Abstract
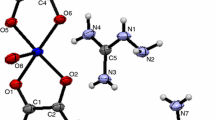
The reaction of CuSO4⋅5H2O with glycine and 4-cyanopyridine in aqueous alkaline medium resulted in polymeric complex [Cu(ox)2(isn)2]·(CONH2)2]n (1) (where ox is oxalate, isn is isonicotinamide). Complex 1 has been studied by single crystal X-ray diffraction. Crystal structure reveals that complex 1 is a polymeric structure bridged by oxalate ions. Glycine has been oxidized to oxalate during the course of the reaction along with the in-situ hydrolysis of 4-cyanopyridine to isonicotinamide resulting in complex 1. Complex 1 was further characterized by molar conductance, UV-Vis and FT-IR spectroscopic techniques. The conversion of glycine to oxalate occurs spontaneously in aqueous solution under ambient conditions.





Similar content being viewed by others
REFERENCES
R. Huber, Angew. Chem. Int. Ed. Engl. 28, 848 (1989). https://doi.org/10.1002/anie.198908481
P. Kyritsis, A. Messerschmidt, R. Huber, et al., J. Chem. Soc., Dalton Trans. 731 (1993). https://doi.org/10.1039/DT9930000731
W. Kaim and J. Rall, Angew. Chem. Int. Ed. Engl. 35, 43 (1996). https://doi.org/10.1002/anie.199600431
U. M. Sundaram, H. H. Zhang, B. Hedman, et al., J. Am. Chem. Soc. 119, 12525 (1997). https://doi.org/10.1021/ja972039i
O. Kahn, Angew. Chem. Int. Ed. Engl. 24, 834 (1985). https://doi.org/10.1002/anie.198508341
M. A. Uvarova and S. E. Nefedov, Russ. J. Coord. Chem. 47, 399 (2021). https://doi.org/10.1134/S1070328421060087
E. O. Andriychenko, V. I. Zelenov, A. V. Bespalov, et al., Russ. J. Gen. Chem. 91, 1697 (2021). https://doi.org/10.1134/S1070363221090139
E. Canpolat and M. Kaya, Russ. J. Coord. Chem. 31, 790 (2005). https://doi.org/10.1007/s11173-005-0170-7
J. D. Ranford, P. J. Sadler, and D. A. Tocher, Dalton Trans. 3393 (1993). https://doi.org/10.1039/DT9930003393
V. Rajendiran, R. Karthik, M. Palaniandavar, et al., Inorg. Chem. 46, 8208 (2007). https://doi.org/10.1021/ic700755p
F. Arjmand, M. Muddassir, and I. Yousuf, J. Photochem. Photobiol. B 136, 62 (2014). https://doi.org/10.1016/j.jphotobiol.2014.04.024
S. Jain, T. A. Khan, Y. P. Patil, et al., J. Photochem. Photobiol. B 174, 35 (2017). https://doi.org/10.1016/j.jphotobiol.2017.06.035
Y. P. Tupolova, L. D. Popov, S. A. Borodkin, et al., Russ. J. Gen. Chem. 91, 1687 (2021). https://doi.org/10.1134/S1070363221090127
D. Laloo and M. K. Mahanti, J. Phys. Org. Chem. 3, 799 (1990). https://doi.org/10.1002/poc.610031205
V. Soni, R. S. Sindal, and R. N. Mehrotra, Polyhedron 24, 1167 (2005). https://doi.org/10.1016/j.poly.2005.03.057
T. P. Jose and S. M. Tuwar, J. Mol. Struct. 827, 137 (2007). https://doi.org/10.1016/j.molstruc.2006.05.015
E. R. Stadtman and B. S. Berlett, J. Biol. Chem. 266, 17201 (1991). https://doi.org/10.1016/S0021-9258(19)47359-6
B. S. Berlett, P. B Chock, M. B. Yim, et al., Proc. Natl. Acad. Sci. USA 87, 389 (1990). https://doi.org/10.1073/pnas.87.1.389
