Abstract
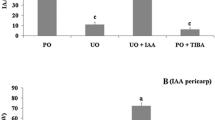
The orchid flowers may stay fresh in unpollinated state from few weeks to months but show rapid senescence upon pollination. Metabolic changes related to this phenomenon are less well understood in orchid flowers. Presently, two orchid species, Aerides multiflora Roxb. and Rhynchostylis retusa (L.) Bl., varying in their floral life span were evaluated for their postpollination-induced responses, involving the oxidative stress. The unpollinated flowers of A. multiflora stayed fresh for 17 days and attained senescence in 5 days after pollination (DAP), while those of R. retusa. remained fresh for 24 days and showed senescence in 7 DAP. After pollination, wilting began in 2 to 3 days in A. multiflora and 3 to 4 days in R. retusa. There was a higher electrolyte leakage accompanied by a concomitant increase in the levels of malondialdehyde (MDA) and hydrogen peroxide (H2O2), indicators of oxidative damage in all the organs after pollination while ascorbic acid decreased significantly. The flowers of A. multiflora showed a greater electrolyte leakage, MDA and H2O2 contents as compared to those of R. retusa. Ascorbic acid content, on the other hand, was lower in A. multiflora than in R. retusa, suggesting a higher oxidative damage to the floral organs in the former species. An application of triiodobenzoic acid ( an auxin inhibitor; 0.25 mM) and silver nitrate (ethylene inhibitor; 0.25 mM) to pollinated flowers partially prevented the oxidative damage and consequently the senescence, suggesting the involvement of these hormones. AgNO3 was more effective in delaying senescence.
Similar content being viewed by others
Abbreviations
- ACC:
-
1-aminocyclopropane-1-carboxylic acid
- ASC:
-
ascorbic acid
- DAP:
-
days after pollination
- HAP:
-
hours after pollination
- MDA:
-
malondialdehyde
- TIBA:
-
triiodobenzoic acid
References
O’Neill, S.D., Nadeau, J.A., Zhang, X.S., Bui, A.Q., and Halevy, A.H., Inter-Organ Regulation of Ethylene Biosynthetic Genes by Pollination, Plant Cell, 1993, vol. 5, pp. 419–432.
Zhang, X.S. and O’Neill, S.D., Ovary and Gametophyte Development Are Co-Ordinately Regulated Following Pollination by Auxin and Ethylene, Plant Cell, 1993, vol. 5, pp. 403–418.
O’Neill, S.D., Pollination Regulation of Flower Development, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1997, vol. 48, pp. 547–574.
Ketsa, S. and Rugkong, A., Senescence of Dendrobium “Pompadour” Flowers Following Pollination, J. Hortic. Sci. Biotech., 1999, vol. 74, pp. 608–613.
Suttle, J.C. and Kende, H., Ethylene and Senescence in Petals of Tradescantia, Plant Physiol., 1978, vol. 65, pp. 1067–72.
Celikel, F.G. and van Doorn, W.G., Solute Leakage, Lipid Peroxidation and Protein Degradation during the Senescence of Iris Tepals, Physiol. Plant., 1995, vol. 94, pp. 515–521.
Thompson, J.E., Froese, C.D., Madey, E., Smith, M.D., and Hong, Y., Lipid Metabolism during Plant Senescence, Prog. Lipid Res., 1998, vol. 37, pp. 119–141.
Hilioti, Z., Richards, C., and Brown, K.M., Regulation of Pollination Induced Ethylene and Its Role in Petal Abscission of Pelargonium hortum, Physiol. Plant., 2000, vol. 109, pp. 322–332.
Bui, A.Q. and O’Neill, S.D., Three 1-Aminocyclopropane-1-Carboxylate Synthase Genes Regulated by Primary and Secondary Pollination Signals in Orchid Flowers, Plant Physiol., 1998, vol. 116, pp. 419–428.
Van Doorn, W.G., Effect of Pollination on Floral Attraction and Longevity, J. Exp. Bot., 1997, vol. 48, pp. 1615–1622.
Lutts, S., Kinet, J.M., and Bouharmont, J., NaCl-Induced Senescence in Leaves of Rice (Oryza sativa L.) Cultivars Differing in Salinity Resistance, Ann. Bot., 1996, vol. 78, pp. 389–398.
Dhindsa, R.S., Dhindsa, P.P., and Thorpe, T.A., Leaf Senescence Correlated with Increase Levels of Membrane Permeability and Lipid Peroxidation and Decrease Level of Superoxide Dismutase and Catalase, J. Exp. Bot., 1981, vol. 32, pp. 93–101.
Mukherjee, S.P. and Choudhuri, M.A., Implication of Water Stress Induced Changes in the Level of Endogenous Ascorbic Acid and H2O2 in Vigna Seedlings, Physiol. Plant., 1983, vol. 58, pp. 166–170.
Teranishi, Y., Tanaka, A., Osumi, M., and Fukui, S., Catalase Activity of Hydrocarbon Utilizing Candida Yeast, Agric. Biol. Chem., 1974, vol. 38, pp. 1213–1216.
