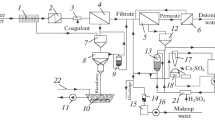
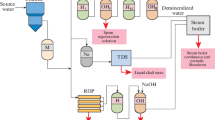
Abstract
Reverse electrodialysis (RED) technology is one of the promising directions of alternative energy based on electrochemical transformations. In this study, an industrial experiment on the utilization of highly mineralized alkaline liquid waste from a chemical desalination water treatment plant by RED technology is performed on an experimental pilot electromembrane unit at an operating TPP. The RED process with ion-selective cation- and anion-exchange membranes proceeds in the diffusion dialysis mode under the action of the concentration gradient of ionic current carriers. As a result, the specific power of the unit per unit area of the membrane pair of 0.36 W/m2 is recorded. The rate of diffusion dialysis transport of the electrolyte substance is 0.3–0.6 kg/h on average per an apparatus. The efficiency of the RED process with respect to the energy generation is 47%, the theoretical electromotive force is 0.12 V per chamber, and the potential energy storage (specific generated energy) is 1.8 kWh/m3 of highly mineralized liquid waste at f = 1/2.




Similar content being viewed by others
REFERENCES
N. V. Korovin, Fuel Cells and Electrochemical Power Plants (Izdatel’stvo MEI, Moscow, 2005) [in Russian].
G. L. Wick, Energy 3, 95 (1978).
J. W. Post, H. V. M. Hamelers, and C. J. N. Buisman, Environ. Sci. Technol. 42, 5785 (2008).
M. Ye, M. Pasta, X. Xie, Y. Cui, and C. S. Criddle, Energy Environ. Sci. 7, 2295 (2014).
D. Brogioli, R. Ziano, R. A. Rica, D. Salerno, O. P. Kozynchenko, and H. V. Hamelers, Energy Environ. Sci. 5, 9870 (2012).
Y. Mei and C. Y. Tang, Desalination 425, 156 (2018).
J. W. Post, C. H. Goeting, J. Valk, and S. Goinga, J. Veerman, and H. V. M. Hamelers, Desalin. Water Treat. 16, 182 (2012).
M. Turek and B. Bandura, Desalination 205, 67 (2007).
P. Dlugole, C. Ag, K. Nijmeijer, and M. Wessling, Environ. Sci. Technol. 43, 6888 (2009).
M. Tedesco, C. Scalici, D. Vaccari, A. Cipollina, A. Tamburini, and G. Micale, J. Membr. Sci. 500, 33 (2016).
M. Tedesco, A. Cipollina, A. Tamburini, and G. Micale, J. Membr. Sci. 522, 226 (2017).
J. Y. Nam, K. S. Hwang, H. C. Kim, H. Jeong, H. Kim, and E. Jwa, Water Res. 148, 261 (2019).
E. G. Novitsky, E. A. Grushevenko, V. P. Vasilevsky, and A. V. Volkov, Membr. Membr. Technol. 2, 109 (2020).
A. V. Semenyuk, V. V. Knyazhev, and S. A. Garmash, Nauch. Probl. Transp. Sib. Dal’nego Vost. 2, 340 (2010).
R. A. Tufa, S. Pawlowski, J. Veerman, K. Bouzek, E. Fontananova, and G. di Profio, Appl. Energy 225, 290 (2018).
D. A. Vermaas, J. Veerman, N. Y. Yip, M. Elimelech, M. Saakes, and K. Nijmeijer, ACS Sustainable Chem. Eng. 1, 1295 (2013).
H. Tian, Y. Wang, Y. Pei, and J. C. Crittenden, Appl. Energy 262, 114482 (2020).
A. M. Benneker, T. Rijnaarts, R. G. H. Lammertink, and J. A. Wood, J. Membr. Sci. 548, 421 (2018).
E. Yu. Safronova, D. V. Golubenko, N. V. Shevlyakova, M. G. D’yakova, V. A. Tverskoi, L. Dammak, D. Grande, and A. B. Yaroslavtsev, J. Membr. Sci. 515, 196 (2016).
