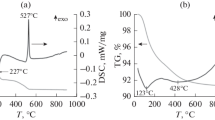
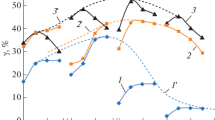
Abstract
The in situ formation of a catalytic heterohomogeneous system containing Al–M alloy (M is Ni, Co, Cu) and Al(M)/Cl complex in a benzene–ethylene medium at a temperature of 80°C and a pressure of 0.2–0.3 MPa is studied. The characteristic patterns of interaction between Al–M alloys activated with a liquid metal Ga–In eutectic and a chlorinating agent (CCl4) with the formation of catalytically active metal–aluminum chloride Al(M)/Cl complexes are established. Results from spectrokinetic measurements show the order of the reactivity of activated alloys with respect to excess CCl4 is Al–Cu ≈ Al–Ni > Al > Al–Co. The highest catalytic activity is displayed by nickel–aluminum chloride complexes whose selectivity toward ethylbenzene is 48%. Data from IR and UV-VIS spectroscopy show that the structure and composition of metal chloride complexes formed in situ in the aromatic reaction medium is determined by a combination of coupled ionic pairs \([{\text{AlC}}{{{\text{l}}}_{4}}]_{{{\text{tetr}}}}^{ - }{\text{/[NiC}}{{{\text{l}}}_{{\text{6}}}}]_{{{\text{oct}}}}^{{4-}}\) and \(\left[ {{\text{AlC}}{{{\text{l}}}_{{\text{4}}}}} \right]_{{{\text{tetr}}}}^{ - }/\left[ {{\text{CuC}}{{{\text{l}}}_{{\text{2}}}}} \right]_{{{\text{lin}}}}^{ - }\), which are stabilized by (C6H5)3C+ carbocation.



Similar content being viewed by others
REFERENCES
Singhal, S., Agarwal, S., Singh, M., Rana, S., Arora, S., and Singhal, N., J. Mol. Liq., 2019, vol. 285, pp. 299–313.
Yan, G.X., Wang, A., Wachs, I.E., and Baltrusaitis, J., Appl. Catal., A, 2019, vol. 572, pp. 210–225.
Lavrenov, A.V., Bogdanets, E.N., and Duplyakin, V.K., Katal. Prom-sti, 2009, no. 1, pp. 28–38.
Li, R., Xing, S., Zhang, S., and Han, M., RSC Adv., 2020, vol. 10, no. 17, pp. 10006–10016.
Olivier-Bourbigou, H., Magna, L., and Morvan, D., Appl. Catal, A, 2010, vol. 373, pp. 1–56.
Shi, Y., Xing, E., Xie, W., Zhang, F., Mu, X., and Shu, X., J. Mol. Catal. A: Chem., 2016, vols. 418/419, pp. 86–94.
Rao, S.M., Saraci, E., Glaser, R., and Coppens, M.O., Chem. Eng. J., 2017, vol. 329, pp. 45–55.
Wang, Y., Gao, Y., Xie, S., Liu, S., Chen, F., Xin, W., Zhu, X., Li, X., Jiang, N., and Xu, L., Catal. Today, 2018, vol. 316, pp. 71–77.
Polubentseva, M.F., Duganova, V.V., and Mikhailenko, G.A., Russ. J. Gen. Chem., 1996, vol. 66, no. 4, pp. 614–618.
Trenikhin, M.V., Kozlov, A.G., Nizovskii, A.I., Drozdov, V.A., Lavrenov, A.V., Bubnov, A.V., Fine-vich, V.P., and Duplyakin, V.K., Russ. J. Gen. Chem., 2007, vol. 77, no. 12, pp. 2320–2327.
Fischman, J., Godart, P., and Hart, D., Int. J. Hydrogen Energy, 2020, vol. 45, no. 35, pp. 17118–17130.
Slocum, J.T., Eagar, T.W., Taylor, R., and Hart, D.P., Appl. Energy, 2020, vol. 279, paper no. 115712.
Arbuzov, A.B., Drozdov, V.A., Likholobov, V.A., Trenikhin, M.V., Talsi, V.P., and Kudrya, E.N., Kinet. Catal., 2010, vol. 51, no. 3, pp. 354–358.
