Abstract
Background
Every genome contains a large number of uncharacterized proteins that may encode entirely novel biological systems. Many of these uncharacterized proteins fall into related sequence families. By applying sequence and structural analysis we hope to provide insight into novel biology.
Results
We analyze a previously uncharacterized Pfam protein family called DUF4424 [Pfam:PF14415]. The recently solved three-dimensional structure of the protein lpg2210 from Legionella pneumophila provides the first structural information pertaining to this family. This protein additionally includes the first representative structure of another Pfam family called the YARHG domain [Pfam:PF13308]. The Pfam family DUF4424 adopts a 19-stranded beta-sandwich fold that shows similarity to the N-terminal domain of leukotriene A-4 hydrolase. The YARHG domain forms an all-helical domain at the C-terminus. Structure analysis allows us to recognize distant similarities between the DUF4424 domain and individual domains of M1 aminopeptidases and tricorn proteases, which form massive proteasome-like capsids in both archaea and bacteria.
Conclusions
Based on our analyses we hypothesize that the DUF4424 domain may have a role in forming large, multi-component enzyme complexes. We suggest that the YARGH domain may play a role in binding a moiety in proximity with peptidoglycan, such as a hydrophobic outer membrane lipid or lipopolysaccharide.
Similar content being viewed by others
Background
A significant percentage of proteins encoded by all known genomes consist of uncharacterized proteins that have never been studied experimentally and do not show significant sequence similarity to any known proteins. A frequent first step in the analysis of such proteins is their classification into protein families. Many research groups are focusing on the identification and definition of new protein families that are then deposited into protein family databases and used for annotation of proteins by resources such as UniProtKB. Some protein families consist entirely of uncharacterized proteins, and therefore are typically defined as domains of unknown function (DUF) or uncharacterized protein families (UPFs). The Pfam database now contains a large collection of these families [1]. In this work we have analyzed the DUF4424 family [Pfam:PF14415] and the YARHG domain [2] [Pfam:PF13308]. The YARHG domain is also experimentally uncharacterized, but it was named for its highly conserved characteristic motif found in many of the sequences.
In a synergistic effort, the NIH Protein Structure Initiative (PSI) centers are systematically targeting uncharacterized proteins with the goal of providing structural information for a significant portion of the protein universe, often using Pfam for guidance in target selection. In this instance, Joint Center for Structural Genomics (JCSG) has solved the crystal structure of a hypothetical protein (lpg2210) [UniProtKB:Q5ZTF2] encoded in the genome of L. pneumophila subsp. Pneumophila str. Philadelphia 1 as a representative of the DUF4424 family, and deposited the coordinates in the Protein Data Bank as [PDB:4g2a]. L. pneumophila invades and replicates within human monocytes and alveolar macrophages in humans, and also within Amoebae, and is the established causative agent of legionellosis or Legionnaires’ disease.
Little is known about the lpg2210 protein. In a gene expression study, lpg2210 was found to be induced in the post-exponential growth phase, when L. pneumophila is known to express a variety of virulence factors in vitro[3]. Thus, it is plausible that lpg2210 and other members of this family, all of which have a signal sequence and are secreted proteins, may play a role in virulence.
Results, Methods and Discussion
Overall structure
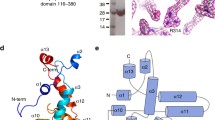
The crystal structure of lpg2210 from L. pneumophila subsp.pneumophila str philadelphia 1 was determined by three-wavelength MAD phasing at 2.33 Å resolution. Full details of data collection, model, and refinement statistics can be found in the Additional file 1. This protein contains two domains: an N-terminal DUF4424 domain that is mainly composed of a 19-stranded beta-sandwich fold and a YARHG domain that consists of a four-helical bundle in its C-terminal. The expressed protein contained a single N-terminal glycine (Gly 0) that remains after cleavage of the expression and purification tag, followed by residues 29-349 of the full-length protein. The asymmetric unit consists of one molecule of lpg2210. The final model includes residues Asn29 - Lys349, 14 sulphate molecules, and 160 water molecules. Electron density was disordered for Gly 0. The Matthews coefficient Vm is 2.23 Å3/Da and the estimated solvent content is 44.9%. The Ramachandran plot produced by Molprobity shows that 97.8% of the residues are in favored regions with no Ramachandran outliers. The protomer is composed of 19 β-strands, three β-sheets, six α-helices, five 310-helices, 18 β-turns, two γ-turns, and one disulphide bond (Figure 1). PISA results suggest that the monomer may be the natural oligomerization state (http://www.ebi.ac.uk/msd-srv/prot_int/).
