Abstract
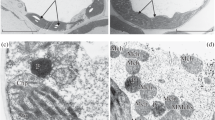
Under controlled conditions, the effect of high (40°C, 2 h) and positive low (4°C, 2 h) temperatures on the ultrastructure of mesophyll cells of the leaf and the content of photosynthetic pigments, phenols, and flavonoids in 2-week-old Triticum spelta plants was studied. The ultrastructure of the mesophyll cells of the leaf of the control plants was typical: a developed thylakoid system was clearly seen that was immersed in a fine-grained stroma in the chloroplasts of regular lenticular shape. Short-term hyperthermia caused a partial destruction of thylakoid membranes. Wave-shaped packing of grana thylakoids, significant expansion of lumen intervals, disturbance of the structural bond between the grana thylacoids and stroma thylakoids was noted. With hyperthermia, the mitochondria noticeably “swelled,” while the cristae membranes became less contrasting. The number of lipid droplets increased in the cytoplasm of cells. In the leaves, the content of chlorophylls and carotenoids decreased, however, the number of common phenols and flavonoids increased. Short-term hypothermia caused intense formation of plastoglobules, and an increase in the number and size of starch grains. Destruction of thylakoid membranes was not observed. Some of the mitochondria were rounded (40%), with their size being close to the control values, and some organelles were lenticular, “dumbbell,” and “cup-shaped.” Under hyper- and hypothermia, the T. spelta leaf mesophyll cells showed a tendency to increase the degree of chromatin condensation in the nucleus. Under hypothermia, the content and ratio of chlorophylls and carotenoids in leaves did not differ much from the control plants, and no significant quantitative changes in the total phenols and flavonoids were recorded.



Similar content being viewed by others
REFERENCES
Amarowicz, R., Weidner, S., Wojtowicz, I., Karmacґ, M., Kosinґska, A., and Rybarczyk, A., Influence of low-temperature stress on changes in the composition of grapevine leaf phenolic compounds and their antioxidant properties, Funct. Plant Sci. Biotechnol., 2010, vol. 4, pp. 90–96.
Armstrong, A.F., Logan, D.C., Tobin, A.K., O’Toole, P., and Atkin, O.K., Heterogeneity of plant mitochondrial responses underpinning respiratory acclimation to the cold in Arabidopsis thaliana Leaves, Plant Cell Environ., 2006, vol. 29, pp. 940–949.
Asada, K., Production and scavenging of reactive oxygen species in chloroplasts and their functions, Plant Physiol., 2006, vol. 141, pp. 391–396.
Austin, J.R., Frost, E., Vidi, P.A, Kessler, F., and Staehelin, L.A., Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes, Plant Cell, 2006, vol. 18, pp. 1693–1703.
Babenko, L.M., The effect of stress temperatures on the activity of lipoxygenases in Triticum spelta, Bull. Kharkiv Nat. Agrar. Univ., Ser. Biol., 2018, vol. 1, no. 43, pp. 40–46.
Babenko, L.M., Kosakivska, I.V., Akimov, Yu.A., Klymchuk, D.O., and Skaternya, T.D., Effect of temperature stresses on pigment content, lipoxygenase activity and cell ultrastructure of winter wheat seedlings, Gen. Plant Physiol., 2014, vol. 4, pp. 117–125.
Babenko, L.M., Scherbatiuk, N.N., Klimchuk, D.A, and Kosakovskaya, I.V., Structural-functional peculiarities of leaf mesophyll cells of Triticum aestivum cultivars with different cold/heat tolerance under short-term temperature stresses, Tsitologiia, 2018, vol. 60, no. 2, pp. 128–135.
Bobo-García, G., Davidov-Pardo, G., Arroqui, C., Vírseda, P., Marín-Arroyo, M.R., and Navarro, M., Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods, J. Sci. Food Agric., 2015, vol. 95, pp. 204–209.
