Abstract
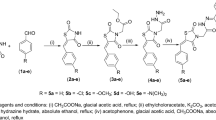
The challenges of the twenty-first century for the pharmaceutical industry are to deliver new and safe medicines within a short period of time. Novel drug discovery is a complex and expensive process with decades of the venture. With the present technologies and inventions, the task is much faster in recent years. Computer simulations give the dynamic picture of the reactions along with the potential drug molecule. Diabetes is a carbohydrate disorder caused due to the effect of environment, which results in increased hepatic glucose production, decreased insulin secretion. It hinders the function of eyes, kidneys, heart, nerves and blood vessels. The most commonly available drug for diabetes involves Metformin, Sulfonylureas, Meglitinide and Thiazolidinedione which cause severe damage to internal organs and many diseases like bladder cancer, hypoglycemia, risk of liver disease and many more. Docking techniques ease the identification of potential drug molecules to specific target. The present work aims at molecular docking studies on derivatives of pioglitazone while taking pioglitazone as reference. Peroxisome Proliferator Activated Receptor gamma (PPAR-γ) was taken as receptor and the derivatives were docked by using Autodock software. Docking results showed that pioglitazone derivatives were active against PPAR-γ with enhanced binding affinity when compared to standard marketed drug i.e. Pioglitazone. Molecular docking studies on the pioglitazone derivatives indicate that they may be used as a promising antidiabetic drug.
Similar content being viewed by others
REFERENCES
Katsarou, A., Gudbjörnsdottir, S., Rawshani, A., Dabelea, D., Bonifacio, E., Anderson, B.J., Jacobsen, L.M., Schatz, D.A., and Lernmark, Å., Nat. Rev. Dis. Prim., 2017, vol. 3 (1), pp. 1–17. https://doi.org/10.1038/nrdp.2017.16
Rines, A.K, Sharabi, K., Tavares, C.D., and Puigserver, P., Nat. Rev. Drug Dis., 2016, vol. 15 (11), pp. 786–804. https://doi.org/10.1038/nrd.2016.151
Hsing, H.Y., Rathnasamy, S., Dianita, R., and Wahab, H.A., Biomed. Res. Ther., 2020, vol. 7 (1), pp. 3579–3592. https://doi.org/10.15419/bmrat.v7i1.585
Vaishnav, Y., Dewangan, D., Verma, S., Mishra, A., Takur, A.S., Kashyap, P., and Kumar Verma, S., J. Heterocycl. Chem., 2020, vol. 57 (5), pp. 2213–2224. https://doi.org/10.1002/jhet.3941
Stumvoll, M. and Häring, H.U., Ann. Med., 2002, vol. 34 (3), pp. 217–224.
Kumar Celestina, S., Sundaram, K., and Ravi, S., Russ. J. Bioorg. Chem., 2019, vol. 45, pp. 405–415. https://doi.org/10.1134/S1068162019050066
Hatting, M., Tavares, C.D., Sharabi, K., Rines, A.K., and Puigserver, P., Ann. N.-Y. Acad. Sci., 2018, vol. 1411 (1), pp. 21–35. https://doi.org/10.1111/nyas.13435
Stanojevic, V. and Habener, J.F., Best Pract. Res. Clin. Endocrinol. Metabol., 2015, vol. 29 (6), pp. 859–871. https://doi.org/10.1016/j.beem.2015.10.002
Lee, M.A., Tan, L., Yang, H., Im, Y.G., and Im Y.J., Sci. Rep., 2017, vol. 7, article no. 16837. https://doi.org/10.1038/s41598-017-17082-x
Karak, M., Bal, N.C., Bal, C., Sharon, A., Curr. Diabetes Rev., 2013, vol. 9 (4), pp. 275–285.
Goldstein, B.J., Rev. Cardiovasc Med., 2003, vol. 4 (suppl. 6), pp. S3–S10.
Bansal, G., Singh, S., Monga, V., Thanikachalam, P.V., and Chawla, P., Bioorg. Chem., 2019, vol. 92, pp. 103271. https://doi.org/10.1016/j.bioorg.2019.103271
Guest, P.C., Ed., Reviews on Biomarker Studies of Metabolic and Metabolism-Related Disorders (Advances in Experimental Medicine and Biology, volume 1134), Springer Cham., 2019. https://doi.org/10.1007/978-3-030-12668-1
Yekta, R., Dehghan, G., Rashtbari, S., Sheibani, N., and Moosavi-Movahedi, A.A., J. Mol. Recognit., 2017, vol. 30 (12), p. e2648. https://doi.org/10.1002/jmr.2648
Greenberg, A.S. and McDaniel, M.L., Eur. J. Clin. Inv., 2002, vol. 32, pp. 24–34.
