Abstract
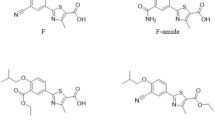
This paper demonstrates the study of the use of single or multiple (2–4) internal standards for the improvement of the repeatability of the micellar electrokinetic capillary chromatographic measurements of temozolomide in model solution and in serum. The dimethylformamide, dimethylsulfoxide, paracetamol and benzyl alcohol were selected as internal standards. The best precision data (RSD less than 0.036%) of the migration times could be reached with two internal standards applying weighted correction according to the distance of the internal standards from the analyte. We also investigated the effects of the use of new capillaries, buffer renewal and postcondition procedures on the repeatability
Similar content being viewed by others
References
Validation of Analytical Procedures: Text and Methodology, ICH Guidelines, 1994. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/ Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. Accessed March 15, 2015.
Guideline for Industry: Text on Validation of Analytical Procedures, ICH Q2A, USFood and Drug Administration, Rockville, 1995. http://www.fda.gov/downloads/Drugs/Guidances/ucm073381.pdf. Accessed March 15, 2015.
Fabre, H. and Altria, K.D., LC GCEur., 2001, no. 5, p. 1.
Guidance for Industry, Bioanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration, CDER, 2013. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf. Accessed March 15, 2015.
Guideline on Bioanalytical Method Validation, European Medicines Agency, 2012. http://www.ema.europa.eu/ docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed March 15, 2015.
Altria, K.D., Analysis of Pharmaceuticals by Capillary Electrophoresis, Braunschweig: Vieweg, 1998.
Faller, T. and Engelhardt, H., J. Chromatogr. A, 1999, vol. 853, nos. 1–2, p. 83.
Mayer, B.X., J. Chromatogr. A, 2001, vol. 907, nos. 1–2, p. 21.
Hasemann, P., Balk, M., and Watzig, H., Electrophoresis, 2007, vol. 28, nos. 11, p. 1798.
Altria, K.D., LC GCEur., 2002, no. 9, p. 1.
Yang, J., Bose, S., and Hage, D.S., J. Chromatogr. A, 1996, vol. 735, nos. 1–2, p. 209.
Altria, K.D., Clayton, N.G., Hart, M., Harden, R.C., Hevizi, J., Makwana, J.V., and Portsmouth, M.J., Chromatographia, 1994, vol. 39, nos. 6, p. 180.
Altria, K.D. and Fabre, H., Chromatographia, 1995, vol. 40, nos. 6, p. 313.
Schaeper, J.P. and Sepaniak, M.J., Electrophoresis, 2000, vol. 21, nos. 7, p. 1421.
Hettiarachchi, K. and Cheung, A.P., J. Chromatogr. A, 1995, vol. 717, nos. 1–2, p. 191.
Tagliaro, F., Smith, F.P., Turrina, S., Equisetto, V., and Marigo, M., J. Chromatogr. A, 1996, vol. 735, nos. 1–2, p. 227.
Baalbaki, B., Blanchin, M.D., and Fabre, H., Anal. Chim. Acta, 2002, vol. 463, nos. 1, p. 15.
Thi, T.D., Pomponio, R., Saevels, J., Van Hove, B., Van Ael, W., Matthijs, N., Vander Heyden, Y., Marini Djan’geing’a, R., Chiap, P., Hubert, P., Crommen, J., Fabre, H., Dehouck, P., Hoogmartens, J., and Van Schepdael, A., Electrophoresis, 2006, vol. 27, nos. 12, p. 2317.
Li, X.F., Ren, H., Le, X., Qi, M., Ireland, I.D., and Dovichi, N.J., J. Chromatogr. A, 2000. vol. 869, nos. 1–2, p. 375.
Riekkola, M.J. and Jumpannen, J.H., J. Chromatogr. A, 1996, vol. 735, nos. 1–2, p. 151.
Darkes, M.J.M., Plosker, G.L., and Jarvis, B., Am. J. Cancer, 2002, vol. 1, nos. 1, p. 55.
Andrási, M., Bustos, R., Gáspár, A., Gomez, F.A., and Klekner, A., J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2010, vol. 878, nos. 21, p. 1801.
Andrási, M., Törzsök, B., Klekner, Á., and Gáspár, A., J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2011, vol. 879, nos. 23, p. 2229.
Mayer, B.X., Hollenstein, U., Brunner, M., Eichler, H.G., and Müller, M., Electrophoresis, 2000, vol. 21, nos. 8, p. 1558.
Mayer, B.X. and Müller, M., LC GCEur., 2001, vol. 01, p. 1.
Smith, S.C., Strasters, J.K., and Khaledi, M.G., J. Chromatogr. A, 1991, vol. 559, nos. 1–2, p. 57.
Ross, G.A., J. Chromatogr. A, 1995, vol. 718, nos. 1–2, p. 444.
Pugsley, H.R., Swearingen, K.E., and Dovichi, N.J., J. Chromatogr. A, 2009, vol. 1216, nos. 15, p. 3418.
Andrasi, M., Gáspár, A., Kovács, O., Baranyai, Zs., Klekner, A., and Brücher, E., Electrophoresis, 2011, vol. 32, nos. 16, p. 2223.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrási, M., Zékány, L. & Gáspár, A. Study on repeatability of the determination of temozolomide by micellar electrokinetic capillary chromatography using internal standards. J Anal Chem 70, 1360–1367 (2015). https://doi.org/10.1134/S1061934815110118
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934815110118