period, and it is therefore not possible to exclude the influence of environmental factors (e.g. stress, specific foods). However, it should be noted that 38 (88%) of 43 participants who developed migraine-like headache did so during the in-hospital phase. Third, a prospective headache diary with daily entries was not used to record the number of migraine-like days in the preceding month. It would be useful if future studies investigated whether the occurrence of CGRP-induced migraine-like headache is related to the frequency of migraine-like headache in the preceding month or time since the last episode with migraine-like headache. Lastly, the study population was limited to people with persistent post-traumatic headache, and our findings can therefore not be extended to conclude on the possible involvement of CGRP in acute post-traumatic headache.
Conclusion
Among participants with persistent post-traumatic headache attributed to mild TBI, most developed migraine-like headache during the 12-hour observational period following intravenous infusion of CGRP. Thus, it seems evident that CGRP is an important signaling molecule in the pathogenesis of persistent post-traumatic headache. The involvement of other signaling molecules should be investigated in future studies to discover pathogenic drivers of post-traumatic headache.
Data Availability
Qualified researchers can request access to patient-level data and related study documents, including the study protocol. Patient-level data will be deidentified and study documents will be redacted to protect the privacy of study participants.
Abbreviations
- CGRP:
-
Calcitonin gene-related peptide.
- TBI:
-
Traumatic brain injury.
- ICHD-3:
-
International Classification of Headache Disorders, 3rd edition.
- TTH:
-
Tension-type headache.
- IQR:
-
Interquartile range.
- SD:
-
Standard deviations.
- AUC:
-
Area under the curve.
- mAb:
-
Monoclonal antibody.
References
Ashina H, Eigenbrodt AK, Seifert T, Sinclair AJ, Scher AI, Schytz HW, Lee MJ, de Icco R, Finkel AG, Ashina M (2021) Post-traumatic headache attributed to traumatic brain injury: classification, clinical characteristics, and treatment. Lancet Neurol 20(6):460–469. https://doi.org/10.1016/S1474-4422(21)00094-6
Lieba-Samal D, Platzer P, Seidel S, Klaschterka P, Knopf A, Wober C (2011) Characteristics of acute posttraumatic headache following mild head injury. Cephalalgia 31(16):1618–1626. https://doi.org/10.1177/0333102411428954
Lucas S, Hoffman JM, Bell KR, Dikmen S (2014) A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 34(2):93–102. https://doi.org/10.1177/0333102413499645
van der Naalt J, Timmerman ME, de Koning ME, van der Horn HJ, Scheenen ME, Jacobs B et al (2017) Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol 16(7):532–540. https://doi.org/10.1016/S1474-4422(17)30117-5
Voormolen DC, Haagsma JA, Polinder S, Maas AIR, Steyerberg EW, Vuleković P, Sewalt CA, Gravesteijn BY, Covic A, Andelic N, Plass AM, von Steinbuechel N (2019) Post-Concussion Symptoms in Complicated vs. Uncomplicated Mild Traumatic Brain Injury Patients at Three and Six Months Post-Injury: Results from the CENTER-TBI Study. J Clin Med 8(11):1921. https://doi.org/10.3390/jcm8111921
Ingebrigtsen T, Waterloo K, Marup-Jensen S, Attner E, Romner B (1998) Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J Neurol 245(9):609–612. https://doi.org/10.1007/s004150050254