Structure comparison
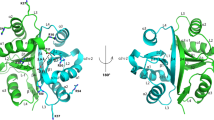
To learn more about the potential function of the DUF4424 and YARHG domains we carried out structure comparisons of each domain against the PDB database using the DALI server [4]. The YARHG domain comparison yielded no significant similarities to any other structure. The DUF4424 domain search yielded the highest scoring alignments to the N-terminal region of leukotriene A-4 hydrolase (LTA4H) [PDB:3fun], with a significant Z score of 11.3 (Figure 2A), and to the N-terminus of a tricorn protease-interacting factor F3 [PDB:1z5h] with a Z score of 10.9. In both these cases this N-terminal domain is playing an auxiliary role in assisting the catalytic core, possibly by binding substrate [5]. SCOP [6] classifies the first subunit of [PDB:3fun] into an N-terminal region of two beta-sandwiches of similar topologies fused together into a single three beta-sheet domain, the second domain as a central catalytic region that is a catalytic metallopeptidase (“zincin”), and the third C-terminal helical region as part of the ARM superfamily. The organization of the first and third domains of LTA4H is reminiscent of the orientation of the two domains present in lpg2210, although only the first beta-sandwich domain is structurally similar. The YARHG domain is an all-helical domain that is structurally unlike the LTA4H C-terminal domain (Figure 2B). It is possible that the lpg2210 protein binds to another enzymatic domain that is analogous to the LTA4H metallopeptidase domain. According to the three-dimensional structure analysis of LTA4H, the N-terminal domain of this enzyme contains a large concave surface exposed to the solvent (Figure 2A) that could participate in the recognition of specific substrates [5]. It is possible that the equivalent surface on DUF4424 might also participate in substrate-recognition.
Ribbon representation of structural alignment of the N-termini of human LTA4H and lpg2210. (A) The N-terminal domains of human leukotriene A4-hydrolase [PDB:3fun] is shown in cyan and lpg2210 protein from L. pneumophila [PDB:4g2a] is shown in yellow. The alignment has 165 equivalent positions with an RMSD of 3.13 Å, and a P-value of 5.15e-04 determined using FATCAT [7] with a 5.2% sequence identity. (B) The arrangements of the N- and C-terminal domains in human leukotriene A4 hydrolase [PDB:3fun] is reminiscent of that seen in lpg2210. The central, catalytic domain in the human leukotriene A4 hydrolase (gray in the figure) is missing in lpg2210. The C-terminal, helical domain of LTA4H has a four helical bundle structure, with no statistically significant similarity to the lpg2210 YARHG domain.
Although there were no structural similarities of the YARHG domain to any other structure, the structure is informative with respect to features of the domain itself. The YARHG domain family contains a subfamily, called YASKG that carries four conserved cysteine residues suggested to form two disulphide bridges (Figure 3B). The structure of the YARHG domain now allows us to evaluate this hypothesis and suggest a plausible bonding-pattern for the cysteines. The YASKG subfamily domains are relatively short, and carry only the YARHG domain with no other associated domains; these proteins are approximately 90 residues in length. The predicted positions of the cysteines on the [PDB:4g2a] version of YARHG indicate that only one pair share spatial proximity and could form a disulphide bridge. Cysteine 1 can potentially bond with cysteine 4. If cysteine 2 bonded with cysteine 3 it would induce significant structural rearrangements due to their distances from each other. The conformation of the shorter domains will be partly stabilized by the disulphide bridge in the absence of any other associated domains. The unbonded cysteines could also serve as redox sensors that regulate the binding of ligands by this domain.