Brüggemann, W., Klaucke, S., and Maas-Kantel, K., Long-term chilling of young tomato plants under low light. V. Kinetic and molecular properties of two key enzymes of the Calvin cycle in Lycopersicon esculentum Mill. and L. peruvianum Mill, Planta, 1994, vol. 194, pp. 160–168.
Ciamporova, M., and Mistrik, I., The ultrastructural response of root cells to stressful conditions, Environ. Exp. Bot., 1993, vol. 33, pp. 11–26.
Commuri, P.D. and Jones, R.J., Ultrastructural characterization of maize (Zea mays L.) kernels exposed to high temperature during endosperm cell division, Plant Cell Environ., 1999, vol. 22, pp. 375–385.
Es-Safi, N.E., Ghidouche, S., and Ducrot, P.H., Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity, Molecules, 2007, vol. 12, pp. 2228–2258.
Hatfield, J. and Prueger, J., Temperature extremes: effect on plant growth and development, Weather Climate Extremes, 2015, vol. 10, pp. 4–10.
Holaday, A.S., Martindale, W., and Alred, R., Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature, Plant Physiol., 1992, vol. 98, pp. 1105–1114.
Hurry, V.M., Strand, Å., and Tobiæson, M., Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content, Plant Physiol., 1995, vol. 109, pp. 697–706.
Kislyuk, I.M., Bubolo, L.S., Kamentseva I.E., Kotlova, E.R., and Sherstneva, O.A., Heat shock increases thermotolerance of photosynthetic electron transport and the content of chloroplast membranes and lipids in wheat leaves, Russ. J. Plant Physiol., 2007, vol. 54, pp. 456–463.
Kislyuk, I.M., Bubolo, L.S., Bykov, O.D., Kamentse-va, I.E., and Sherstneva, O.A., Protective and injuring action of visible light on photosynthetic apparatus in wheat plants during hyperthermia treatment, Russ. J. Plant Physiol., 2008, vol. 55, pp. 613–621.
Kleine, T., Voigt, C., and Leister, D., Plastid signalling to the nucleus: messengers still lost in the mists?, Trends Genet., 2009, vol. 25, pp. 185–192.
Klymchuk, D.O., Kosakivska, I.V., Akimov, Yu.M., Shcherbatyuk, M.M., and Vorobyova, T.V., Structure-functional peculiarities of Brassica campestris and Amarantus caudathus leaf cells under low positive temperature, Bull. Kharkiv Nat. Agrar. Univ., Ser. Biol., 2011, vol. 3, no. 24, pp. 15–24.
Klymchuk, D.O., Kosakivska, I.V., Akimov, Yu.M., Shcherbatyuk, M.M., and Vorobyova, T.V., Structural and functional peculiarities of Brassica campestris and Amaranthus caudatus leaf cells under high temperature, Bull. Kharkiv Nat. Agrar. Univ., Ser. Biol., 2012, vol. 2, no. 26, pp. 61–70.
Kosakivska, I.V., Klymchuk, D.O., Negretzky, V.A., Bluma, D.A., and Ustinova, A.Yu., Stress proteins and ultrastructural characteristics of leaf cells in plants with different types of ecological strategies, Gen. Appl. Plant Physiol., 2008, vol. 34, pp. 405–418.
Kosakovskaya, I.V., Babenko, L.M, Skaternaya, T.D., and Ustinova, A.Yu., Influence of hypo- and hyperthermia on the activity and content of pigments and soluble proteins in Triticum aestivum L. seedlings of Yatran 60, Fiziol. Rast. Genet., 2014, vol. 46, no. 3, pp. 212–220.
Krol, A., Amarowiczb, R., and Weidnera, S., The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves, J. Plant Physiol., 2015, vol. 189, pp. 97–104.
Li, T.A., Xu, S.L., Oses, Prieto, J.A., Putil, S., Xu, P., Wang, R.L., Li, K.H., Maltby, D.A., An, L.H., Burlingame, A.L., Deng, Z.P., and Wang, Z.Y., Proteomics analysis reveals posttranslational mechanisms for cold-induced metabolic changes in Arabidopsis, Mol. Plant., 2011, vol. 4, pp. 361–374.