Xu H, Pi H, Ma L, Su X, Wang J (2016) Incidence of Headache After Traumatic Brain Injury in China: A Large Prospective Study. World Neurosurg 88:289–296. https://doi.org/10.1016/j.wneu.2015.12.028
Ashina H, Iljazi A, Al-Khazali HM, Ashina S, Jensen RH, Amin FM et al (2020) Persistent post-traumatic headache attributed to mild traumatic brain injury: deep phenotyping and treatment patterns. Cephalalgia 40(6):554–564. https://doi.org/10.1177/0333102420909865
Ashina H, Porreca F, Anderson T, Amin FM, Ashina M, Schytz HW, Dodick DW (2019) Post-traumatic headache: epidemiology and pathophysiological insights. Nat Rev Neurol 15(10):607–617. https://doi.org/10.1038/s41582-019-0243-8
Ashina H, Dodick DW (2022) Post-traumatic Headache: Pharmacologic Management and Targeting CGRP Signaling. Curr Neurol Neurosci Rep 22(2):105–111. https://doi.org/10.1007/s11910-022-01175-w
Ashina H, Iljazi A, Al-Khazali HM, Christensen CE, Amin FM, Ashina M et al (2020) Hypersensitivity to Calcitonin Gene-Related Peptide in Post-Traumatic Headache. Ann Neurol 88(6):1220–1228. https://doi.org/10.1002/ana.25915
Ashina H, Moskowitz MA (2021) Shared biological foundations of post-traumatic headache and migraine. Headache 61(3):558–559. https://doi.org/10.1111/head.14084
Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38(1):1–211
Iljazi A, Ashina H, Zhuang ZA, Lopez Lopez C, Snellman J, Ashina M, Schytz HW (2021) Hypersensitivity to calcitonin gene-related peptide in chronic migraine. Cephalalgia 41(6):701–710. https://doi.org/10.1177/0333102420981666
Younis S, Christensen CE, Toft NM, Søborg T, Amin FM, Hougaard A, Ashina M (2019) Investigation of distinct molecular pathways in migraine induction using calcitonin gene-related peptide and sildenafil. Cephalalgia 39(14):1776–1788. https://doi.org/10.1177/0333102419882474
Vollesen ALH, Snoer A, Beske RP, Guo S, Hoffmann J, Jensen RH, Ashina M (2018) Effect of Infusion of Calcitonin Gene-Related Peptide on Cluster Headache Attacks: A Randomized Clinical Trial. JAMA Neurol 75(10):1187–1197. https://doi.org/10.1001/jamaneurol.2018.1675
Ashina M (2020) Migraine. N Engl J Med 383(19):1866–1876. https://doi.org/10.1056/NEJMra1915327
Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA (2019) Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol 18(8):795–804. https://doi.org/10.1016/S1474-4422(19)30185-1
Iyengar S, Johnson KW, Ossipov MH, Aurora SK (2019) CGRP and the Trigeminal System in Migraine. Headache 59(5):659–681. https://doi.org/10.1111/head.13529
Iyengar S, Ossipov MH, Johnson KW (2017) The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 158(4):543–559. https://doi.org/10.1097/j.pain.0000000000000831
Kopruszinski CM, Turnes JM, Swiokla J, Weinstein TJ, Schwedt TJ, Dodick DW, Anderson T, Navratilova E, Porreca F (2021) CGRP monoclonal antibody prevents the loss of diffuse noxious inhibitory controls (DNIC) in a mouse model of post-traumatic headache. Cephalalgia 41(6):749–759. https://doi.org/10.1177/0333102420981688
Navratilova E, Rau J, Oyarzo J, Tien J, Mackenzie K, Stratton J, Remeniuk B, Schwedt T, Anderson T, Dodick D, Porreca F (2019) CGRP-dependent and independent mechanisms of acute and persistent post-traumatic headache following mild traumatic brain injury in mice. Cephalalgia 39(14):1762–1775. https://doi.org/10.1177/0333102419877662
Bree D, Levy D (2018) Development of CGRP-dependent pain and headache related behaviours in a rat model of concussion: Implications for mechanisms of post-traumatic headache. Cephalalgia 38(2):246–258. https://doi.org/10.1177/0333102416681571