Arditti, J., Fundamentals of Orchid Biology, New York: John Wiley and Sons, 1992.
Weiss, M.R., Floral Colour Changes as Cues for Pollinators, Nature, 1991, vol. 354, pp. 227–229.
Porat, R., Borochov, A., Halevy, A.H., and O’Neill, S.D., Pollination Induced Senescence of Phalaenopsis Petals. The Wilting Process, Ethylene Production and Sensitivity to Ethylene, Plant Growth Regul., 1994, vol. 15, pp. 129–136.
Xu-Yan, M.R. and Hanson, Y.X., Programmed Cell Death during Pollination Induced Petal Senescence in Petunia, Plant Physiol., 2000, vol. 122, pp. 1323–1333.
Fobel, M., Lynch, D.V., and Thompson, J.E., Membrane Deterioration in Senescing Carnation Flowers, Plant Physiol., 1987, vol. 85, pp. 204–211.
Lesham, Y.Y., Membrane-Associated Phospholytic and Lipolytic Enzymes, Plant Membranes: A Biophysical Approach to Structure, Development and Senescence, Lesham, Y.Y., Ed., Dordrecht: Kluwer, 1992, pp. 174–191.
Thompson, J.E., Legge, R.L., and Barber, R.F., The Role of Free Radical in Senescence and Wounding, New Phytol., 1987, vol. 105, pp. 317–344.
Panavas, T. and Rubinstein, B., Oxidative Events during Programmed Cell Death of Daylilly (Hemerocallis Hybrid) Petals, Plant Sci., 1998, vol. 133, pp. 125–138.
Fukuchi-Mizutani, M., Ishiguro, K., Nakayama, T., Utsunomiya, Y., Tanaka, Y., Kusumi, T., and Ueda, T., Molecular and Functional Characterization of a Rose Lipoxygenase cDNA Related to Flower Senescence, Plant Sci., 2000, vol. 160, pp. 129–137.
Asada, K. and Takahanshi, M., Production and Scavenging of Active Oxygen in Photosynthesis, Photoinhibition, Kyle, D.J., Osmand, C.B., and Amtzen, C.J., Eds., Amsterdam: Elsevier, 1987, pp. 227–287.
Halliwel, B., Oxidative Damage, Lipid Peroxidation and Antioxidant Protection in Chloroplasts, Chem. Phys. Lipids, 1987, vol. 44, pp. 327–340.
Bowler, C., van Montagu, M., and Inze, D., Superoxide Dismutase and Stress Tolerance, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1992, vol. 43, pp. 83–116.
Lin, J.N. and Kao, C.H., Effect of Oxidative Stress Caused by Hydrogen Peroxide on Senescence of Rice Leaves, Bot. Bull. Acad. Sinica, 1998, vol. 39, pp. 161–165.
Noctor, G. and Foyer, C.H., Ascorbate and Glutathione: Keeping Active Oxygen under Control, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1998, vol. 49, pp. 249–279.
Prochazkova, D., Sairam, R.K., Srivastava, G.C., and Singh, D.V., Oxidative Stress and Antioxidant Activity as the Basis of Senescence in Maize Leaves, Plant Sci., 2001, vol. 161, pp. 765–771.
Jones, M.L. and Woodson, W.R., Pollination Induced Ethylene in Carnation. Role of Stylar Ethylene in Corolla Senescence, Plant Physiol., 1997, vol. 115, pp. 205–212.
Ketsa, S., Rugkong, A., Saichol, K., and Adirak, R., Ethylene Production, Senescence and Ethylene Sensitivity of Dendrobium ‘Pampadour’ Flowers Following Pollination, J. Hortic. Sci. Biotech., 2000, vol. 75, pp. 149–153.
Atti, L.K., Nayyar, H., Bhanwra, R.K., and Viy, S.P., Post Pollination Related Biochemical Change in Floral Organs of Rhynchostylis retusa (L.) Bl. and Aerides multiflora Roxb. (Orchidaclal), J. Plant Biol., 2007, vol. 50(5), pp. 548–556.
Atti, L.K., Nayyar, H., Bhanwra, R.K., and Pehwal, A., Pollination_Jnduced Oxidative Atress in Floral Organs of Cymbidium pendulum (Roxb.) Sw. and Cymbidium alofolium (L.) Sw. (Orchidaclal): A Biochemical Jnvestigation, Sci. Hortic., 2008, vol. 116, pp. 311–317.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.K. Attri, H. Nayyar, R.K. Bhanwra, S.P. Vij, 2008, published in Fiziologiya Rastenii, 2008, Vol. 55, No. 6, pp. 908–915.
This text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Attri, L.K., Nayyar, H., Bhanwra, R.K. et al. Pollination-induced floral senescence in orchids: Status of oxidative stress. Russ J Plant Physiol 55, 821–828 (2008). https://doi.org/10.1134/S1021443708060125
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443708060125