Abstract
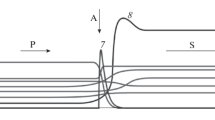
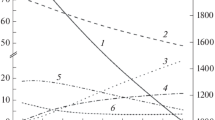
The possibility of converting low-caloric fuels into synthesis gas by their partial oxidation in a moving porous layer of a granular heat carrier is theoretically studied. The process is considered in a new version of the reactor with a counterflow of a solid granular heat carrier and reacting gases. A feature of the reactor is the presence of an additional heat exchanger, where the oxidizer gas is preheated due to the residual heat of the solid heat carrier discharged from the reactor. Theoretically, in the approximation of the absence of heat loss and established thermodynamic equilibrium in the products, the macrokinetic modes of the process are considered depending on the main control parameters: the flow rate of the oxidizer gas, the flow rate of steam, the flow rate of the solid heat carrier, and the fuel composition. A quantitative calculation of the temperature and composition of the products in various combustion modes is carried out for the air-steam conversion of isopropanol and the air conversion of low-caloric pyrolysis gas. It is shown that the scheme under consideration provides a possibility to convert low-caloric gas at a temperature above 1500 K with a chemical efficiency of gasification over 90%.








Similar content being viewed by others
Notes
Strictly speaking, since isopropanol decomposes at a high temperature, we should use the enthalpy of the decomposition products which reach the combustion zone. In this case, the enthalpy of the pyrolysis reaction will be a term in the heat capacity of isopropanol.
REFERENCES
V. S. Arutyunov, L. N. Strekova, V. I. Savchenko, I. V. Sedov, A. V. Nikitin, O. L. Eliseev, M. V. Kryuchkov, and A. L. Lapidus, Pet. Chem. 59, 370 (2019). https://doi.org/10.1134/S0965544119040029
H. N. Abubackar, M. C. Veiga, and C. Kennes, in Sustainable Resource Recovery and Zero Waste Approaches, Ed. by M. J. Taherzadeh, K. Bolton, J. Wong, and A. Pandey (Elsevier, UK, 2019), p. 207. https://doi.org/10.1016/B978-0-444-64200-4.00015-3
J. Huang, K. G. Schmidt, and Z. Bian, Energies 4, 1163 (2011). https://doi.org/10.3390/en4081163
W. Zhang, H. Liu, I. Ul Hai, Y. Neubauer, P. Schröder, H. Oldenburg, A. Seilkopf, and A. Kölling, Int. J. Low-Carbon Technol. 7 (2), 69 (2012). https://doi.org/10.1093/ijlct/ctr046
V. G. Sister, A. A. Borisov, K. Ya. Troshin, et al., Khim. Fiz. 25 (1), 61 (2006).
V. S. Arutyunov and L. N. Strekova, Russ. J. Phys. Chem. B 6, 486 (2012).
S. M. Aldoshin, V. S. Arutyunov, V. I. Savchenko, I. V. Sedov, A. V. Nikitin and I. G. Fokin, Russ. J. Phys. Chem. B 15, 498 (2021).
A. M. Tereza, G. L. Agafonov, E. K. Anderzhanov, and S. P. Medvedev, Russ. J. Phys. Chem. B 15, 678 (2021).
A. M. Tereza, G. L. Agafonov, E. K. Anderzhanov, S. P. Medvedev, S. V. Khomik, S. K. Petrov, and M. V. Chernyshov, Russ. J. Phys. Chem. B 14, 654 (2020).
S. V. Glazov, V. M. Kislov, A. V. Razmyslov, and M. V. Salganskaya, Russ. J. Appl. Chem. 92, 1020 (2019). https://doi.org/10.1134/S107042721907019X
S. V. Glazov, Theor. Found. Chem. Eng. 53, 51 (2019). https://doi.org/10.1134/S0040579519010032
S. Dorofeenko and E. Polianczyk, Chem. Eng. J. 292, 183 (2016). https://doi.org/10.1016/j.cej.2016.02.013
E. Polianczyk and S. Dorofeenko, Int. J. Hydrogen Energy 44, 4079 (2019). https://doi.org/10.1016/j.ijhydene.2018.12.117
S. S. Kostenko, E. V. Polianchik, A. A. Karnaukh, A. N. Ivanova, and G. B. Manelis, Khim. Fiz. 25 (5), 43 (2006).
L. V. Zyubin and K. D. Bakanov, RF Patent No. 2733777, Rospatent No. 28 (2020).
S. Dorofeenko and E. Polianczyk, Int. J. Hydrogen Energy 44, 30039 (2019). https://doi.org/10.1016/j.ijhydene.2019.09.208
V. S. Arutyunov, Khim. Fiz. 24 (9), 76 (2005).
Thermodynamical Properties of Individual Substances, The Handbook, Ed. by V. P. Glushko, L. V. Gurvich, G. A. Bergman, et al. (Nauka, Moscow, 1978, 1981) [in Russian].
G. P. Smith, D. M. Golden, M. Frenklach, et al., GRI-Mech 3.0 (2018). http://combustion.berkeley.edu/gri-mech/version30/text30.html.
J. Chao and F. D. Rossini, J. Chem. Eng. Data 10, 374 (1965). https://doi.org/10.1021/je60027a022
D. I. Mendeleev, Basics of the Factory Industry (Tipogr. V. Demakova, St. Petersburg, 1897), No. 1, p. 90 [in Russian].
M. B. Ravich, Fuel Efficiency (Nauka, Moscow, 1977), p. 36 [in Russian].
Funding
This study was partly supported by the Russian Foundation for Basic Research in cooperation with the TUBITAK agency as part of research project no. 21-51-46007. This study was carried out in relation to a state order (registration number AAAA-A19-119022690098-3, topic 0089-2019-0018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorofeenko, S.O., Polianczyk, E.V. Conversion of Low Caloric Fuels to Synthesis Gas in the Filtration Combustion Mode in a Moving Layer of a Granulated Heat Carrier. Russ. J. Phys. Chem. B 16, 242–252 (2022). https://doi.org/10.1134/S199079312202004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199079312202004X