Daiutolo BV, Tyburski A, Clark SW, Elliott MB (2016) Trigeminal Pain Molecules, Allodynia, and Photosensitivity Are Pharmacologically and Genetically Modulated in a Model of Traumatic Brain Injury. J Neurotrauma 33(8):748–760. https://doi.org/10.1089/neu.2015.4087
Ashina M, Terwindt GM, Al-Karagholi MA, de Boer I, Lee MJ, Hay DL, Schulte LH, Hadjikhani N, Sinclair AJ, Ashina H, Schwedt TJ, Goadsby PJ (2021) Migraine: disease characterisation, biomarkers, and precision medicine. Lancet 397(10283):1496–1504. https://doi.org/10.1016/S0140-6736(20)32162-0
Al-Karagholi MA, Hansen JM, Guo S, Olesen J, Ashina M (2019) Opening of ATP-sensitive potassium channels causes migraine attacks: a new target for the treatment of migraine. Brain 142(9):2644–2654. https://doi.org/10.1093/brain/awz199
Al-Karagholi MA, Ghanizada H, Waldorff Nielsen CA, Skandarioon C, Snellman J, Lopez-Lopez C, Hansen JM, Ashina M (2021) Opening of BKCa channels causes migraine attacks: a new downstream target for the treatment of migraine. Pain 162(10):2512–2520. doi: https://doi.org/10.1097/j.pain.0000000000002238
Hansen JM, Hauge AW, Olesen J, Ashina M (2010) Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 30(10):1179–1186. https://doi.org/10.1177/0333102410368444
Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M (2009) PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 132(Pt 1):16–25. https://doi.org/10.1093/brain/awn307
Ghanizada H, Al-Karagholi MA, Arngrim N, Olesen J, Ashina M (2020) PACAP27 induces migraine-like attacks in migraine patients. Cephalalgia 40(1):57–67. https://doi.org/10.1177/0333102419864507
Ghanizada H, Al-Karagholi MA, Walker CS, Arngrim N, Rees T, Petersen J, Siow A, Mørch-Rasmussen M, Tan S, O’Carroll SJ, Harris P, Skovgaard LT, Jørgensen NR, Brimble M, Waite JS, Rea BJ, Sowers LP, Russo AF, Hay DL, Ashina M (2021) Amylin Analog Pramlintide Induces Migraine-like Attacks in Patients. Ann Neurol 89(6):1157–1171. https://doi.org/10.1002/ana.26072
Ghanizada H, Al-Karagholi MA, Arngrim N, Mørch-Rasmussen M, Walker CS, Hay DL, Ashina M (2021) Effect of Adrenomedullin on Migraine-Like Attacks in Patients With Migraine: A Randomized Crossover Study. Neurology 96(20):e2488–e2499. https://doi.org/10.1212/WNL.0000000000011930
Guo S, Olesen J, Ashina M (2014) Phosphodiesterase 3 inhibitor cilostazol induces migraine-like attacks via cyclic AMP increase. Brain 137(Pt 11):2951–2959. https://doi.org/10.1093/brain/awu244
Christensen CE, Younis S, Lindberg U, de Koning P, Tolnai D, Paulson OB, Larsson HBW, Amin FM, Ashina M (2021) Intradural artery dilation during experimentally induced migraine attacks. Pain 162(1):176–183. https://doi.org/10.1097/j.pain.0000000000002008
Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, Ashina M, van den Maagdenberg AMJM, Dodick DW (2022) Migraine. Nat Rev Dis Primers 8(1):2. https://doi.org/10.1038/s41572-021-00328-4
Melo-Carrillo A, Strassman AM, Nir RR, Schain AJ, Noseda R, Stratton J, Burstein R (2017) Fremanezumab-A Humanized Monoclonal Anti-CGRP Antibody-Inhibits Thinly Myelinated (Aδ) But Not Unmyelinated (C) Meningeal Nociceptors. J Neurosci 37(44):10587–10596. https://doi.org/10.1523/JNEUROSCI.2211-17.2017
Noseda R, Schain AJ, Melo-Carrillo A, Tien J, Stratton J, Mai F, Strassman AM, Burstein R (2020) Fluorescently-labeled fremanezumab is distributed to sensory and autonomic ganglia and the dura but not to the brain of rats with uncompromised blood brain barrier. Cephalalgia 40(3):229–240. https://doi.org/10.1177/0333102419896760