D. V. Golubenko, B. V. Bruggen, and A. B. Yaroslavtsev, J. Appl. Polym. Sci. 137, 48656 (2020).
D. V. Golubenko, G. Pourcelly, and A. B. Yaroslavtsev, Sep. Purif. Technol. 207, 329 (2018).
E. Güler, R. Elizen, D. A. Vermaas, M. Saakes, and K. Nijmeijer, J. Membr. Sci. 446, 266 (2013).
D. A. Vermaas, M. Saakes, and K. Nijmeijer, J. Membr. Sci. 385–386, 234 (2011).
D. A. Vermaas, D. Kunteng, J. Veerman, M. Saakes, and K. Nijmeijer, Environ. Sci. Technol. 48, 3065 (2014).
G. Z. Ramon, B. J. Feinberg, and E. M. V. Hoek, Energy Environ. Sci. 4, 4423 (2011).
E. Fontananova, W. Zhang, I. Nicotera, C. Simari, W. van Baak, and G. Di Profio, J. Membr. Sci. 459, 177 (2014).
L. J. J. Janssen, J. Appl. Electrochem. 37, 1383 (2007).
R. A. Tufa, E. Rugiero, D. Chanda, J. Hnat, W. van Baak, and J. Veerman, J. Membr. Sci. 514, 155 (2016).
R. A. Tufa, J. Hnát, M. Němeček, R. Kodým, E. Curcio, and K. Bouzek, J. Cleaner Prod. 203, 418 (2018).
M. C. Hatzell, X. Zhu, and B. E. Logan, ACS Sustainable Chem. Eng. 2, 2211 (2014).
X. Chen, C. Jiang, Y. Zhang, Y. Wang, and T. Xu, J. Membr. Sci. 544, 397 (2017).
X. Chen, C. Jiang, M. A. Shehzad, Y. Wang, H. Feng, and Z. Yang, ACS Sustainable Chem. Eng. 7, 130230 (2019).
J. H. Han, H. Kim, K. S. Hwang, N. Jeong, and C. S. Kim, J. Electrochem. Sci. Technol. 10, 302 (2019).
X. Zhu, T. Kim, M. Rahimi, C. A. Gorski, and B. E. Logan, ChemSusChem 10, 797 (2017).
R. S. Kingsbury, K. Chu, and O. Coronell, J. Membr. Sci. 495, 502 (2015).
W. J. van Egmond, M. Saakes, S. Porada, T. Meuwissen, C. J. N. Buisman, and H. V. M. Hamelers, J. Power Sources 325, 129 (2016).
O. Scialdone, A. D’Angelo, E. De Lume, and A. Galia, “Cathodic Reduction of Hexavalent Chromium Coupled with Electricity Generation Achieved by Reverse-Electrodialysis Processes Using Salinity Gradients,” Electrochim. Acta 137, 258 (2014).
O. Scialdone, A. D’Angelo, and A. Galia, J. Electroanal. Chem. 738, 61 (2015).
Y. Zhou, K. Zhao, C. Hu, H. Liu, Y. Wang, and J. Qu, Electrochim. Acta 269, 128 (2018).
S. Lee, et al., Ain Shams Eng. J. 12, 159 (2021).
D. M. Kader and N. V. Alekseeva, Yuzhno-Sib. Nauch. Vest. 2, 161 (2019).
D. A. Vermaas, E. Guler, M. Saakes, and K. Nijmeijer, Energy Proc. 20, 170 (2012).
D. A. Vermaas, M. Saakes, and K. Nijmeijer, Environ. Sci. Technol. 45, 7089 (2011).
H. I. Jeong, H. J. Kim, and D. K. Kim, Energy 68, 229 (2014).
N. Y. Yip, D. A. Vermaas, K. Nijmeijer, and M. Elimelech, Environ. Sci. Technol. 48, 4925 (2014).
A. A. Filimonova, E. K. Arakelyan, A. A. Chichirov, N. D. Chichirova, and A. I. Minibaev, Nov. Elektroenerg. 3, 29 (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Filimonova, A.A., Chichirov, A.A. & Chichirova, N.D. The Utilization of Highly Mineralized Liquid Waste from a Chemical Desalination Water Treatment Plant of a TPP with the Generation of Electrical Energy by Reverse Electrodialysis. Membr. Membr. Technol. 3, 344–350 (2021). https://doi.org/10.1134/S251775162105005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S251775162105005X