Kuranov, S.O., Luzina, O.A., and Salakhutdinov, N.F., Russ. J. Bioorg. Chem., 2020, vol. 46, pp. 972–988. https://doi.org/10.1134/S1068162020060151
Frkic, R.L., He, Y., Rodriguez, B.B., Chang, M.R., Kuruvilla, D., Ciesla, A., Abell, A.D., Kamenecka, T.M., Griffin, P.R., and Bruning, J.B., J. Med. Chem., 2017, vol. 60 (11), pp. 4584–4593. https://doi.org/10.1021/acs.jmedchem.6b01727
Cariou, B., Charbonnel, B., and Staels, B., Trends Endocrinol. Metabol., 2012, vol. 23 (5), pp. 205–215. https://doi.org/10.1016/j.tem.2012.03.001
Kubota, N., Terauchi, Y., Kubota, K.H., Itoh, S., Moroi, M., et al. J. Biolchem., 2006, vol. 281, pp. 8748–8755. https://doi.org/10.1074/jbc.M505649200
Prabhu, D.S. and Rajeswari, V.D., Mol. Biol. Rep., 2020, vol. 47 (7), pp. 5273–5283. https://doi.org/10.1007/s11033-020-05605-1
Biovia DS, Discovery Studio Visualizer, 2017, San Diego, CA, USA. https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-discovery-studio/visualization/.
Szychowski, K.A., Leja, M.L., Kaminskyy, D.V., Kryshchyshyn, A.P., Binduga, U.E., Pinyazhko, R., Lesyk, R.B., Tobiasz, J., and Gmiński, J., Eur. J. Med. Chem., 2017, vol. 141, pp. 162–168. https://doi.org/10.1016/j.ejmech.2017.09.071
Szychowski, K.A., Skóra, B., Kryshchyshyn-Dylevych, A., Kaminskyy, D., Khyluk, D., and Lesyk, R., Biomed. Pharm., 2021, vol. 139, article no. 111684. https://doi.org/10.1016/j.biopha.2021.111684
Patagar, D., Uttarkar, A., Patra, S.M., Patil, J.H., Kusanur, R., Niranjan, V., and Ashok Kumar, H.G., Russ. J. Bioorg. Chem., 2021, vol. 47, pp. 390–398. https://doi.org/10.1134/S1068162021020199
Kotha, S., Swapna, B., Kulkarni, V.M., Setty, R., Harish Kumar, B., and Harisha, R., J. Biomol. Struct. Dynam., 2021, vol. 39 (6), pp. 2210–2229. https://doi.org/10.1080/07391102.2020.1747543
Faridbod, F., Ganjali, M.R., Larijani, B., Riahi, S., Saboury, A., Hosseini, M., Norouzi, P., and Pillip, C., Spectrochim. Acta Part A Mol. Biomol. Spectr., 2011, vol. 78 (1), pp. 96–101. https://doi.org/10.1016/j.saa.2010.09.001
Lee, M.A., Tan, L., Yang, H., Im, Y.G., and Im, Y.J., Sci. Rep., 2017, vol. 7 (1), article no. 16837. https://doi.org/10.1038/s41598-017-17082-x
Rajapaksha, H., Bhatia, H., Wegener, K., Petrovsky, N., and Bruning, J.B., Biochim. Biophys. Acta Gen. Sub., 2017, vol. 1861 (8), pp. 1981–1991. https://doi.org/10.1016/j.bbagen.2017.05.008
Rajapaksha, H., Bhatia, P., Wegener, K., Petrovsky, N., and Bruning, J.B., Biochim. Biophys. Acta Gen. Sub., 2017, vol. 1861 (8), pp. 1981–1991. https://doi.org/10.1016/j.bbagen.2017.05.008
Lehman, J.M., Moore, L.B., Smith-Oliver, T.A., Wilkison, W.O., Willson, T.M., and Kliewer, S.A., J. Biol. Chem., 1995, vol. 270, pp. 12953–12956. https://doi.org/10.1074/jbc.270.22.12953
Ferre, P., Diabetes, 2004, vol. 53, pp. 43–50. https://doi.org/10.2337/diabetes.53.2007.S43
Geetha, B., Swarnalatha, G., and Reddy, G.S., Rasayan J. Chem., 2019, vol. 12 (3), pp. 1063–1071. https://doi.org/10.31788/RJC.2019.1235165
Sanner, M.F., J. Mol. Graph. Mod., 1999, vol. 17, pp. 57–61.