Arbuzov, A.B., Drozdov, V.A., Shlyapin, D.A., and Lavrenov, A.V., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2018, vol. 61, nos. 9/10, pp. 64–69.
Arbuzov, A.B., Shilova, A.V., Trenikhin, M.V., and Drozdov, V.A., Chem. Sustainable Dev., 2017, vol. 25, no. 2, pp. 133–137.
Arbuzov, A.B., Drozdov, V.A., Trenikhin, M.V., Leont’eva, N.N., Shilova, A.V., Kireeva, T.V., and Lavrenov, A.V., Prot. Met. Phys. Chem. Surf., 2015, vol. 51, no. 4, pp. 587–592.
Arbuzov, A.B., Drozdov, V.A., Kazakov, M.O., Lavrenov, A.V., Trenikhin, M.V., and Likholobov, V.A., Kinet. Catal., 2012, vol. 53, no. 3, pp. 357–362.
Arbuzov, A.B., Drozdov, V.A., Talsi, V.P., Babenko, A.V., Izmailov, R.R., and Yurpalov, V.L., AIP Conf. Proc., 2019, vol. 2143, paper no. 020044.
Arbuzov, A.B., Drozdov, V.A., and Talsi, V.P., AIP Conf. Proc., 2020, vol. 2301, paper no. 040002.
Arbuzov, A.B., Drozdov, V.A., and Talsi, V.P., AIP Conf. Proc., 2020, vol. 2301, paper no. 040001.
Arbuzov, A.B., Drozdov, V.A., Leont’eva, N.N., Shilova, A.V., Kireeva, T.V., Trenikhin, M.V., and Lavrenov, A.V., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, no. 4, pp. 653–657.
Arbuzov, A.B., Drozdov, V.A., Trenikhin, M.V., Muromtsev, I.V., Izmailov, R.R., and Kireeva, T.V., Russ. J. Appl. Chem., 2017, vol. 90, no. 12, pp. 1998–2003.
Drozdov, V.A., Arbuzov, A.B., Trenikhin, M.V., Lavrenov, A.V., Kazakov, M.O., and Likholobov, V.A., Chem. Sustainable Dev., 2011, vo. 19, no. 1, pp. 45–52.
Pulletikurthi, G., Bödecker, B., Borodin, A., Weidenfeller, B., and Endres, F., Prog. Nat. Sci.: Mater. Int., 2015, vol. 25, no. 6, pp. 603–611.
Lever, A.B.P., Inorganic Electronic Spectroscopy, Amsterdam: Elsevier, 1984.
Estager, J., Holbrey, J.D., and Swadźba-Kwaśny, M., Chem. Soc. Rev., 2014, vol. 43, no. 3, pp. 847–886.
Roeper, D.F., Pandya, K.I., Cheek, G.T., and O’Grady, W.E., J. Electrochem. Soc., 2011, vol. 158, no. 3, pp. F21–F28.
Eide, O.K., Ystenes, M., Støvneng, J.A., and Eilertsen, J.L., Vib. Spectrosc., 2007, vol. 43, no. 1, pp. 210–216.
Sovremennye problemy khimii karbonievykh ionov (Contemporary Problems of Carbonium Ions Chemistry) Koptyug, V.A, Ed., Novosibirsk: Nauka, 1975.
Makarychev, Yu.B., Akimov, A.G., and Azarov, A.A., Poverkhn.: Fiz., Khim., Mekh., 1985, no. 7, pp. 143–147.
Domen, K. and Chuang, T.J., J. Am. Chem. Soc., 1987, vol. 109, no. 17, pp. 5288–5289.
ACKNOWLEDGMENTS
The authors are grateful to engineer S.N. Evdokimov and chief engineer E.N. Kudrya for participating in our NMR and GC/MS studies.
Funding
This work was supported by the RF Ministry of Science and Higher Education as part of a state taks to the Boreskov Institute of Catalysis, project no. AAAA-A21-121011890074-4.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Arbuzov, A.B., Drozdov, V.A., Lavrenov, A.V. et al. Creating a Heterohomogeneous Catalytic System for the Alkylation of Benzene with Ethylene through the Reaction beteen Carbon Tetrachloride and Aluminum Alloys. Catal. Ind. 14, 363–368 (2022). https://doi.org/10.1134/S207005042204002X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207005042204002X