Multiple sequence alignments of representative sequences in the DUF4424 and YARHG families. The secondary structure of [PDB:4g2a], which corresponds to [UniProtKB:Q5ZTF2] is shown above the alignment, with alpha helices colored red and beta strands colored yellow. The secondary structure elements are numbered as in Figure 1. (A) Multiple sequence alignment of representative DUF4424 domain sequences. Sequences were chosen based on both domain architecture and taxonomic distribution. The following sequences contain a C-terminal YARHG domain: [UniProtKB:Q5ZTF2] (Legionella pneumophila), [UniProtKB:Q7P768] (Fusobacterium nucleatum). The following sequences all from different Proteobacterial classes contain only the DUF4424 domain: [UniProtKB:C6B3I8] (Rhizobium leguminosarum bv. trifolii), [UniProtKB:C6M949] (Neisseria sicca), [UniProtKB:E8RIM1] (Desulfobulbus propionicus), [UniProtKB:B5NMH4] (Salmonella enterica). [UniProtKB:Q64EJ4] contains a C-terminal CARDB domain (Archaea metagenomics). (B) Multiple sequence alignment of representative YARHG domains. The alignment has been split to show examples of the classical YARHG at the top and the YASKG subfamily at the bottom. The classical YARHG representatives are: [UniProtKB:B5CPT2] (Ruminococcus lactaris ATCC 29176), [UniProtKB:C3BHH4] (Bacillus pseudomycoides), [UniProtKB:C3WK84] (Fusobacterium sp. 2_1_31), [UniProtKB:C7NDL9] (Leptotrichia buccalis), [UniProtKB:D8E0N2] (Prevotella bryantii), [UniProtKB:E2N0N0] (Capnocytophaga sputigena), [UniProtKB:H0MP08] (Salmonella enterica subsp. enterica serovar Montevideo str), [UniProtKB:Q7P768] (Fusobacterium nucleatum subsp. vincentii), [UniProtKB:Q8XP49] (Clostridium perfringens), [UniProtKB:A9G0S1] (Phaeobacter gallaeciensis) The YASKG subfamily contains four conserved cysteine residues, that are marked by a gray circle below the column. The sequences shown for this subfamily are: [UniProtKB:B0VPK4] (Acinetobacter baumannii), [UniProtKB:C6RJI6] (Acinetobacter radioresistens), [UniProtKB:D0C4A2] (Acinetobacter sp. RUH2624), [UniProtKB:G7GH70] (Acinetobacter sp. NBRC 100985).
The first structure of the YARHG domain gives us the opportunity to look at the reason that the YARHG motif is conserved among members of the family. Detailed examination of the structure shows that the most conserved sequence-region corresponds to the structural region that contains a rather unusual feature, i.e. that of crossing loops. The underlying loop connects helices H8 and H9 (Figure 1), whereas the next loop, between H9 and H10, crosses over the underlying loop. The YARHG sequence motif (the actual sequence in lpg2210 is YAQYG) maps onto the C-terminal part of H8 with the conserved Gly residue forming its C-terminal cap. The other conserved residues probably contribute to the stabilization of this structural feature. In particular, the small residue (Ala) is packed against several conserved aromatic residues ‘upstream’ of the YARHG motif (F308, F317, W322 and Y323). The sequence and structural conservation of this region suggest again that it might contribute to the binding of a yet unknown specific ligand of this family.
Three lines of evidence support the role of the YARHG as a ligand-binding domain that specializes in sensing extracellular ligands: 1) The YARHG domain is located in a predicted extracellular position with intracellular signaling domains such as a S/T protein kinase domain and three distinct kinds of intracellular Zn-ribbon domains. This architectural theme has been previously observed in several sensory proteins [8] and by analogy suggests that the binding of the ligand is communicated to intracellular domains; 2) The current structure reveals that the alpha-helical bundle adopted by the YARHG domain (Figure 4) is rather open in its configuration. This suggests that it would potentially facilitate interactions with a small molecule via this open pocket. Probing the pocket using 2 solvent radii binding helps better define this potential ligand-binding site; 3) Examination of the residues lining this pocket shows the presence of an unusual overrepresentation of exposed hydrophobic residues that are not involved in stabilizing the core via hydrophobic packing. This observation suggests they are likely to provide an interface for interacting with a hydrophobic ligand via solvent exclusion (Figure 4). In particular the association of the YARHG domain with extracellular peptidoglycan-binding domains such as the bacterial SH3 and PASTA domains is suggestive of a role for the YARGH domain in binding a moiety in proximity with peptidoglycan, such as the hydrophobic outer membrane lipid or lipopolysaccharides. In this capacity it might help anchor a variety of extracellular peptidase domains to the cell-surface.