L. Pecci, G. Montefoschi, G. Musci, et al., Amino Acids 13, 355 (1997). https://doi.org/10.1007/BF01372599
L. Meriwether and F. H. Westheime, J. Am. Chem. Soc. 78, 5119 (1956). https://doi.org/10.1021/ja01600a081
M. Mortl, K. Diederichs, W. Welte, et al., J. Biol. Chem. 279, 29718 (2004). https://doi.org/10.1074/jbc.M401224200
Y. Nishiya and T. Imanaka, FEBS Lett. 438, 263 (1998). https://doi.org/10.1016/S0014-5793(98)01313-1
K. Brandenburg, Diamond. Crystal Impact (version 3.1f) GbR, Bonn, Germany (2008).
D. Sun, Z. H. Wei, C. F. Yang, et al., CrystEngComm 13, 1591 (2011). https://doi.org/10.1039/C0CE00539H
W. Fitzgerald, J. Foley, D. McSweeney, et al., J. Chem. Soc., Dalton Trans. 1117 (1982). https://doi.org/10.1039/DT9820001117
D. Chisca, L. Croitor, E. B. Coropceanu, et al., Inorg. Chem. Commun. 132, 108864 (2021). https://doi.org/10.1016/j.inoche.2021.108864
A. A. Opalade, C. J. Gomez-Garcia and N. Gerasimchuk, Cryst. Growth Des. 19, 678 (2008). https://doi.org/10.1021/acs.cgd.8b01262
B. Cordero, V. Gόmez, A. E. Platero-Prats, et al., Dalton Trans. 2832 (2008). https://doi.org/10.1039/B801115J
G. Verardo, A. G. Giumanini, F. Gorassini, et al., Tetrahedron 49, 10609 (1993). https://doi.org/10.1016/S0040-4020(01)81552-0
F. J. Martínez-Martínez, I. I. Padilla-Martínez, M. A. Brito, et al., J. Chem. Soc., Perkin Trans. 2, 401 (1998). https://doi.org/10.1039/A704640E
R. C. Paul, B. R. Sreenathan and S. L. Chadha, J. Inorg. Nucl. Chem. 28, 1225 (1966). https://doi.org/10.1016/0022-1902(66)80449-9
R. C. Paul and S. L. Chadha, J. Inorg. Nucl. Chem. 31, 2753 (1969). https://doi.org/10.1016/0022-1902(69)80189-2
S. Bayari, A. Ataç and Ş. Yurdakul, J. Mol. Struct. 655, 163 (2003). https://doi.org/10.1016/S0022-2860(03)00256-4
M. Ðaković and Z. Popović, Acta Cryst. C65, m361 (2009). https://doi.org/10.1107/S0108270109031989
S. Yurdakul, A. Atac, E. Sahin and S. Ide, Vib. Spectrosc. 31, 41 (2003). https://doi.org/10.1016/S0924-2031(02)00066-8
H. Icbudak, H. Olmez, O. Z. Yesilel, et al., J. Mol. Struct. 657, 255 (2003). https://doi.org/10.1016/S0022-2860(03)00404-6
D. Sutton, Electronic Spectra of Transition Metal Complexes (McGraw-Hill, London, 1968).
Y. Asano and K. Yasukawa, Curr. Opin. Chem. Biol. 49, 76 (2019). https://doi.org/10.1016/j.cbpa.2018.10.020
N. N. Murthy, M. Mahroof-Tahir and K. D. Karlin, J. Am. Chem. Soc. 115, 10404 (1993). https://doi.org/10.1021/ja00075a084
C. C. Chang and A. H. C. Huang, Plant Physiol. 67, 1003 (1981). https://doi.org/10.1104/pp.67.5.1003
P. J. Thureen, M. R. Narkewicz, F. C. Battaglia, et al., Pediatr. Res. 38, 775 (1995). https://doi.org/10.1203/00006450-199511000-00023
R. Biswas and M. Koley, World J. Chem. Educ. 5, 185 (2017). https://doi.org/10.12691/wjce-5-5-8
ACKNOWLEDGMENTS
The authors thank SAIF, Gauhati University for single crystal X-ray diffraction data.
Funding
Linkon Bharali thanks DST for providing Inspire scholarship (Registration number: 201500053206).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Anshuman Gogoi, Linkon Bharali Conversion of Glycine to Oxalate in Presence of CuSO4⋅5H2O and Isonicotinamide. Russ. J. Inorg. Chem. 67, 608–615 (2022). https://doi.org/10.1134/S0036023622050072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622050072