Abstract
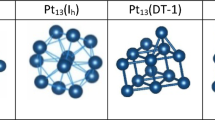
The catalytic activity of Pt clusters is dependent not only on the nanoparticle size and its composition, but also on its internal structure. To determine the real structure of the nanoparticles used in catalysis, the boundaries of the thermal structure stability of Pt clusters to 8.0 nm in diameter interacting with carbon substrates of two types: a fixed α-graphite plane and a mobile substrate with the diamond structure. The effect of a substrate on the processes melting of Pt nanoclusters is estimated. The role of the cooling rate in the formation of the internal structure of Pt clusters during crystallization is studied. The regularities obtained in the case of “free” Pt clusters and Pt clusters on a substrate are compared. It is concluded that platinum nanoparticles with diameter D ≤ 4.0 nm disposed on a carbon substrate conserve the initial fcc structure during cooling.
Similar content being viewed by others
References
Ch. Pool and F. Owens, Nanotechnologies (Tekhnosfera, Moscow, 2006; Wiley, Hoboken, NJ, 2003).
M. Metzler, A. Thorwart, S. Zeller, T. Diemant, R. J. Behm, and T. Jacob, Catal. Today 244, 3 (2014).
I. F. Golovnev, E. I. Golovneva, and V. M. Fomin, Fiz. Mezomekh. 11 (2), 51 (2008).
C. Mottet, G. Rossi, F. Baletto, and F. Ferrando, Phys. Rev. Lett. 95, 035501 (2005).
H. Akbarzadeh, H. Yaghoubi, A. N. Shamkhali, and F. Taherkhani, J. Phys. Chem. C 117, 26287 (2013).
S. L. Gafner, L. V. Redel, and Yu. Ya. Gafner, J. Exp. Theor. Phys. 108, 784 (2009).
Yu. Ya. Gafner, Zh. V. Goloven’ko, and S. L. Gafner, J. Exp. Theor. Phys. 116, 252 (2013).
G. Liu, K. Yang, J. Li, W. Tang, J. Xu, H. Liu, R. Yue, and Y. Chen, J. Phys. Chem. C 118, 22719 (2014).
I. Janowska, M. S. Moldovan, O. Ersen, H. Bulou, K. Chizari, M. J. Ledoux, and C. Pham-Huu, Nano Res. 4, 511 (2011).
P. Xu, L. Dong, M. Neek-Amal, M. L. Ackerman, J. Yu, S. D. Barber, J. K. Schoelz, D. Qi, F. Xu, P. M. Thibado, and F. M. Peeters, ACS Nano 8, 2697 (2014).
M. S. Moldovan, H. Bulou, Y. J. Dappe, I. Janowska, D. Bégin, C. Pham-Huu, and O. Ersen, J. Phys. Chem. C 116, 9274 (2012).
E. Yoo, T. Okada, T. Akita, M. Kohyama, I. Honma, and J. Nakamuraa, J. Power Sources 196, 110 (2011).
A. I. Frenkel, S. Nemzer, I. Pister, L. Soussan, T. Harris, Y. Sun, and M. H. Rafailovich, J. Chem. Phys. 123, 184701 (2005).
X. W. Zhou, R. A. Johnson, and H. N. G. Wadley, Phys. Rev. B 69, 144113 (2004).
B. H. Morrow and A. Striolo, Mol. Simul. 35, 795 (2009).
Subramanian, K. R. S. Sankaranarayanan, V. R. Bhethanabotla, and B. Joseph, Phys. Rev. B 72, 195405 (2005).
J. Tersoff, Phys. Rev. B 38, 9902 (1988).
S. H. Lee, S. S. Han, J. K. Kang, J. H. Ryu, and H. M. Lee, Surf. Sci. 602, 1433 (2008).
J. D. Honeycutt and H. C. Anderson, J. Chem. Phys. 91, 4950 (1987).
V. S. Baidyshev, Yu. Ya. Gafner, V. M. Samsonov, and A. G. Bembel, Crystallogr. Rep. 60, 95 (2015).
R. Kelsall, I. Hamley, and M. Geoghegan, Nanoscale Science and Technology (Wiley, Chichester, 2005).
V. M. Samsonov, S. A. Vasilyev, I. V. Talyzin, and Yu. A. Ryzhkov, JETP Lett. 103, 94 (2016).
D. Schebarchov, S. C. Hendy, and W. Polak, J. Phys.: Condens. Matter. 21, 144204 (2009).
K. K. Nanda, Pramana—J. Phys. 72, 617 (2009).
L. V. Redel’, S. L. Gafner, Yu. Ya. Gafner, I. S. Zamulin, and Zh. V. Goloven’ko, Phys. Solid State 59, 413 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.S. Baidyshev, Yu.Ya. Gafner, S.L. Gafner, L.V. Redel, 2017, published in Fizika Tverdogo Tela, 2017, Vol. 59, No. 12, pp. 2483–2489.
Rights and permissions
About this article
Cite this article
Baidyshev, V.S., Gafner, Y.Y., Gafner, S.L. et al. Thermal stability of Pt nanoclusters interacting to carbon sublattice. Phys. Solid State 59, 2512–2518 (2017). https://doi.org/10.1134/S1063783417120071
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783417120071