Sequence analysis
To investigate further the potential functions of the DUF4424 domain we examined all the differing multiple domain architectural contexts in which the domain is found, as shown in Figure 5. The family DUF4424 in the current release of Pfam (27.0) consists of 165 sequences from UniProtKB [9]. Almost all proteins in the DUF4424 family carry a predicted signal peptide at the N-terminus indicating that they are secreted proteins. Some 70% carry only the DUF4424 domain, with no other associated domains. The remaining 30% have an associated YARHG domain at their C-terminal end. The proteins range in length between approximately 300 and 360 amino acids. There is one sequence, [UniProtKB:Q64EJ4], that is longer than the average, and, although not carrying a YARHG domain, has a CARDB domain at the C-terminus. The CARDB domain is related to bacterial cell-adhesion.
Representative domain architectures of sequences carrying a DUF4424 domain. Four different domain compositions or architectures are found amongst the sequences in the DUF4424 family. The signal-peptide is shown in pink, the DUF in blue and the associated, YARHG, domain in yellow, the CARDB domain in green.
The sequence alignment of the DUF4424 domain does not suggest any strongly conserved short motifs suggestive of a particular function, and is shown for a representative set in Figure 3A.
Species distribution
Pfam family DUF4424 is found predominantly in Gram-negative bacteria, in particular in Fusobacteria and Proteobacteria species, where it is found in Alpha-, Beta-, Gamma- and Delta-proteobacteria. Many of these species make up part of the natural gut and oral flora of a human, but can also be involved in human disease. For example, Fusobacterium nucleatum is an oral bacterium, indigenous to the human oral cavity, that plays a role in periodontal disease. The organism is commonly recovered from different monomicrobial and mixed infections in humans and animals. It is a key component of periodontal plaque due to its abundance and its ability to co-aggregate with other species in the oral cavity [10]. The sequence containing the CARDB domain came from a genomic analysis of deep-sea sediments [11] and is annotated as being of archaeal origin.
Genomic context
In bacterial families it has been shown that analysis of the gene-neighborhood can give hints about the function of a protein family [12]. Our analysis of genomic contexts using MicrobesOnline [13] and STRING [14] did not show any clearly recurrent associations with other genes that might give a hint towards function.
Conclusions
In this work we present the novel structure of the lpg2210 protein from the bacterium Legionella pneumophila. The structure confirms the domain organization that was inferred through careful sequence analysis in the Pfam database. The N-terminal domain was found to share structural similarity to a variety of peptidase-associated domains. Based on these structural similarities we suggest that the lpg2210 protein is a part of a multiple-component enzyme, possibly pairing with a catalytic partner. Our analysis is also suggestive of a role for the YARGH domain in binding a moiety in proximity with peptidoglycan. This could be a hydrophobic outer membrane lipid or lipopolysaccharide. In this capacity it might help anchor a variety of extracellular peptidase domains to the cell-surface.
References
Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer ELL, Eddy SR, Bateman A, Finn RD: The Pfam protein families database. Nucleic Acids Res. 2012, 40: D290-D301. 10.1093/nar/gkr1065.
Coggill P, Bateman A: The YARHG domain: an extracellular domain in search of a function. PLoS ONE. 2012, 7: e35575-10.1371/journal.pone.0035575.
Edwards RL: PhD thesis. Metabolic cues and regulatory proteins that govern Legionella Pneumophila differentiation and virulence. 2008, : University of Michigan, Cellular and Molecular Biology Department
Holm L, Rosenström P: Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010, 38: W545-W549. 10.1093/nar/gkq366.