Logan, D.C., Mitochondrial fusion, division and positioning in plants, Biochem. Soc. Trands., 2010, vol. 38, pp. 789–779.
Olenichenko, N.A., Zagoskina, N.V., Astakhova, N.V., Trunova, T.I., and Kuznetsov, Yu.V., Primary and secondary metabolism of winter wheat during cold hardening and the action of antioxidants, Appl. Biochem. Microbiol., 2008, vol. 44, no. 5, pp. 589–594.
Pareek, A., Singla, S., and Grover, A., Short-term salinity and high temperature stress associated ultrastructural alterations in young leaf cells of Oryza sativa L., Ann. Bot., 1997, vol. 80, pp. 629–639.
Pavlyuchkova, S.M., Spivak, E.A., Vershilovskaya, I.V., Nedved, E.L., and Shkraba, E.V., Effect of exogenous 5-aminolevulinic acid on the functioning of the antioxidant system of potato plants (Solanum tuberosum) under low-temperature stress, Vestsi Nat. Acad. Nauk. Belarusi, Ser. Biyal., 2014, vol. 3, pp. 46–51.
Popov, V.N., Antipina, O.V., and Astakhova, N.V., Changes in chloroplast ultrastructure of tobacco plants in the course of protection from oxidative stress under hypothermia, Russ. J. Plant Physiol., 2016, vol. 63, pp. 301–307.
Posmyk, M.M., Bailly, C., Szafranґska, K., Jasan, K.M., and Corbinea, F., Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedlings, J. Plant Physiol., 2005, vol. 162, pp. 403–412.
Reddy, A.R., Chaitanya, K.V., and Vivekanandan, M., Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants, J. Plant Physiol., 2004, vol. 161, pp. 1189–1202.
Ristic, Z. and Ashworth, E., Changes in leaf ultrastructure and carbohydrates in Arabidopsis thaliana L. (Heynh) cv. Columbia during rapid cold acclimation, Protoplasma, 1993, vol. 172, pp. 111–123.
Rivero, R., Ruiz, J., Garcıa, P., Lopez-Lefebre, L., Sanchez, E., and Romero, L., Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants, Plant Sci., 2001, vol. 160, pp. 315–321.
Rudikovskaya, E.G., Fedorova, G.A., Dudareva, L.V., Makarova, L.E., and Rudokovskij, A.V., Effect of growth temperature on the composition of phenols in pea roots, Russ. J. Plant Physiol., 2008, vol. 55, pp. 712–715.
Rurek, M., Plant mitochondria under a variety of temperature stress conditions, Mitochondrion, 2014, vol. 19, pp. 289–294.
Salem-Fnayou, A.B., Bouamama, B., Ghorbel, A., and Mliki, A., Investigations on the leaf anatomy and ultrastructure of grapevine (Vitis vinifera) under heat stress, Microsc. Res. Tech., 2011, vol. 74, pp. 756–762.
Smirnov, O., Kosyan, A., Kosyk, O., and Taran, N., Response of phenolic metabolism induced by aluminium (Al3+) toxicity in Fagopyrum esculentum Moench plants, Ukr. Biochem. J., 2015, vol. 87, pp. 129–135.
Sopher, C.R., Krol, M., and Huner, N.P.A., Chloroplastic changes associated with paclobutrazol-induced stress protection in maize seedlings, Can. J. Bot., 1999, vol. 77, pp. 279–290.
Stefanowska, M., Kura, M., and Kacperska, A., Low temperature induced modifications in cell ultrastructure and localization of phenolics in winter oilseed rape (Brassica napus L, var. oleifera) leaves, Annu. Bot., 2002, vol. 90, pp. 637–645.
Swigonska, S., Amarowicz, R., Kryl, A., Mostek, A., Badowiec, A., and Weidner, S., Influence of abiotic stress during soybean germination followed by recovery on the phenolic compounds of radicles and their antioxidant capacity, Acta Soc. Bot. Pol., 2014, vol. 83, pp. 209–218.
