Abstract
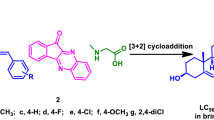
Istaroxime 1, a novel cardiotonic agent with high efficiency and low toxicity was synthesized from dehydroepiandrosterone 2 using a novel approach that included epoxidation, ring-opening, substitution, and oximation. The new protocol without gas protection was milder than the reported approaches. The overall yield of the method was 24.1%.
Similar content being viewed by others
References
Khatibzadeh, S., Farzadfar, F., Oliver, J., Ezzati, M., and Moran, A., Int. J. Cardiol., 2013, vol. 168, no. 2, p. 1186. doi 10.1016/j.ijcard.2012.11.065
Cheng, M.J., Zhou, J.C., and Zhang, S.C., J. China Prescription Drug, 2015, vol. 13, no. 1, p. 52.
Gheorghiade, M., Adams, K.F., and Colucci, W.S., Circulation, 2004, vol. 109, no. 24, p. 2959. doi 10.1161/01.CIR.0000132482.95686.87
Mattera, G.G., Giudice, P.L., Loi, F.M.P., Vanoli, E., Gagnol, J.P., Borsini, F., and Carminati, P., Am. J. Cardiol., 2007, vol. 99, no. 2, p. 33. doi 10.1016/j.amjcard.2006.09.004
Rocchetti, M., Besana, A., Mostacciuolo, G., Micheletti, R., Ferrari, P., Sarkozi, S., Szegedi, C., Jona, I., and Zaza, A., J. Pharmacol. Exp. Ther., 2005, vol. 313, no. 1, p. 207. doi 10.1124/jpet.104.077933
Ferrari, P., Micheletti, R., Valentini, G., and Bianchi, G., Med. Hypotheses, 2007, vol. 68, no. 5, p. 1120. doi 10.1016/j.mehy.2006.08.045
Alemanni, M., Rocchetti, M., Re, D., and Zaza, A., J. Mol. Cell. Cardiol., 2011, vol. 50, no. 5, p. 910. doi 10.1016/j.yjmcc.2011.02.008
Farmakis, D., and Filippatos, G., Cardiovasc. Drugs Ther., 2011, vol. 25, no. 2, p. 115. doi 10.1007/s10557-011-6295-7
Parissis, J.T., Rafouli-Stergiou, P., Stasinos, V., Psarogiannakopoulos, P., and Mebazaa, A., Curr. Opin. Crit. Care, 2010, vol. 16, no. 5, p. 432. doi 10.1097/MCC.0b013e32833e10fb
Shah, S.J., Blair, J.E.A., Filippatos, G.S., Macarie, C., Ruzyllo, W., Korewicki, J., Bubenek-Turconi, S.I., Ceracchi, M., Bianchetti, M., Carminati, P., Kremastinos, D., Grzybowski, J., Valentini, G., Sabbah, H.N., and Gheorghiade, M., Am. Heart J., 2009, vol. 157, no. 6, p. 1035. doi 10.1016/j.ahj.2009.03.007
Ferrandi, M., Barassi, P., Tadini-Buoninsegni, F., Bartolommei, G., Molinari, I., Tripodi, M. G., Reina, C., Moncelli, M.R., Bianchi, G., and Ferrari, P., Brit. J. Pharmacol., 2013, vol. 169, no. 8, p. 1849. doi 10.1111/bph.12278
De Munari, S., Folpini, E., Sputore, S., Frigerio, M., Melloni, P., and Serra, F., DE Patent 19633349, 1998.
Gobbini, M., Carzana, G., and Sputore, S., EP Patent 1156058, 2001.
De Munari, S., Cerri A., Gobbini, M., Almirante, N., Banfi, L., Carzana, G., Ferrari, P., Giuseppe, M.G., Micheletti, R., Schiavone, A., Sputore, S., Torri, M., Pia Zappavigna, M., and Melloni P., J. Med. Chem., 2003, vol. 46, no. 17, p. 3644. doi 10.1021/jm030830y
Baregama, L. K., Sharma, R., and Ahmed, M., Orient. J. Chem., 2003, vol. 19, no. 3, p. 605.
Glover, S.A., Goosen, A., McClei, C.V., and Schoonraad, J.L., Tetrahedron, 1987, vol. 43, no. 11, p. 2577. doi 10.1016/S0040-4020(01)86337-7
Cui, J.G., Huang, L.L., Huang, Y.M., and Fan, J.C., Chin. J. Org. Chem., 2009, vol. 29, no. 6, p. 971.
Cui, J.G., Zeng, L.M., Su, J.Y., and Lin, C.W., Chem. Res. Chinese U., 2002, vol. 18, no. 4, p. 400.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Liang, GP., Guo, JH. & Jiang, RW. Novel approach to the synthesis of istaroxime. Russ J Gen Chem 87, 2643–2647 (2017). https://doi.org/10.1134/S1070363217110196
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363217110196