Thunnissen MM, Nordlund P, Haeggström JZ: Crystal structure of human leukotriene A(4) hydrolase, a bifunctional enzyme in inflammation. Nat Struct Biol. 2001, 8: 131-135. 10.1038/84117.
Andreeva A, Murzin AG: Structural classification of proteins and structural genomics: new insights into protein folding and evolution. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010, 66: 1190-1197. 10.1107/S1744309110007177.
Ye Y, Godzik A: Flexible structure alignment by chaining aligned fragment pairs allowing twists. Bioinformatics. 2003, 19 (Suppl 2): ii246-ii255. 10.1093/bioinformatics/btg1086.
Anantharaman V, Aravind L: Application of comparative genomics in the identification and analysis of novel families of membrane-associated receptors in bacteria. BMC Genomics. 2003, 4: 34-10.1186/1471-2164-4-34.
Consortium UP: The Universal Protein Resource (UniProt) 2009. Nucleic Acids Res. 2009, 37: D169-D174.
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE: Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005, 43: 5721-5732. 10.1128/JCM.43.11.5721-5732.2005.
Hallam SJ, Putnam N, Preston CM, Detter JC, Rokhsar D, Richardson PM, DeLong EF: Reverse methanogenesis: testing the hypothesis with environmental genomics. Science. 2004, 305: 1457-1462. 10.1126/science.1100025.
Snel B, Bork P, Huynen MA: The identification of functional modules from the genomic association of genes. Proc Natl Acad Sci USA. 2002, 99: 5890-5895. 10.1073/pnas.092632599.
Dehal PS, Joachimiak MP, Price MN, Bates JT, Baumohl JK, Chivian D, Friedland GD, Huang KH, Keller K, Novichkov PS, Dubchak IL, Alm EJ, Arkin AP: MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Res. 2010, 38: D396-D400. 10.1093/nar/gkp919.
Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, Mering C, Jensen LJ: STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41: D808-D815. 10.1093/nar/gks1094.
Acknowledgements
We are grateful to the Sanford Burnham Medical Research Institute for hosting the DUF annotation jamboree in June 2013, which allowed the authors to collaborate on this work. We would like to thank all the participants of this workshop for their intellectual contributions to this work, who, in addition to the authors, were: Padmaja Natarajan, Marco Punta, Neil Rawlings, Daniel Rigden, Mayya Sedova, Anna Sheydina, John Wooley. We thank the members of the JCSG high-throughput structural biology pipeline for their contribution to this work.
Funding
Wellcome Trust (grant numbers WT077044/Z/05/Z); Howard Hughes Medical Institute; Work by LA is supported by the intramural funds of the National Library of Medicine, USA.; NIH (R01GM101457); This work was supported in part by National Institutes of Health Grant U54 GM094586 from the NIGMS Protein Structure Initiative to the Joint Center for Structural Genomics. The DUF annotation jamboree was supported by National Science Foundation (IIS-0646708 and IIS-1153617); Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). Work by AGM was supported by the UK Medical Research Council [MC_U105192716]. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS, NCRR or NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PC and AGB wrote the manuscript; PC, RYE, AGB, RDF and YC carried out the sequence-analysis; LJ, AG, DD, QP, HLA carried out strucuture-determination; LA and AGM performed sequence- and structural-analyses. All authors read and approved the final manuscript.
Penelope Coggill and Alex Bateman contributed equally to this work.
Electronic supplementary material
12859_2013_6052_MOESM1_ESM.docx
Additional file 1: Experimental Details [PDB:4g2a] [UniProtKB:Q5ZTF2]. This section contains the detailed Materials and Methods with an appropriate Table of results for the determination of the structure under investigation. (DOCX 32 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Coggill, P., Eberhardt, R.Y., Finn, R.D. et al. Two Pfam protein families characterized by a crystal structure of protein lpg2210 from Legionella pneumophila. BMC Bioinformatics 14, 265 (2013). https://doi.org/10.1186/1471-2105-14-265
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2105-14-265