Abstract
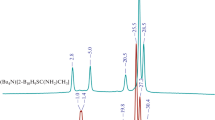
Currently, the chemistry of carbonyl derivatives of the closo-borate anions is actively developing. It is of great interest to obtain protonated complexes of these types of clusters for further directed functionalization. The aim of this study is to develop an approach to the preparation of the protonated complex of the monocarbonyl derivative [B10H9CO⋅Hfac]0. In the course of the work, the target complex has been obtained by protonation of the [B10H9CO]– anion with an excess of trifluoromethanesulfonic acid CF3SO3OH. In this reaction, the boron cluster is quantitatively protonated. When a base is added, the protonation process becomes reversible.




Similar content being viewed by others
REFERENCES
X. Mu, J. C. Axtell, N. A. Bernier, et al., Chem. 5, 2461 (2019). https://doi.org/10.1016/j.chempr.2019.07.018
V. V. Avdeeva, E. A. Malinina, K. Y. Zhizhin, et al., Russ. J. Inorg. Chem. 65, 514 (2020). https://doi.org/10.1134/S0036023620040026
F. Ali, N. S. Hosmane, and Y. Zhu, Molecules 25, 828 (2020). https://doi.org/10.3390/molecules25040828
T. Jelinek, B. Štibr, F. Mareš, et al., Polyhedron 6, 1737 (1987). https://doi.org/10.1016/S0277-5387(00)86544-4
Z. Laila, O. Yazbeck, F. A. Ghaida, et al., J. Organomet. Chem. 910, 121132 (2020). https://doi.org/10.1016/j.jorganchem.2020.121132
M. Y. Stogniy, S. A. Erokhina, I. B. Sivaev, et al., Phosphorus, Sulfur Silicon Relat. Elem. 194, 983 (2019). https://doi.org/10.1080/10426507.2019.1631312
D. Naoufal, B. Bonnetot, and H. Mongeot, Appl. Organomet. Chem. 17, 244 (2003). https://doi.org/10.1002/aoc.424
I. B. Sivaev, V. I. Bregadze, and S. Sjöberg, Collect. Czech. Chem. Commun. 67 (6), 679 (2002). https://doi.org/10.1135/cccc20020679
A. Jankowiak, A. Balinski, J. E. Harvey, et al., J. Mater. Chem. C 1, 1144 (2013). https://doi.org/10.1039/c2tc00547f
I. N. Klyukin, A. S. Kubasov, I. P. Limarev, et al., Polyhedron 101, 215 (2015). https://doi.org/10.1016/j.poly.2015.09.025
S. G. Shore, E. J. M. Hamilton, A. N. Bridges, et al., Inorg. Chem. 42, 1175 (2003). https://doi.org/10.1021/ic020540s
V. K. Kochnev, V. V. Avdeeva, E. A. Malinina, et al., Russ. J. Inorg. Chem. 59, 706 (2014). https://doi.org/10.1134/S0036023614070079
M. F. Hawthorne, I. J. Mavunkal, and C. B. Knobler, J. Am. Chem. Soc. 9, 4427 (1992).
A. V. Ezhov, F. Y. Vyal’ba, I. N. Kluykin, et al., Macroheterocycles 10, 505 (2017). https://doi.org/10.6060/mhc171254z
F. Abi-Ghaida, Z. Laila, and G. Ibrahim, Dalton Trans. 13087 (2014). https://doi.org/10.1039/c4dt00772g
M. Diab, A. Mateo, J. Al Cheikh, et al., Dalton Trans. 49, 4685 (2020). https://doi.org/10.1039/C9DT04676C
I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, et al., Mendeleev Commun. 30, 88 (2020). https://doi.org/10.1016/j.mencom.2020.01.029
I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, et al., Polyhedron 187, 114682 (2020). https://doi.org/10.1016/j.poly.2020.114682
D. S. Wilbur, M. K. Chyan, D. K. Hamlin, et al., Bioconjug. Chem. 18, 1226 (2007). https://doi.org/10.1021/bc060345s
D. S. Wilbur, M. Chyan, H. Nakamae, et al., Bioconjug. Chem. 23, 409 (2012).
K. Shelly, C. B. Knobler, and M. F. Hawthorne, Inorg. Chem. 31, 2889 (1992). https://doi.org/10.1021/ic00039a041
ACKNOWLEDGMENTS
The studies were carried out using the equipment at the Center for Collective Use of the Physical Methods of Investigation of the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, which functions with the support of the State Assignment of the Kurnakov Institute RAS in the field of fundamental scientific research.
Funding
This work was supported by the Russian Science Foundation (grant no. 20-73-00326).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Klyukin, I.N., Kolbunova, A.V., Selivanov, N.A. et al. Study of Protonation of the Monocarbonyl Derivative of the closo-Decaborate Anion [B10H9CO]–. Russ. J. Inorg. Chem. 66, 1798–1801 (2021). https://doi.org/10.1134/S003602362112007X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602362112007X