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S., and Olson, A.J., J. Comp. Chem., 2009, vol. 16, pp. 2785–27891. https://doi.org/10.1002/jcc.21256
Schrodinger, LLC., The PyMOL Molecular Graphics System, Version 2.2.3. 2010
Trott, O. and Olson, A.J., J. Comp. Chem., 2010, vol. 31 (2), pp. 455–461. https://doi.org/10.1002/jcc.21334
Fukumitsu, S., Aida, K., Ueno, N., Ozawa, S., Takahashi, Y., and Kobori, M., British J. Nutr., 2008, vol. 100 (3), pp. 669– 676. https://doi.org/10.1017/S0007114508911570
Variya, B.C., Modi, S.J., Savjani, J., and Patel, S., Int. J. Pharm. Pharm. Sci., 2016, vol. 9 (1), p. 102.
Izrailev, S., Stepaniants, S., Balsera, M., Oono, Y., and Schulten, K., Biophys. J., 1997, vol. 72, pp. 1568–1581. https://doi.org/10.1016/S0006-3495(97)78804-0
Wang, W., Donini, O., Reyes, C.M., and Kollman, P.A., Ann. Rev. Biophys. Biomol. Struct., 2001, vol. 30, pp. 211–243. https://doi.org/10.1146/annurev.biophys.30.1.211
Simonson, T., Archontis, G., and Karplus, M., Acc. Chem. Res., 2002, vol. 35, pp. 430–437. https://doi.org/10.1021/ar010030m
Gervasio, F.G., Laio, A., and Parrinello, M., J. Am. Chem. Soc., 2005, vol. 127, pp. 2600–2607. https://doi.org/10.1021/ja0445950
Woo, H.J. and Roux, B., Proc. Natl. Acad. Sci. U.S.A., 2005, vol. 102, pp. 6825–6830. https://doi.org/10.1073/pnas.0409005102
Zhang, D., Gullingsgrud, J., and McCammon, J.A., J. Am. Chem. Soc., 2006, vol. 128, pp. 3019–3026. https://doi.org/10.1021/ja057292u
Gabdoulline, R.R. and Wade, R.C., Biophys. J., 1997, vol. 72, pp. 1917–1929. https://doi.org/10.1016/S0006-3495(97)78838-6
Fu, W., Cui, M., Briggs, J.M., Huang, X., Xiong, B., Zhang, Y., Luo, X., Shen, J., Ji, R., Jiang, H., Chen, K., Biophys. J., 2002, vol. 83, pp. 2370–2385. https://doi.org/10.1016/S0006-3495(02)75251-X
Gross, E.L. and Pearson, D.C., Jr., Biophys. J., 2003, vol. 85, pp. 2055–2068. https://doi.org/10.1016/S0006-3495(03)74633-5
Halperin, I., Ma, B., Wolfson, H., and Nussinov, R., Proteins, 2002, vol. 47, pp. 409–443. https://doi.org/10.1002/prot.10115
Brooijmans, N. and Kuntz, I.D., Ann. Rev. Biophys. Biomol. Struct., 2003, vol. 32, pp. 335–373. https://doi.org/10.1146/annurev.biophys.32.110601.142532
Sousa, S.F., Fernandes, P.A., and Ramos, M.J., Proteins, 2006, vol. 65, pp. 15–26. https://doi.org/10.1002/prot.21082
ACKNOWLEDGMENTS
The authors are thankful to the Principal, RV College of Engineering, Bengaluru, Karnataka, India for providing the computational facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article doesnot contain any studies involving human participants performed by any of the authors and doesnot contain any studies involving animals performed by any of the author.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Rao, P., S, A., Masood, G. et al. Bioinformatics Study of Pioglitazone Analogues as Potential Anti-Diabetic Drugs. Russ J Bioorg Chem 48, 976–989 (2022). https://doi.org/10.1134/S106816202205017X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202205017X