Theocharis, A., Clement, C., and Barka, E.A., Physiological and molecular changes in plants grown at low temperatures, Planta, 2012, vol. 235, pp. 1091–1105.
Treutter, D., Significance of flavonoids in plant resistance: a review, Environ. Chem. Lett., 2006, vol. 4, pp. 147–157.
Vassileva, V., Signarbieux, C., Anders, I., and Feller, U., Genotypic variation in drought stress response and subsequent recovery of wheat (Triticum aestivum L.), J. Plant Res., 2011, vol. 124, no. 1, pp. 147–154.
Vella, G.F., Joss, T, V., and Roberts, T.H., Chilling-induced ultrastructural changes to mesophyll cells of arabidopsis grown under short days are almost completely reversible by plant rewarming, Protoplasma, 2012, vol. 249, pp. 1137–1149.
Venzhik, Yu.V., Titov, A.F., Talanova, V.V., Mirosla-vov, E.A., and Koteeva, N.K., Structural and functional reorganization of the photosynthetic apparatus in adaptation to cold of wheat plants, Cell Tissue Biol., 2013, vol. 7, no. 2, pp. 168–176.
Venzhik, Yu.V., Titov, A.F., and Talanova, V.V., Short-term chilling of wheat seedlings or roots affects the ultrastructure of mesophyll cells, Tr. Karel. Nauch. Tsentra, Ross. Akad. Nauk, 2017, vol. 5, pp. 66–78.
Veselova, S., Farhutdinov, R., Mitrichenko, A., Symonyan, M., and Hartung, W., The effect of root cooling on hormone content and root hydraulic conductivity of durum wheat seedlings (Triticum durum L.), Bulg. J. Plant Physiol., Special Issue, 2003 pp. 360–366.
Voskresenskaya, O.L., Voskresenskiy, V.S., Sabaeva, E.V., and Yagdarova, O.A., Influence of ultraviolet radiation and microclimate parameters on the content of pigments in leaves of birch which grows under the conditions of the city, Vestn. Udmurt. Univ., Ser. Biol. Nauki Zemle, 2014, vol. 3, pp. 39–45.
Weidner, S., Kordala, E., Brosowska-Arendt, W., Karamacґ, M., Kosinґska, A., and Amarowicz, R., Phenolic compounds and properties of antioxidants in grapevine roots followed by recovery, Acta Soc. Bot. Pol., 2009, vol. 78, pp. 279–286.
Wellburn, A., The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution, J. Plant Physiol., 1994, vol. 144, pp. 307–313.
Wise, R.R. and Naylor, A.W., Chilling-enhanced photooxidation. The peroxidative destruction of lipids during chilling injury to photosynthesis and ultrastructure, Plant Physiol., 1987, vol. 8, pp. 272–277.
Yamada, K., and Osakabe, Y., Sugar compartmentation as an environmental stress adaptation strategy in plants, Semin. Cell Dev. Biol., 2017, vol. 72, pp. 1–10.
ACKNOWLEDGMENTS
This work was supported by the National Academy of Sciences of Ukraine, the project “The Phytohormonal System of New Genotypes of Triticum aestivum L. and Its Wild Ancestors under the Influence of Extreme Climatic Factors.”
We are thankful to D.A. Klimchuk, Ph.D., the head of the Center for Electron Microscopy, N. Kholodny Institute of Botany, National Academy of Sciences of Ukraine, for his attention and useful discussion of the results when this publication was prepared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by T. Borisova
Rights and permissions
About this article
Cite this article
Babenko, L.M., Vodka, M.V., Akimov, Y.N. et al. Specific Features of the Ultrastructure and Biochemical Composition of Triticum spelta L. Leaf Mesophile Cells in the Initial Period of Stress Temperature Action. Cell Tiss. Biol. 13, 70–78 (2019). https://doi.org/10.1134/S1990519X19010024
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X19010024