De Logu F, Nassini R, Hegron A, Landini L, Jensen DD, Latorre R, Ding J, Marini M, Souza Monteiro de Araujo D, Ramírez-Garcia P, Whittaker M, Retamal J, Titiz M, Innocenti A, Davis TP, Veldhuis N, Schmidt BL, Bunnett NW, Geppetti P (2022) Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat Commun 13(1):646. doi: https://doi.org/10.1038/s41467-022-28204-z
Ashina H, Iljazi A, Al-Khazali HM, Eigenbrodt AK, Larsen EL, Andersen AM, Hansen KJ, Bräuner KB, Mørch-Jessen T, Chaudhry B, Antic S, Christensen CE, Ashina M, Amin FM, Schytz HW (2020) Efficacy, tolerability, and safety of erenumab for the preventive treatment of persistent post-traumatic headache attributed to mild traumatic brain injury: an open-label study. J Headache Pain 21(1):62. https://doi.org/10.1186/s10194-020-01136-z
Amin FM, Hougaard A, Schytz HW, Asghar MS, Lundholm E, Parvaiz AI, de Koning PJ, Andersen MR, Larsson HB, Fahrenkrug J, Olesen J, Ashina M (2014) Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 137(Pt 3):779–794. https://doi.org/10.1093/brain/awt369
Benemei S, Labastida-Ramírez A, Abramova E, Brunelli N, Caronna E, Diana P, Gapeshin R, Hofacker MD, Maestrini I, Pías EM, Mikulenka P, Tikhonova O, Martelletti P, MaassenVanDenBrink A, European Headache Federation School of Advanced Studies (EHF-SAS) (2020) Persistent post-traumatic headache: a migrainous loop or not? The preclinical evidence. J Headache Pain 14(1):90. doi: https://doi.org/10.1186/s10194-020-01135-0
Labastida-Ramírez A, Benemei S, Albanese M, D’Amico A, Grillo G, Grosu O, Ertem DH, Mecklenburg J, Fedorova EP, Řehulka P, di Cola FS, Lopez JT, Vashchenko N, MaassenVanDenBrink A, Martelletti P(2020) ; Persistent post-traumatic headache: a migrainous loop or not? The clinical evidence. J Headache Pain. 2020 May 24;21(1):55. doi: https://doi.org/10.1186/s10194-020-01122-5
Acknowledgements
Not applicable.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this Article: The study was supported by an investigator-initiated research grant from Novartis Healthcare A/S.
Author information
Authors and Affiliations
Contributions
Study concept and design: HA, FMA, HWS.
Acquisition of data: HA, AI, HMA, AKE, ELL, AMA; KJH, KBB, BC.
Analysis and interpretation: HA, TPD, FMA, HWS.
Drafting the manuscript: HA.
Critical revision of the manuscript for important intellectual content: AI, HMA, AKE, ELL, AMA; KJH, KBB, BC, FMA, HWS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed written consent was obtained from each patient before conducting any study-related procedures. The study protocol was approved by the relevant ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: H.A. reports personal fees from Teva. F.M.A. has been a consultant, speaker, or scientific adviser for Eli Lilly, Lundbeck, Novartis, and Teva, outside of the submitted work. H.W.S. has been a consultant, speaker, or scientific adviser for Novartis, Eli Lilly, Lundbeck, Abbvie, and Teva, outside of the submitted work. All other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ashina, H., Iljazi, A., Al-Khazali, H.M. et al. CGRP-induced migraine-like headache in persistent post-traumatic headache attributed to mild traumatic brain injury. J Headache Pain 23, 135 (2022). https://doi.org/10.1186/s10194-022-01499-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-022-01499-5